Introduction
Resistance to antibiotics is a worldwide problem, which may be reduced by prescribing less antibiotics and reducing inappropriate prescriptions (Wise et al., Reference Wise, Hart, Cars, Streulens, Helmuth, Huovinen and Sprenger1998; Butler et al., Reference Butler, Dunstan, Heginbothom, Mason, Roberts, Hillier, Howe, Palmer and Howard2007). More than 90% of antibiotics in Europe are prescribed in primary care (Goossens et al., Reference Goossens, Ferech, Vander Stichele and Elseviers2005). For urinary tract infections (UTIs) the initial prescription of antibiotics is usually ‘blindfolded’, without knowing the causing uropathogen and its susceptibility to antibiotics. This is considered sufficient for healthy non-pregnant women, because of the high prevalence of uropathogen Escherichia coli and its susceptibility to nitrofurantoin and fosfomycin (Den Heijer et al., Reference Den Heijer, Donker, Maes and Stobberingh2010). However, there are several groups of patients, including pregnant women and all men, who are at high risk for UTI complications (pyelonephritis, prostatitis or urosepsis) because of different uropathogens or immunosuppression. Therefore, the guideline of Dutch College of General Practitioners (GPs) on UTI (NHG-guideline) (Van Pinxteren et al., Reference Van Pinxteren, Knottnerus, Geerlings, Visser, Klinkhamer, Van der Weele, Verduijn, Opstelten, Burgers and Van Asselt2013) recommends a urine culture and specific antibiotics for these high-risk patients. If a culture is ordered, the guideline recommends that antibiotics should be started empirically in anticipation of the culture result, after which a switch can be made if resistance is seen. This Dutch guideline is very similar to the UK guideline for GPs (Health Protection Agency, 2011), with the exception of two risk factors: recurrent UTI is not considered a risk factor for complications in the Dutch guideline, whereas, on the other hand, immune disorders are not mentioned in the UK guideline (Health Protection Agency, 2011). The two guidelines are quite similar regarding antibiotic recommendations. Both guidelines advise nitrofurantoin and trimethoprim as first choice antibiotics for low-risk patients, however, the Dutch guideline also includes fosfomycin. In addition, in the Dutch guideline co-amoxiclav is the second choice instead of amoxicillin for any patient without tissue invasion and co-trimoxazole for patients with tissue invasion.
In Dutch general practice, resistance to the recommended antibiotics varied from 2% (nitrofurantoin) to 26% (trimethoprim) for the most common uropathogen E. coli in cultures (De Greeff et al., Reference De Greeff, Mouton and Schoffelen2015).
In most countries, studies mainly focus on treatment of UTI and rarely on use of cultures in general practice. A Spanish GP study shows that for 33% of low-risk patients a culture is ordered, even though not meeting the national guideline criteria for a culture (Llor et al., Reference Llor, Rabanaque, López and Cots2011). A study among GPs in the UK shows a culture was ordered in 40% of low-risk patients and in 39–98% of high-risk patients, depending on risk factor.
When prescribing antibiotics for UTI, only 42% (Braspenning et al., Reference Braspenning, Schellevis and Grol2004) to 50% (Van Bergeijk and Berger, Reference Van Bergeijk and Berger2008) of Dutch GPs appear to adhere to the therapeutic recommendations in the national guideline. The current guideline on UTI is well-known among GPs in The Netherlands, but a study also showed that Dutch GPs still perceive several barriers implementing this guideline, like unavailable diagnostic materials or unavailable dosages of medication (Lugtenberg et al., Reference Lugtenberg, Burgers, Zegers-van Schaick and Westert2010).
To support a more rational prescription of antibiotics in primary care, more data are needed on current use of cultures and related to this, antibiotic prescriptions. Therefore, our aim is to identify subgroups of UTI patients in which the use of urine cultures and antibiotic prescriptions deviates from the guidelines. We also investigate the influence of established risk factors for UTI complications (eg pregnancy, immunodeficiency), common comorbidities (like cardiovascular or pulmonary diseases), age and a history of recurrent UTI on urine culture requests.
Methods
This is an observational study using anonymous electronic patient records (EPR) from seven practices (24 GPs and 30,452 patients) from the Family Medicine Network (FaMe-Net). This is a practice-based research network from the Radboud University Medical Centre in Nijmegen, The Netherlands. FaMe-Net originates from a fusion between the Continuous Morbidity Registration (Van Weel, Reference Van Weel2008) and Transitionproject (Okkes et al., Reference Okkes, Oskam, Van Boven and Lamberts2005). Of seven practices, three practices are located in Nijmegen (Southeast; urban and semi-urban area), two practices in Amstelveen (West; semi-urban area), one in Olst (East; rural area) and one in Franeker (North; rural area). The study practices are representative to Dutch practices in terms of average patient population size, male-to-female GP ratio and proportion of practices that include GP trainees (CBS Statistics Netherlands; Netherlands Institute for Health Services Research (NIVEL)). However, the majority of our study practices are group practices consisting of three or more GPs, whereas fewer solo or duo practices are included in comparison to the general practices across The Netherlands. The patient population in this network is representative of the general Dutch population in terms of age and sex (CBS Statistics Netherlands). Participating GPs have special interest in primary care research and code each episode of care according to the International Classification of Primary Care (ICPC) (Hofmans-Okkes and Lamberts, Reference Hofmans-Okkes and Lamberts1996). An episode of care is defined as a health problem in an individual from the first to the last visit related to the specified health problem. All actions from the GP, including physical examination, diagnostic tests and prescriptions, are systematically coded. The validity of registration is high, as participating GPs meet regularly to discuss registration and diagnostic criteria. Moreover, the system warns the GP in case of error or inconsistency in registration. Research with FaMe-Net data is exempted from ethical review by the CCMO (Dutch Central Committee on research involving human subjects).
The study population included all patients with ICPC codes U71 (cystitis), U70 (pyelonephritis) or Y73 (prostatitis) that presented to their GP in 2015 (n=1295). If patients had multiple UTIs, only the first diagnosis in 2015 was included.
Definition of risk for UTI complication
The risk of UTI complications was classified according to the Dutch national guideline on UTI and is summarized in Table 1 (Van Pinxteren et al., Reference Van Pinxteren, Knottnerus, Geerlings, Visser, Klinkhamer, Van der Weele, Verduijn, Opstelten, Burgers and Van Asselt2013). High-risk patients had at least one of the risk factors. Consequently, low-risk patients include all patients with an UTI without any of these high-risk criteria.
Table 1 Characteristics of study population and likelihood of urine culture request in subgroups (n=1295)
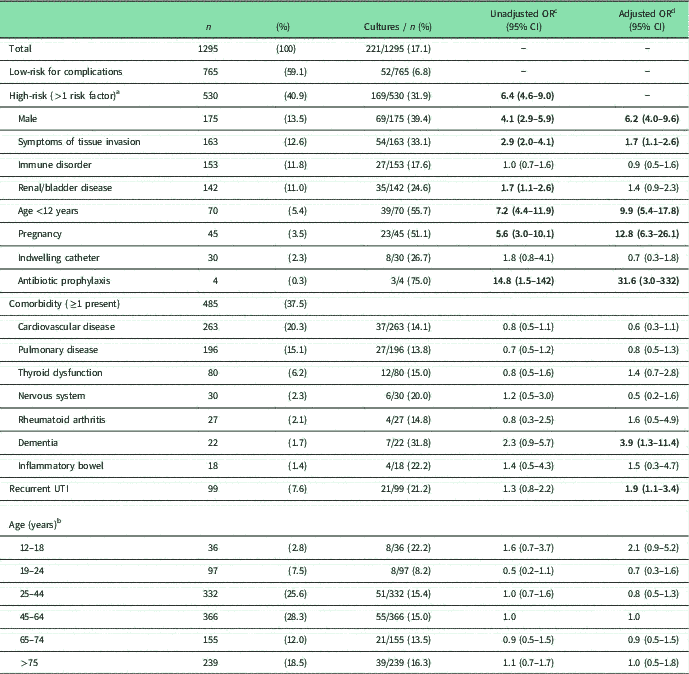
a Patients with multiple risk factors are included for each risk factor.
b For age 0–12 months, see risk factor ‘Age <12 years’.
c Statistically significant results are printed in bold. OR present the likelihood of a urine culture being requested in patients with that characteristic compared with all other patients without that specific characteristic.
d OR are adjusted: models include all risk factors, all comorbidities, a history of recurrent UTI and age groups compared with the largest age group of 45–64 years simultaneously.
Data extraction
Data from the EPR were extracted in two steps. In step one, a data set including all ICPC codes registered in 2015 were extracted to identify patients that presented with a UTI to their GP in 2015. In step two, complete data from all available years before 2015 were extracted for all selected UTI patients. Data were extracted from the EPR, which contains ICPC codes (diagnosis), Anatomical Therapeutic Chemical (ATC) codes (medication prescriptions using Anatomical Therapeutic Chemical classification system), intervention codes, laboratory codes, written lines by the GP and letters from other (medical) professions. For each subject the characteristics that matched the criteria for a risk group were classified, using a combined list of ICPC, ATC and laboratory codes and keywords (see Appendices I, II and III for full list).
Urine cultures
We extracted data on all urine cultures ordered one day before UTI diagnosis until 14 days after, by an electronic search of the written lines in the EPR and by screening the letters from microbiologists and laboratories.
Medical history
We retrieved data on common comorbidities (other than risk factors for complications of UTI) with a list of ICPC codes (see Appendix II) using full medical history data in the EPR. Comorbidities included cardiovascular and pulmonary disease, inflammatory bowel diseases, thyroid dysfunctions, rheumatoid arthritis, dementia and diseases of the nervous system.
Recurrent UTI was defined as three or more UTIs in the preceding 12 months (Epp et al., Reference Epp, Larochelle, Lovatsis, Walter, Easton, Farrell, Girouard, Gupta, Harvey, Robert, Ross, Schachter, Schulz, Wilkie, Ehman, Domb, Gagnon, Hughes, Konkin, Lynch and Marshall2010).
Statistical analysis
The main outcome was the order of a culture, and the main determinants were subgroups at high risk for complications of UTI, comorbidities, history of recurrent UTI and age groups compared with the largest age group of 45–64 years. We categorized age of patients into seven groups for analyses. We analysed the association between urine culture orders and each of the above-described patient characteristics individually with an unadjusted logistic regression analysis. An adjusted logistic regression model, where all the above-described characteristics were entered simultaneously, was used to identify which factors contributed independently. We evaluated the compliance of GPs to the national guideline (Van Pinxteren et al., Reference Van Pinxteren, Knottnerus, Geerlings, Visser, Klinkhamer, Van der Weele, Verduijn, Opstelten, Burgers and Van Asselt2013) for the use of urine cultures and the type of prescribed antibiotics with descriptive statistics and χ2-tests. All analyses were performed using SPSS version 22. A P-value of <0.05 was considered statistically significant.
Results
Patient characteristics
In total, 530 (40.9%) patients had one or more risk factors for complications of UTI (Table 1). The most common high-risk characteristic was male gender (13.5%), followed by symptoms of tissue invasion (12.6%), immune disorders (11.8%) and renal/bladder diseases (11.0%). Selected comorbidities were present in 37.5% of patients, and 7.6% of patients had a history of recurrent UTI.
Urine cultures
A urine culture was ordered in 221 patients (17.1%) (Table 1). Urine cultures were more commonly ordered in high-risk (31.9%) than low-risk patients (6.8%) (OR=6.4, 95% CI 4.6–9.0). Relatively more cultures were ordered in patients that used antibiotic prophylaxis (75.0%), followed by patients aged under 12 (55.7%), and pregnant women (51.1%), although fewer cultures were ordered in patients with an indwelling catheter (26.7%), renal/bladder disease (24.6%) or immune disorder (17.6%).
As expected, urine culture orders were more common among several subgroups of patients at high risk for complications. Adjusted analyses (including all risk factors for complications, comorbidities, a history of recurrent UTI and age) show that urine cultures were significantly more often ordered in patients with antibiotic prophylaxis (OR=31.6), pregnancy (OR=12.8), age <12 years (OR=9.9), male (OR=6.2) or symptoms of tissue invasion (OR=1.7) compared with patient groups without these characteristics, respectively (see Table 1). Strikingly, urine cultures were not more often ordered in patients with immune disorder, renal/bladder disease or an indwelling catheter, whereas these patients are also at high risk for complications of UTI, and the national guideline recommends urine cultures in these patients. In addition, culture orders were significantly more common in patients with recurrent UTI (OR=1.9) and dementia (OR=3.9), compared with those without recurrent UTI or dementia, respectively. There was no statistically significant influence of age or other comorbidities on urine culture orders.
In 6.8% of low-risk patients, a urine culture was ordered. In the low-risk group urine cultures were more common in patients with dementia (OR=8.3; 95% CI 1.8–38.3, results not shown). None of the other comorbidities, age or recurrent UTI had a statistically significant effect on urine culture orders.
Among high-risk patients, we found no influence of comorbidities, age or recurrent UTI on urine culture orders (results not shown).
Antibiotics
Almost all UTI patients (94%) were prescribed antibiotics, mostly nitrofurantoin (68.7%), followed by ciprofloxacin (8.8%) and fosfomycin (6.5%). In the low-risk group, 31 (4.1%) patients did not receive antibiotics, compared with 47 (8.9%) in the high-risk group (P-value <0.001). As shown in Table 2, recommended antibiotics (first, second or third choice in the guideline) were prescribed to 91.4% of low-risk patients. For high-risk patients adherence to the recommended antibiotics varied strongly by risk factor. Higher adherence was seen in children and pregnant women without tissue invasion, 91.7 and 80.0%, respectively. Lower adherence was observed in men without tissue invasion (50%) and high-risk (non-pregnant female) patients with tissue invasion (range 28.6–50.0%) (see Table 2).
Table 2 Antibiotic prescriptions for UTI in subgroups at risk for UTI complications, compared with recommended prescriptions in the Dutch GP guideline (Van Pinxteren et al., Reference Van Pinxteren, Knottnerus, Geerlings, Visser, Klinkhamer, Van der Weele, Verduijn, Opstelten, Burgers and Van Asselt2013)
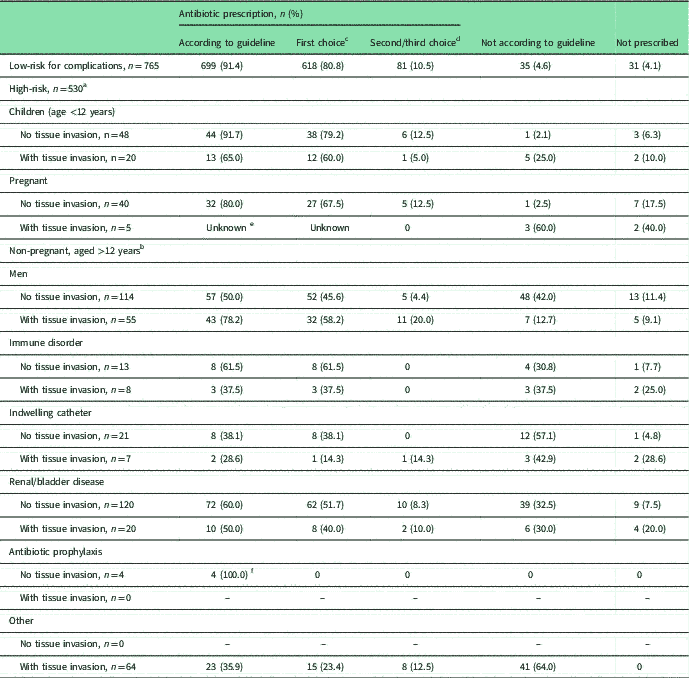
(NHG=Nederlands Huisartsen Genootschap/Dutch College of General Practitioners)
a For each high-risk group, patients with and without tissue invasion are distinguished because of different antibiotic recommendations in NHG-guideline.
b Patients with multiple risk factors are included for each risk factor.
c First choice antibiotic is nitrofurantoin for all subgroups, except for patients with tissue invasion where first choice is co-amoxiclav in children aged <12 years, and ciprofloxacin in other patients with tissue invasion. Pregnant women with tissue invasion are recommended to be referred to hospital.
d Second choice antibiotic is fosfomycin (low-risk patients), co-amoxiclav (children without tissue invasion/pregnant women without tissue invasion/non-pregnant patients aged >12 years with tissue invasion), co-trimoxazole (children with tissue invasion), trimethoprim (non-pregnant patients aged >12 years without tissue invasion); third choice antibiotic is only listed for low-risk patients (ie trimethoprim) and non-pregnant patients aged >12 years with tissue invasion (ie co-trimoxazole).
e There were no data available due to referrals from hospital (as recommended in guideline).
f National GP guideline advises a switch in antibiotics.
Discussion
Most important findings
This study shows a low percentage (32%) of urine culture ordered in patients with risk factors for complications of UTI. Culture orders were particularly low in patients with immune disorder (18%), renal/bladder disease (25%) or an indwelling catheter (27%). Antibiotics were prescribed to the majority of patients (94%), but compliance to the national guideline varied broadly by risk factor for complications (29–92%).
Strengths and limitations
One of the major strengths is the high-quality of recording in this cohort, as inconsistency in registration is flagged by the system and GPs from the network discuss registration issues regularly. Also, the number of patients with an UTI included in our study was high (n=1295) and we had access to individual patient data from the EPR. This is the first study in The Netherlands that examined urine culture orders for UTI in general practice, identifying patient characteristics in whom a culture was more frequently ordered.
There are several limitations worth mentioning. First, the time period in which temporary risk factors (pregnancy and indwelling catheter) were present had to be estimated. Therefore, we compared the prevalence of these risk factors with those found in the literature, and were found to correspond to our figures. Van Bergeijk and Berger (Reference Van Bergeijk and Berger2008) found 3.1% pregnant patients with UTI in Dutch general practice (compared with 3.5% in our study). Indwelling catheter appeared in 3% of female patients in the study of Hummers-Pradier et al. (Reference Hummers-Pradier, Ohse, Koch, Heizmann and Kochen2005), compared with 2.3% in our study. For indwelling catheters, we assumed a standard use of 12 weeks. Patients who had their catheter removed sooner are potentially misclassified as high risk, which may (partly) explain the lower rate of urine culture orders in this group. Second, it was not possible to identify patients with ‘failure of two blindfolded antibiotic prescriptions’, which is listed as an additional risk factor for complications of UTI in the national guideline, because we were not able to distinguish in the EPR if symptoms had disappeared or continued between two prescriptions for antibiotics. Third, the result of slightly more patients in the high-risk group that did not receive antibiotics (8.9% compared with 4.1% in the low-risk group) was unexpected. This finding might be partially explained, by having misclassified some patients as high risk (ie not all patients with malignancies received chemotherapy/radiotherapy, as we have assumed). Also, some high-risk patients may have been referred to hospital and were prescribed antibiotics by a medical specialist, which is not registered in the EPR in general practice. Lastly, the GPs in our database were all part of the FaMe-Net practices, which are GPs with special interest in research and have frequent meetings to optimize accurate registration in the EPR, so perhaps the compliance with the guideline is higher than among other GPs.
Comparison to other literature
In comparison with the study by Llor et al. (Reference Llor, Rabanaque, López and Cots2011) in Spain, who found that GPs ordered urine cultures in 33% of low-risk patients, the GPs in our study performed better by ordering fewer cultures in low-risk patients (7%). This might be because of the absence of dipslides in the Spanish study population, which means the only available diagnostic tool is a culture.
Ironmonger et al. (Reference Ironmonger, Edeghere, Gossain and Hawkey2016) questioned GPs in the UK on the use of urine culture in patients with and without risk factors. As we did, they found a notable difference in culture orders in low-risk (40%) and high-risk patients, with differences by risk factor (38% for indwelling catheter to 98% for male patients). However, they investigated only a selection of risk factors in five hypothetical patients, making it difficult to compare the reported percentages with our data. In comparison with a Dutch study by Den Heijer et al. (Reference Den Heijer, Donker, Maes and Stobberingh2010), who investigated the antibiotic prescription rate by GPs for low-risk patients with UTI and the susceptibility of E. coli to common antibiotics, the percentage of antibiotic prescriptions corresponding to the guideline for low-risk patients in our study is higher, namely 91% compared with 77%. This might be partially due to the continuing decrease in use of quinolones for low-risk patients, as has been advised in the national guideline since 2005.
Implications
Both diagnostic and therapeutic adequacy could be improved, which is implicated by the relatively low percentage of culture orders and high use of inappropriate antibiotics (ie non-recommended in the guideline) in several subgroups of high-risk patients. This is worrying because resistance to commonly prescribed antibiotics in the high-risk group is often seen (De Greeff et al., Reference De Greeff, Mouton and Schoffelen2015). Not ordering a urine culture in high-risk patients can have several reasons, for example, the GP is not aware of all risk factors for complications of UTI, the GP might not see the benefit of a culture owing to experience and the low incidence of complications, or possibly the patient does not want a culture because of financial costs. The underlying motives for not ordering cultures should be further explored.
The percentage of culture orders in low-risk patients is relatively low (7%), but not 0% as would have been expected with perfect compliance to guidelines. This group might still contain some high-risk patients with risk factor ‘failure of two blindfolded given antibiotics’, which we have not been able to identify. Furthermore, we found that patients with dementia are more likely to get a culture order. In case of an unexplained fever in patients with dementia, a urine culture might be routinely performed to find the cause. Moreover, owing to a difficult anamnesis, the efficacy of antibiotics is hard to monitor in these patients, so a urine culture may contribute in evaluating treatment effects. As dementia is not specifically mentioned in the guideline, it would be of interest to investigate the reasons of GPs for performing more cultures in this patient group, and its potential value.
For high-risk patients the adherence to recommended antibiotics varied strongly by risk factor. Reasons for non-adherence should be further explored, focussing both on GP-related factors and patient influences. It is unknown whether the lower rates of adherence in some subgroups of patients are based on intentional or non-intentional decisions by GPs or driven by patient requests.
The Dutch GP guideline on UTI is currently under revision, and our findings imply the need of critical review of current policies, particularly in the highlighted subgroups. As non-adherence is low in subgroups of patients, future revisions of the guideline should evaluate current policies and include information on the cost-effectiveness of urine cultures in specific subgroups of patients and address issues related to non-adherence.
In conclusion, in The Netherlands urine cultures are ordered by GPs in only a third of patients at high risk for complications of UTI, even though the guideline recommends cultures in all high-risk patients. Antibiotics were prescribed to nearly all patients, but compliance to the national guideline varied a lot by risk factor. Diagnostic and therapeutic adequacy can be improved in UTI patients at high risk for complications by ordering more cultures and adjust prescriptions to antibiotic susceptibility of the uropathogen, and improve antibiotic prescriptions adhering to the national guideline. This may contribute to containing antibiotic resistance in UTI.
Acknowledgements
We thank all GPs from FaMe-Net for enabling research with data from their EPR and the staff from Radboudumc Technology Center – Health Data for providing this data.
Financial Support
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
Conflict of Interests
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guidelines on human experimentation (Dutch Central Committee on Research Involving Human Subjects) and with the Helsinki Declaration of 1975, as revised in 2008.
Appendix I: Additional information on methods
Full definition for immunocompromised patients
Immune disorders include diabetes mellitus, the use of chemotherapy or radiotherapy (defined as a malignancy in the previous two years* for which those are commonly received), use of immunosuppressants or immunostimulants, and a medical history with immune deficiencies, AIDS/HIV, splenectomy or a received organ transplantation.
*According to the Health Council of The Netherlands (Van Pinxteren et al., Reference Van Pinxteren, Knottnerus, Geerlings, Visser, Klinkhamer, Van der Weele, Verduijn, Opstelten, Burgers and Van Asselt2013), most of the treatment and short-term follow-up with specialized care in the hospital takes place within the first and second year after diagnosis of a malignancy. Therefore, we consider patients within the first two years after diagnosis of a malignancy for which chemotherapy or radiotherapy is often given, to be immunocompromised, owing to the high chance of receiving either treatments.
Electronic search with keywords
Risk factors, comorbidities and diagnostic tests in the written lines of the EPR appeared in the database as free text and were searched using the following protocol to optimize standardized data extraction. The free text was searched electronically for keywords, with the use of iterative processing (ie including additional keywords based on interim checks) to fine tune the result. To find the absence or presence of a symptom or test, different positive and negative prefixes and suffixes were used in addition to searches with the single keywords. Spelling errors in keywords were anticipated and overcome by testing possible errors. The final result of the electronic search was checked with random samples and proved to be of good quality.
Classifying diabetes
In the NHG-guideline, it is described that otherwise healthy women with diabetes may be considered as low risk for complications of UTI. After selection of all female patients with diabetes, but with no other comorbidities, no risk factor and aged under 60 (Health Protection Agency, 2011), we identified only three patients and we have therefore chosen to classify all diabetic patients as high risk.
Other
∙ The date of all given ICPC and ATC codes for risk factors and comorbidities had to occur before the date of the UTI of that patient, in order to be included in the analysis.
∙ All patients had to be subscribed to the practice for the full period of data collection.
∙ In case a patient had a culture found in multiple ways, the most extensive or detailed one was used.
Appendix II: ICPC codes used to identify comorbidities
Appendix III: Definitions of risk factors for complications of UTI, according to the NHG-guideline and description of data extraction







