The rationale for the prescription of injectable methadone to opiate-dependent patients is twofold (Reference Farrell, Ward and MattickFarrell et al, 1994). Firstly, that it attracts intravenous opiate-dependent individuals into treatment who would not have been attracted by a prescription for oral methadone. Secondly, that these individuals can be encouraged to change their drug-using behaviour and the prescription can then be changed to oral methadone. We do not know of any studies to date that show how, or indeed whether, change can be achieved. One paper concerning the transition from methadone tablets to mixture describes the process as difficult, with negative consequences for both patients and staff (Reference Steels, Hamilton and McLeanSteels et al, 1992).
Prior to the appointment of a consultant with special responsibility for substance misuse, there was a long history in Shropshire of prescribing injectable methadone for opiate-dependent patients. In 1998, a decision was taken by the consultant to transfer all such patients to oral methadone because of concerns that methadone ampoules were being diverted to the illicit market. This decision was not supported by some of the workers in the community substance misuse teams who predicted that such a change would cause patients to deviate from treatment, to increase their use of illicit opiates and put themselves at greater risk of a fatal or non-fatal overdose. They also argued that some of the patients would not be able to tolerate oral methadone mixture because of nausea or vomiting.
Our experience was that the transition from injectable to oral methadone was completed successfully and safely, with no evidence of intolerance to oral methadone, no loss of cases from treatment due to the transfer, no long-term deterioration in clinical stability and no problems with overdose or death. This paper describes the transition process and the subsequent progress of this group of patients.
Process of transition
At the beginning of 1998, there were 14 patients being prescribed injectable methadone. All were seen by the consultant, informed that their prescription would be changed to oral methadone and given an explanation for this. No specific time limit was placed upon the transition, although it was hoped that it would be achieved within 6 months. Most patients were unhappy about the idea of receiving oral methadone, predicting that it would ‘destabilise’ their treatment. Some suggested that they enjoyed the act of injecting and would therefore have to return to injecting illicit opiates. A few patients saw it as an opportunity to progress through treatment.
All patients were offered a titration at the in-patient unit. This was partly for reasons of safety — injectable methadone prescriptions had not been supervised and there was no guarantee that the medication had been used as prescribed. The titration also ensured that adequate oral doses of methadone would be given. As an alternative to titration, an interim period was offered, during which both intravenous and oral methadone was offered. If patients chose neither of these options, their prescriptions were simply changed at their out-patient appointments.
It should be mentioned that there were no alternative local treatment providers of intravenous methadone and that general practitioners in Shropshire do not prescribe injectable opiates. Patients therefore had little choice but to accept this change.
Case notes were examined at the beginning of 2001, looking at progress since transition and, in particular, at evidence from the results of urine tests. Evidence of injecting was taken from recorded examination for needle marks and patients' self-reporting on the frequency of this.
Characteristics at initial presentation
These patients had presented to the service between 1989 and 1997 as intravenous opiate users. Their age range at presentation was between 23 and 41 years. Many had been prescribed injectable methadone from their initial appointments without first being given a trial of oral methadone. Two patients were injecting into the neck and one into the groin. Some were receiving both intravenous and oral methadone for no clear reason. There had been some previous attempts to alter prescriptions to oral methadone, without success. Evidence that patients had not been using illicit opiates at the time of transition was based on urine tests in the 3 months prior to transition. The patients' characteristics at the time of transition are described in Table 1.
Table 1. Patient characteristics at the time of transition

| Patient | Age | Length of intravenous (i.v.) prescription (years) | Daily dose of i.v. methadone (mg) | Evidence of abstinence from illicit drugs | Duration of transition |
|---|---|---|---|---|---|
| 1 | 36 | 6.5 | 30 (+30 ml oral) | Yes | Day titration |
| 2 | 36 | 4 | 50 (+50 ml oral) | Yes | Day titration |
| 3 | 31 | 3 | 20 (+50 ml oral) | Yes | Immediate |
| 4 | 25 | 2.5 | 50 | No | Immediate |
| 5 | 40 | 2.5 | 50 (+30 ml oral) | Yes | Day titration |
| 6 | 32 | 5 | 120 | Yes | Day titration |
| 7 | 25 | 2 | 60 | No | 3 months of both oral and i.v. |
| 8 | 32 | 5 | 40 | Yes | 6 months of both oral and i.v. |
| 9 | 30 | 7 | 100 | Yes | 1 month of both oral and i.v. |
| 10 | 42 | 1 | 50 (+35 ml oral) | No | Brief admission |
| 11 | 37 | 8 | 50 (+60 ml oral) | Yes | Immediate |
| 12 | 36 | 9 | 100 | Yes | Immediate |
| 13 | 40 | 1.5 | 40 | Yes | Immediate |
| 14 | 29 | 1 | 50 (+60 ml oral) | Yes | Immediate |
Drug use in the 6 months following transition
Eight patients immediately and completely ceased injecting. One continued to inject illicit opiates in addition to their oral methadone at least daily and five injected illicit drugs less than daily. There were no deaths or known complications as a result of this and patients were not lost to treatment during this 6-month period. No one complained about intolerance to methadone mixture.
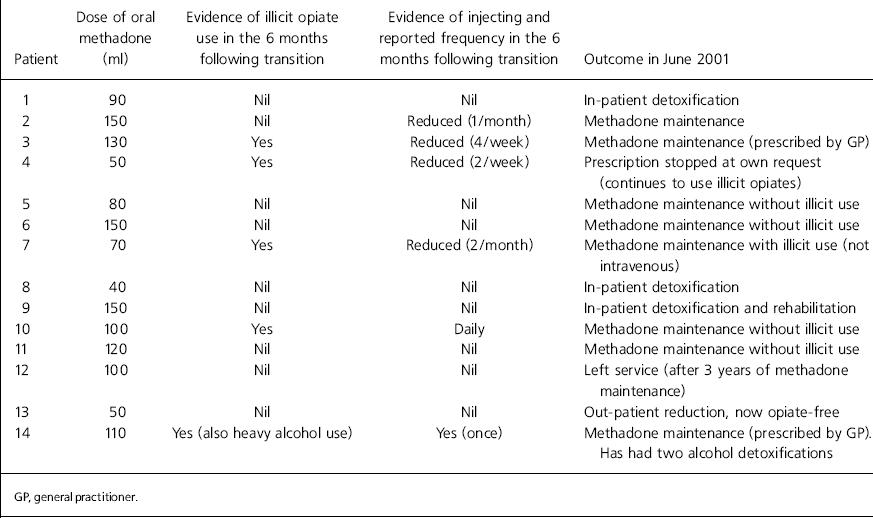
Patients reacted differently to the change. Two individuals were abusive and threatening and two complained vigorously with distress over a period of several months. Some complained briefly and others accepted the change with little or no comment. There were no incidents of physical violence to staff. The outcome of the six months following transition is described in Table 2.
Table 2. Outcome following transition at 6 months and 3 years
| Patient | Dose of oral methadone (ml) | Evidence of illicit opiate use in the 6 months following transition | Evidence of injecting and reported frequency in the 6 months following transition | Outcome in June 2001 |
|---|---|---|---|---|
| 1 | 90 | Nil | Nil | In-patient detoxification |
| 2 | 150 | Nil | Reduced (1/month) | Methadone maintenance |
| 3 | 130 | Yes | Reduced (4/week) | Methadone maintenance (prescribed by GP) |
| 4 | 50 | Yes | Reduced (2/week) | Prescription stopped at own request (continues to use illicit opiates) |
| 5 | 80 | Nil | Nil | Methadone maintenance without illicit use |
| 6 | 150 | Nil | Nil | Methadone maintenance without illicit use |
| 7 | 70 | Yes | Reduced (2/month) | Methadone maintenance with illicit use (not intravenous) |
| 8 | 40 | Nil | Nil | In-patient detoxification |
| 9 | 150 | Nil | Nil | In-patient detoxification and rehabilitation |
| 10 | 100 | Yes | Daily | Methadone maintenance without illicit use |
| 11 | 120 | Nil | Nil | Methadone maintenance without illicit use |
| 12 | 100 | Nil | Nil | Left service (after 3 years of methadone maintenance) |
| 13 | 50 | Nil | Nil | Out-patient reduction, now opiate-free |
| 14 | 110 | Yes (also heavy alcohol use) | Yes (once) | Methadone maintenance (prescribed by GP). Has had two alcohol detoxifications |
Progress from transition to June 2001
Four patients had ceased to use opiates — three after inpatient detoxification and one by methadone reduction as an out-patient. Six patients were receiving methadone maintenance from the service with one of these using illicit opiates but not injecting. Two patients were receiving oral methadone maintenance from their general practitioner and it was not known whether they were using illicit opiates. One patient had his methadone stopped at his own request and was understood to be using illicit opiates. One patient had left the service 3 years after transition.
Patients were counted as having ‘no illicit opiate use’ if the results of their unsupervised urine tests during the 3 months to June 2001 were negative for opiates. The outcome for each patient in June 2001 is shown in Table 2.
Summary
This is a group of 14 patients who had been receiving injectable methadone for several years and who had shown no previous inclination to change formulation. In the period of transition, there was some short-lived additional intravenous illicit opiate use and one case of heavy alcohol use, but the dire predictions from patients and community drug workers did not occur. Generally, the patients settled into their previous pattern, either staying clear of illicit opiates or using them intermittently on top of their prescription. After 3 years of follow-up, 9 of the group whose outcome is known are free from illicit opiate use.
Without a control group, we cannot comment on whether these outcomes would have occurred if transition had not been enforced, but given that these patients had changed little during their preceding 4.7 years of treatment, this seems unlikely.
We believe that we have shown that transition from injectable to oral formulations is not as problematic as may be expected.
Discussion
One community postal survey has shown that 9.3% of all prescriptions of methadone are for the injectable form and that this is being prescribed in higher doses with less frequent pick-up schedules than is the oral form (Reference Strang, Sheridan and BarberStrang et al, 1996). This group of patients may experience poorer physical and mental health, use more illicit drugs and have more social problems than those receiving oral methadone (Reference Felder, Uehlinger and BaumannFelder et al, 1999); problems which probably predate treatment.
It is not known whether this is a static population or whether these individuals are being stabilised and then encouraged to progress to oral methadone. It is not even clear whether transition from injectable to oral methadone is a ‘progression’, although oral methadone obviously reduces the risk of overdose, the harm associated with chronic injecting and the dangers related to the site of injection such as the groin or neck. There is some recent evidence that after 6 months of follow-up, individuals prescribed injectable methadone have similar outcomes to those prescribed oral methadone (Reference Strang, Marsden and CumminsStrang et al, 2000).
The Department of Health guidelines for clinical management of drug misuse and dependence (Department of Health, 1999) state that ‘though there is a very limited place for the prescribing of injectable formulations by specialists, prescribing should generally aim to minimise injecting’. No official guidelines have been published as to a suitable length of time for continuing injectable methadone or the criteria for transition.
Sarfraz and Alcorn (Reference Sarfraz and Alcorn1999) have published a set of suggested guidelines, based on their clinical practice, including aims of treatment, essential features of a prescribing policy, patient eligibility and assessment procedure. They suggest that one of the aims of treatment should be ‘to move away from injectable to oral methadone use’.
This limited, small study suggests that patients prescribed intravenous methadone do not progress readily to oral methadone mixture. However, it is possible to transfer patients to oral methadone without losing them from treatment, without causing a long-term deterioration in clinical stability, without precipitating persistent additional use of illicit opiates and with no episodes of non-fatal or fatal overdose. Importantly, for some patients the transition is associated with an improved clinical condition, not least because daily injecting ceases. For a few, the transition acts as an incentive to become completely drug-free. Any pessimism about attempting to move individuals away from intravenous methadone to oral methadone is not justified. Outcomes are favourable, even when the change is imposed rather than sought, although this requires determination on the part of the practitioner.





eLetters
No eLetters have been published for this article.