There is little research detailing the changes to the human brain as a result of chronic opioid use and in early abstinence. We have previously used [11C]-diprenorphine positron emission tomography (PET) to measure levels of available opioid receptors in the brain of patients on methadone maintenance, but found no detectable occupancy by methadone (Reference Melichar, Hume and WilliamsMelichar et al, 2005). Other PET studies have reported that increased binding of [11C]-carfentanil in withdrawal and abstinence in cocaine and alcohol dependence is associated with craving (Reference Zubieta, Gorelick and StaufferZubieta et al, 1996; Reference Gorelick, Kim and BencherifGorelick et al, 2005; Reference Heinz, Riemold and WraseHeinz et al, 2005). A preliminary study also reported an increase in [11C]-carfentanil binding in people with opioid dependence who were briefly maintained on buprenorphine (Reference Zubieta, Greenwald and LombardiZubieta et al, 2000). In this study we present data on the binding of the opioid receptor PET tracer [11C]-diprenorphine, which labels μ, κ and δ opioid receptors, in people with opioid dependence during early abstinence. We measured [11C]-diprenorphine binding in brain areas implicated in dependence and its relationship to clinical variables. Our hypothesis was that in people with opioid addiction, opioid receptor availability would be increased in early abstinence and that this would be related to craving.
METHOD
Participants
We recruited ten people with opioid dependence (8 male, 2 female; mean age 31.7 years, s.d.=6.3, range 25-45) undergoing in-patient detoxification at Bristol Specialist Drug Service (Bristol, UK). All fulfilled DSM-IV criteria for opioid dependence (American Psychiatric Association, 1994) prior to detoxification but were excluded if they fulfilled diagnostic criteria for another current Axis I disorder (excluding nicotine dependence). The participants were in their 10th day of a lofexidine-assisted in-patient detoxification regime from methadone and were free of opioids or any other drug of misuse as confirmed by urinalysis at the time of scanning. They were typically long-standing opioid users and had been on methadone for a mean of 6.9 years (s.d.=5.2, range 2-17 years); the mean dose at the start of detoxification was 31 mg/day (s.d.=12, range 15-50). The patients had a history of using a variety of other drugs prior to admission (Table 1); all but one participant continued to use heroin in addition to their prescribed methadone in the month prior to detoxification, half were using ‘crack’ cocaine prior to detoxification and all were tobacco smokers. All participants underwent the standard detoxification regimen under the care of the clinical team, independent of the investigators.
Table 1 Drug and alcohol use in the 30 days prior to scanning

| Ever used (n=10) | Any use in past 30 days (n=10) | Days used in past 30 days | |||||
|---|---|---|---|---|---|---|---|
| Drug | Mean (s.d.) | Median | Range | ||||
| Heroin | 10 | 9 | 14.7 (4.7) | 17 | 6–19 | ||
| Intravenous | 10 | 7 | 12.7 (6.4) | 16 | 3–19 | ||
| Smoked | 10 | 4 | 17.5 (3.9) | 17.5 | 10–18 | ||
| Cannabis | 10 | 8 | 10.3 (7.7) | 10.5 | 1–20 | ||
| Crack | 9 | 5 | 8.2 (5.4) | 6 | 3–15 | ||
| Benzodiazepines | 10 | 4 | 15.0 (6.0) | 16 | 8–20 | ||
| Amphetamines | 9 | 0 | – | – | – | ||
| MDMA | 8 | 0 | – | – | – | ||
| LSD | 10 | 0 | – | – | – | ||
| Alcohol | 10 | 5 | 8.2 (6.3) | 5 | 2–16 | ||
| Nicotine | 10 | 10 | 30 | 30 | All 30 | ||
MDMA, methylenedioxymethamphetamine; LSD, lysergic acid diethylamide
Twenty healthy people (18 male, 2 female; mean age 34.8 years, s.d.=8.3, range 25-48) with no history of dependence on any drug except nicotine (all current non-smokers) were recruited as controls. Controls had no history of serious psychiatric or medical disorder as determined by clinical interview. They were recruited for this and other studies to form a common pool, to avoid unnecessary duplication and radiation exposure. Therefore, only 8 of the 20 controls completed all the same questionnaires as participants with opioid dependence. The control group for this study was selected to match the age range of participants with opioid dependence.
Local research ethics committees and the UK Administration of Radioactive Substances Advisory Committee approved all procedures and experimental protocols. After full explanation of the study procedures, volunteers gave written informed consent.
Clinical measures
Subjective measures included the Adjective Checklist (Reference JasinskiJasinski, 1997), which measures effects of opioid agonists (16 items) and opioid withdrawal symptoms (21 items), and the 49-item short form of the Addiction Research Centre Inventory (ARCI), which is scored for euphoria, dysphoria and sedation scales (Reference HaertzenHaertzen, 1970).
Objective evidence of withdrawal (Opiate Withdrawal Scale; OWS) was measured using an adaptation of the Kolb and Himmelsbach point system as previously described (Reference Law, Bailey and AllenLaw et al, 1997). Drug craving was assessed using two tools; the 45-item Heroin Craving Questionnaire (HCQ; Reference Weinstein, Wilson and BaileyWeinstein et al 1997) and the Obsessive Compulsive Drinking Scale (OCDS), adapted to measure opioid compulsive behaviour and obsessive thoughts, and allowing for mode of drug delivery (Reference Anton, Moak and LathamAnton et al 1996). Two experienced addiction clinicians (T.W. and M.D.) independently rated each patient for the amount of opioids used in the month and year prior to scanning, as well as lifetime use, using a structured rating scheme taking into account the combination of opioids used, length and route of use. We also looked for associations with published post-mortem data reporting regional densities of μ opioid receptors (Reference Pfeiffer, Pasi and MehraeinPfeiffer et al, 1982).
Participants also completed the Spielberger State-Trait Anxiety Inventory (SSAI, STAI; Reference SpielbergerSpielberger 1983), the 36-item short form of the General Health Survey (SF-36; Reference McHorney, Ware and LuMcHorney et al, 1994), the revised Eysenck Personality Questionnaire (EPQ-R; Reference Eysenck and EysenckEysenck & Eysenck, 1975) and the Eysenck Impulsiveness Questionnaire (IVE; Reference Eysenck, Pearson and EastingEysenck et al, 1985).
PET scans
All participants underwent [11C]-diprenorphine PET using a CTI/Siemens (Munich, Germany) ECAT 953b brain camera in high-sensitivity three-dimensional mode. A bolus of 370 MBq [11C]-diprenorphine was given intravenously over 30 s. Dynamic emission data were acquired over 90 min, in 18 time frames (27 frames for 9 controls) and reconstructed into 31 contiguous horizontal image planes (Reference Jones, Cunningham and Ha-KawaJones et al, 1994). Radioactivity in arterial blood was assayed continuously online in accordance with a standard protocol and discrete blood samples were taken every 5-10 min for assay of radiolabelled metabolites in plasma (Reference Ranicar, Williams and SchnorrRanicar et al, 1991).
Image processing and statistical analysis
The dynamic PET scans were analysed to produce parametric images of ligand volume of distribution using spectral analysis with in-house receptor parametric mapping software implemented in Matlab (Mathworks Inc., Natick, Massachusetts) (Reference Gunn, Gunn and TurkheimerGunn et al, 2002). Spectral analysis with individual metabolite corrected plasma input function takes account of any difference in tracer delivery between individuals or groups. The volume of distribution is the ratio of total free and bound tissue to free plasma ligand concentration at equilibrium and provides an index of receptor binding. We used Statistical Parametric Mapping (SPM2, Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, UK) running in Matlab to transform the volume of distribution images into a standard space as defined by the Montréal Neurological Institute (Reference Evans, Collins and MillsEvans et al, 1993) using a weighted-mean [11C]-diprenorphine template created inhouse from the PET scans of seven healthy volunteers.
We performed two types of analysis, first using predefined volumes of interest and then using SPM2 for an exploratory analysis. Twenty-one regions for which we had an a priori hypothesis for increased opioid receptor availability based on previous studies were selected for comparison. These areas were the orbito-frontal cortex, anterior cingulate, ventral striatum (including the nucleus accumbens), dorsal striatum (including the caudate nucleus and putamen), thalamus, amygdala and periaqueductal grey matter. All these regions have been shown to have a role in the addiction process. The anterior cingulate cortex, orbitofrontal cortex, nucleus accumbens and amygdala are key in reward and motivation during drug-using from the evaluation of stimuli to reward-based decision-making and learning. The periaqueductal grey matter is an important element of the endogenous opioid system and is involved in conditioned processes in dependent drug use. Similarly the thalamus, caudate and putamen form part of the emotional reward neurocircuitry which has an important role in motivational factors and links to motor pathways, possibly being a route for the development of locomotor sensitisation with continued drug use (Reference Kalivas and VolkowKalivas & Volkow, 2005; Reference Nutt, Robbins and StimsonNutt et al, 2006).
We used statistical parametric mapping to transfer 73 standardised volumes of interest derived from a probabilistic atlas of brain images (Reference Hammers, Allom and KoeppHammers et al, 2003) onto individual scans by inverting the deformations used to spatially normalise the images. The volume of distribution maps were sampled using the individualised atlas for every participant to generate mean volume of distribution values for each volume of interest. These values were then compared between groups using a two-tailed non-paired t-test - unequal variances were assumed. Pearson's correlation statistics were used to assess the association of clinical variables with opioid receptor binding.
In addition, [11C]-diprenorphine volume of distribution images were analysed on a voxel-by-voxel basis using SPM2. Spatially normalised parametric images were smoothed with a 12 mm kernel at full width half maximum. Mean differences between groups were interrogated using non-paired t-tests, and correlations between clinical variables and [11C]-diprenorphine binding were explored using linear regression within the general linear model in SPM2. Proportional scaling was used to normalise global differences throughout. For regions where there existed an a priori hypothesis, results are reported as significant at a threshold of P<0.05 uncorrected. For all other areas familywise error correction was used.
RESULTS
Clinical measures
At the time of scanning, there was no clinically significant opioid withdrawal as measured by the OWS (mean score 1.2, s.d.=1.2, range 0-3). However the subjective ARCI and Adjective Checklist showed higher measures of withdrawal in participants with opioid dependence than in controls. They showed significantly higher state, but not trait, anxiety than controls on the day of their scan. Participants with opioid dependence scored significantly lower than controls on some measures of health (Table 2). On the personality questionnaires participants with opioid dependence scored significantly higher for psychoticism, extraversion, impulsiveness and venturesomeness than controls, but not for neuroticism or empathy.
Table 2 Clinical measures
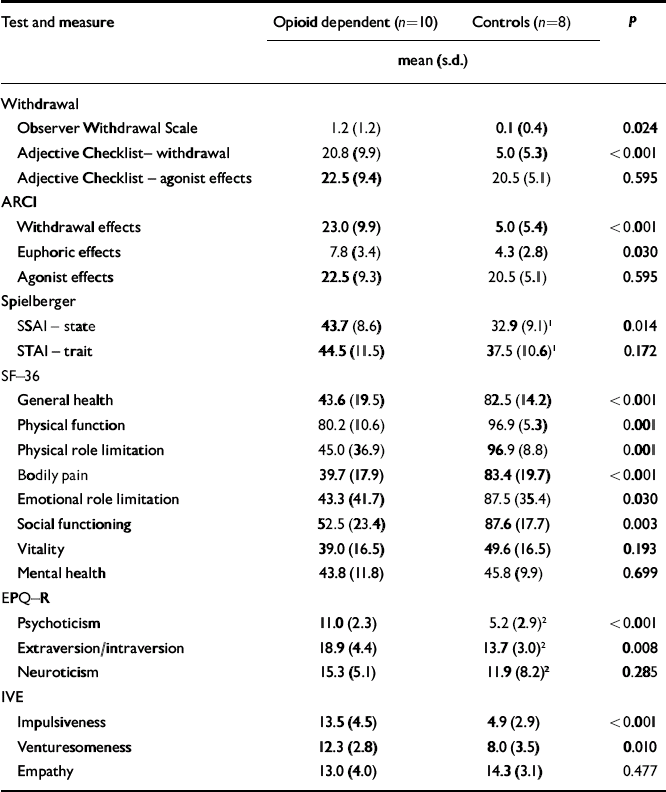
| Test and measure | Opioid dependent (n=10) | Controls (n=8) | P | |
|---|---|---|---|---|
| mean (s.d.) | ||||
| Withdrawal | ||||
| Observer Withdrawal Scale | 1.2 (1.2) | 0.1 (0.4) | 0.024 | |
| Adjective Checklist– withdrawal | 20.8 (9.9) | 5.0 (5.3) | < 0.001 | |
| Adjective Checklist – agonist effects | 22.5 (9.4) | 20.5 (5.1) | 0.595 | |
| ARCI | ||||
| Withdrawal effects | 23.0 (9.9) | 5.0 (5.4) | < 0.001 | |
| Euphoric effects | 7.8 (3.4) | 4.3 (2.8) | 0.030 | |
| Agonist effects | 22.5 (9.3) | 20.5 (5.1) | 0.595 | |
| Spielberger | ||||
| SSAI – state | 43.7 (8.6) | 32.9 (9.1) 1 | 0.014 | |
| STAI – trait | 44.5 (11.5) | 37.5 (10.6) 1 | 0.172 | |
| SF–36 | ||||
| General health | 43.6 (19.5) | 82.5 (14.2) | < 0.001 | |
| Physical function | 80.2 (10.6) | 96.9 (5.3) | 0.001 | |
| Physical role limitation | 45.0 (36.9) | 96.9 (8.8) | 0.001 | |
| Bodily pain | 39.7 (17.9) | 83.4 (19.7) | < 0.001 | |
| Emotional role limitation | 43.3 (41.7) | 87.5 (35.4) | 0.030 | |
| Social functioning | 52.5 (23.4) | 87.6 (17.7) | 0.003 | |
| Vitality | 39.0 (16.5) | 49.6 (16.5) | 0.193 | |
| Mental health | 43.8 (11.8) | 45.8 (9.9) | 0.699 | |
| EPQ–R | ||||
| Psychoticism | 11.0 (2.3) | 5.2 (2.9) 2 | < 0.001 | |
| Extraversion/intraversion | 18.9 (4.4) | 13.7 (3.0) 2 | 0.008 | |
| Neuroticism | 15.3 (5.1) | 11.9 (8.2) 2 | 0.285 | |
| IVE | ||||
| Impulsiveness | 13.5 (4.5) | 4.9 (2.9) | < 0.001 | |
| Venturesomeness | 12.3 (2.8) | 8.0 (3.5) | 0.010 | |
| Empathy | 13.0 (4.0) | 14.3 (3.1) | 0.477 | |
ARCI, Addiction Research Centre Inventory; SF–36, 36-item short form of the General Health Survey; EPQ–R, Eysenck Personality Questionnaire – Revised; IVE, Eysenck Impulsiveness Questionnaire
1. n=10
2. n=9
All participants with opioid dependence reported craving on the HCQ (mean score 15.7, s.d.=6.0) and modified OCDS (mean score 21.67, s.d.=10.6), and the scores were highly correlated (r=0.76, P<0.018). Craving scores were comparable with our previous study of the same stage of detoxification where craving was elicited using an imagery-based procedure (Reference Weinstein, Wilson and BaileyWeinstein et al, 1997).
Image analysis
Participants with opioid dependence showed a significantly higher level of opioid receptor availability, as measured by global [11C]-diprenorphine volume of distribution, when compared with controls (19.3 v. 17.1, 11.4% increase, 95% CI 2.1-20.7, P=0.019). In 15 of the 21 a priori regions studied there was a significant increase in volume of distribution in people with opioid dependence when compared with controls (P<0.05 uncorrected). These were the brain-stem, right amygdala, left medial orbital cortex and bilateral anterior cingulate, putamen, thalamus, and anterior, lateral and posterior orbitofrontal cortex. Only the left lateral orbital area remained significant if these areas are considered independent and the overly conservative Bonferroni correction is applied (P=0.042, corrected). There was no significant association between age and global [11C]-diprenorphine volume of distribution for the whole group (n=30, r=-0.30, P=0.105) or when the control (n=20, r=-0.31, P=0.184) and opiod-dependent groups (n=10, r=-0.02, P=0.962) were analysed separately.

Fig. 1 Global and a priori regional [11C]-diprenorphine binding for people with opioid dependence (▪) and controls (![]() ). *
P<0.05 unpaired t-test.
). *
P<0.05 unpaired t-test.
The re-detoxification daily methadone total dose (mg/kg) and duration of methadone use showed no association with either global or regional [11C]-diprenorphine binding. Furthermore, no relationship with [11C]-diprenorphine binding was found when the participants with opioid dependence were divided into two groups: short-term users (use for under 10 years) and long-term users (use for more than 10 years). We found no significant effect for either alcohol or ‘crack’ cocaine on [11C]-diprenorphine binding by comparing those that had used in the previous 30 days with those that had not. Of interest, although it did not achieve significance, was a trend towards a negative correlation between level of opioid use in the previous month, year or lifetime and global [11C]-diprenorphine binding. There was no correlation between [11C]-diprenorphine volume of distribution and craving, subjective opioid effects or withdrawal, or any of the personality variables.
Regional densities of μ opioid receptors, as reported in post-mortem tissue (Reference Pfeiffer, Pasi and MehraeinPfeiffer et al, 1982), correlated with [11C]-diprenorphine binding in each region for the whole group (r=0.54, P=0.006), and when control and patient groups were analysed separately (r=0.51, P=0.010 and r=0.53, P=0.007 respectively). There was no correlation between [11C]-diprenorphine volume of distribution and κ opioid receptor (combined group r=0.05, P=0.803) or δ opioid receptor regional densities (combined group r=0.31, P=0.141). No differences were apparent between the groups in these correlations.
An exploratory comparison of people with opioid dependence and controls using statistical parametric mapping showed a significant increase in [11C]-diprenorphine binding only in the right fusiform and parahippocampal gyri (MNI coordinates x=38 mm, y=-26 mm, z=-32 mm, cluster size 594 voxels, peak T=5.15, P=0.016 familywise error corrected). No significant correlations were found using statistical parametric mapping between [11C]-diprenorphine volume of distribution and any clinical variables. Using a statistical threshold of P⩽0.05 uncorrected to investigate the a priori areas confirmed the findings of the region of interest analysis.
DISCUSSION
This is the first study, to our knowledge, that has studied opioid receptor binding using [11C]-diprenorphine PET in people with opioid dependence undergoing recent detoxification. We found a significant increase in [11C]-diprenorphine volume of distribution in the majority of a priori regions of interest, although notably not in the nucleus accumbens or caudate nucleus. Exploratory statistical parametric mapping at a threshold for significance applying a full correction for multiple comparisons identified the right fusiform and right parahippocampal gyri as areas of significantly increased binding. At a lower threshold, the mapping confirmed the findings of the volume of interest analysis when applied to regions specified a priori.
Opioid receptor availability in dependence
There is limited preclinical research that helps to interpret the results of this study. Although it is clear that chronic opioid exposure leads to reduced opioid receptor function (tolerance), the mechanisms through which this is achieved are not certain and may include receptor internalisation or reduced receptor-effector coupling (Reference Williams, Christie and ManzoniWilliams et al, 2001). In vitro studies have shown that chronic exposure to an opioid agonist can lead to a downregulation in opioid receptors (Reference Goodman, Emilien and BeckettsGoodman et al, 1996). However, this is not a consistent pattern in in vivo studies, which have reported increases, decreases and no change in opioid receptors, depending on the paradigm used (Reference Zadina, Kastin and HarrisonZadina et al, 1995). In humans, tolerance to opioid agonists is well characterised but there are virtually no data on brain opioid receptor imaging. We have previously demonstrated a dose-related reduction in opioid receptor function in people with opioid dependence who are on methadone maintenance by showing that they are less sensitive to the effects of an opioid agonist, hydromorphone (Reference Melichar, Myles and EapMelichar et al, 2003). However, in a parallel [11C]-diprenorphine PET study, we found no difference in binding compared with a healthy control group, suggesting limited occupancy and no significant changes in receptor number (Reference Melichar, Hume and WilliamsMelichar et al, 2005). This complements a study using [18F]-cyclofoxy PET that also suggested that methadone requires only very low levels of opioid receptor occupancy for efficacy (Reference Kling, Carson and BorgKling et al, 2000). Lastly, post-mortem studies of people with heroin dependence have shown inconsistent changes (reduction or no difference) in μ opioid receptor density compared with healthy controls (Reference Gabilondo, Meana and BarturenGabilondo et al, 1994; Reference Ferrer-Alcon, La Harpe and Garcia-SevillaFerrer-Alcon et al, 2004). These studies suggest that chronic opioid exposure might not alter opioid receptor availability and importantly not increase receptor availability.
Increased [11C]-diprenorphine binding
The increase in [11C]-diprenorphine binding reflects an increase in availability of opioid receptors to this PET tracer. Increased receptor affinity for the tracer could account for this increased availability, but there is no preclinical evidence that chronic opioid administration alters affinity. Therefore, the increase in [11C]-diprenorphine binding might be due to: (a) an increase in opioid receptors during early abstinence from opioid drugs; (b) an increase in opioid receptor number that develops with the chronic use of an opioid agonist; (c) a reduction in competition from endogenous opioids. We believe that it is most likely that our findings reflect a significant increase in opioid receptor number immediately following detoxification from opioids. We know that withdrawal and early abstinence is a time when the brain is under stress, and that such an increase might represent a neuroadaptive response. This would explain the similar findings after cocaine and alcohol dependence (Reference Zubieta, Gorelick and StaufferZubieta et al, 1996; Reference Gorelick, Kim and BencherifGorelick et al, 2005; Reference Heinz, Riemold and WraseHeinz et al, 2005).
Increased [11C]-diprenorphine binding could also reflect increased opioid receptor availability as a result of suppression of endogenous opioid release. Preclinical evidence shows that chronic treatment with methadone does not alter the concentration or function of endogenous opioids, although later studies with other opioids and other drugs of misuse suggest that endogenous opioids play a role in craving or drug-seeking behaviour (for a review see Reference Gerrits, Lesscher and van ReeGerrits et al, 2003). Activation of the endogenous opioid system is associated with the regulation of emotions, physical and emotional pain (Reference Ribeiro, Kennedy and SmithRibeiro et al, 2005). A possibility is that the exogenous opioids used to alter emotions by people with opioid dependence might lead to suppression of the endogenous opioid system and consequently a compensatory upregulation of receptors. This would leave more receptors available for occupancy by [11C]-diprenorphine in early abstinence. We are not aware of any human studies describing the impact of chronic opioid agonist use on levels of endogenous opioids.
Opioid system after abstinence from substances
In addition to being the primary target for opioid drugs, the opioid neurotransmitter system is important in initiating and maintaining dependence on a variety of misused substances (Reference HerzHerz, 1997; Reference Gerrits, Lesscher and van ReeGerrits et al, 2003; Reference Kreek, Schlussman and BartKreek et al, 2004). A number of recent neuroimaging studies in humans using the μ-selective agonist [11C]-carfentanil have reported elevations of tracer binding in early abstinence from cocaine and alcohol, which are associated with craving (Reference Zubieta, Gorelick and StaufferZubieta et al, 1996; Reference Gorelick, Kim and BencherifGorelick et al, 2005; Reference Heinz, Riemold and WraseHeinz et al, 2005). Detoxification from a short course of buprenorphine has been shown in a preliminary study to result in a significant increase in μ opioid [11C]-carfentanil binding in the inferofrontal cortex and anterior cingulate regions compared with controls (Reference Zubieta, Greenwald and LombardiZubieta et al, 2000). Therefore, it appears that similar increases in opioid receptor availability are seen during early abstinence from cocaine and alcohol, and preliminary data suggest a comparable increase in people with opioid dependence.
The evidence to date points to elevations in opioid binding being an acute effect of early abstinence, and our results in opioid dependence complement these findings. It is not clear whether these changes persist or even become additive with progressive detoxifications. In cocaine dependence, opioid receptor binding in some but not all regions returns to control levels within 1 week (Reference Gorelick, Kim and BencherifGorelick et al, 2005). In alcohol dependence the increase appears more persistent, with no reduction evident after 5 weeks of abstinence (Reference Heinz, Riemold and WraseHeinz et al, 2005). It was not possible to scan our participants after a period of abstinence owing to high relapse rates and strict residential rehabilitation programmes, but this would be valuable in future studies.
We found significant increases in [11C]-diprenorphine binding in the majority of regions analysed using the atlas, and significant increases in fusiform/parahippocampal gyri using exploratory voxel-based statistical parametric mapping, although increases were seen throughout the brain when the threshold for significance was lowered. It is not clear why an area incorporating the fusiform/parahippocampal gyri which is involved in processing visual associations and memory was highlighted by statistical parametric mapping. We found no difference in [11C]-diprenorphine binding between people with opioid dependence and controls in several of the a priori areas, notably the periaqueductal grey matter (in the brain-stem), nucleus accumbens and caudate. The template used for the brain-stem region is not precise enough to isolate the periaqueductal grey matter within the brain-stem region of interest, which may account for the lack of association there. However, we are surprised to find no association with the nucleus accumbens and caudate in the light of previous findings of increased receptor number in these areas during withdrawal from cocaine and alcohol. In the two studies of cocaine dependence, significant increases were seen in the ventral striatum and the anterior cingulate, frontal and temporal cortices, caudate and thalamus (Reference Zubieta, Gorelick and StaufferZubieta et al, 1996; Reference Gorelick, Kim and BencherifGorelick et al, 2005), whereas in alcohol dependence, significant increases were restricted to the ventral striatum (Reference Heinz, Riemold and WraseHeinz et al, 2005). In people with opioid dependence the changes were much more widespread, perhaps because of the direct pharmacological effect of opioids and possible changes in the endogenous opioid system.
Opioid receptor availability and clinical variables
We found no correlation between craving and opioid receptor availability, which is at variance with our hypothesis and previous findings in alcohol and cocaine dependence (Reference Zubieta, Gorelick and StaufferZubieta et al, 1996; Reference Gorelick, Kim and BencherifGorelick et al, 2005; Reference Heinz, Riemold and WraseHeinz et al, 2005). Our participants with opioid dependence demonstrated high scores on two rating scales for craving, which were comparable with those in a previous study (Reference Weinstein, Wilson and BaileyWeinstein et al, 1997) and with individuals maintained on methadone who had withdrawal induced by naloxone (Reference Schuster, Greenwald and JohansonSchuster et al, 1995) but were higher than scores for people maintained on methadone (Reference Schuster, Greenwald and JohansonSchuster et al, 1995). Furthermore, our participants experienced levels of and variance in craving scores that were comparable with earlier studies in which craving was induced and resulting brain activation detected (Reference Daglish, Weinstein and MaliziaDaglish et al, 2001). Craving measures vary and so comparison with other studies is hampered. However, in our study we chose two commonly used scales with a total of seven craving subscales, so the absence of a correlation here is robust.
In other studies reporting a relationship between craving and opioid receptor levels, [11C]-carfentanil, a μ-selective tracer was used (Zubieta et al, Reference Zubieta, Gorelick and Stauffer1996, Reference Zubieta, Greenwald and Lombardi2000; Reference Gorelick, Kim and BencherifGorelick et al, 2005; Reference Heinz, Riemold and WraseHeinz et al, 2005). It may be that since [11C]-diprenorphine labels μ, κ and δ opioid receptors, μ receptor-related changes were obscured by alterations in the other subtypes. However we think this unlikely as the [11C]-diprenorphine signal correlated only with the reported μ opioid receptor density in each brain region and not with the κ and δ opioid receptor density. Nevertheless, it would be beneficial to repeat this study using a more selective opioid receptor tracer, such as [11C]-carfentanil, to determine whether the increase in opioid receptor binding demonstrated here is mainly due to increase in any particular subtype.
Opioid receptor binding levels were not related to withdrawal symptoms as found in cocaine and alcohol dependence (Reference Zubieta, Gorelick and StaufferZubieta et al, 1996; Reference Gorelick, Kim and BencherifGorelick et al, 2005). This is consistent with the clinical picture where opioid withdrawal can be ameliorated by non-opioid pharmacotherapy. We did not find a correlation between age and opioid receptor levels in either the group with opioid dependence or controls. In a [11C]-carfentanil PET study of healthy controls with a wider age range, increasing age was associated with higher opioid receptor levels in the neocortex (Reference Zubieta, Dannals and FrostZubieta et al, 1999). Our more limited age range and younger average age likely contributed to the absence of such a correlation. All of our group with dependence were tobacco smokers and controls were current non-smokers, but there was no correlation between quantity of cigarettes smoked and [11C]-diprenorphine binding. Furthermore, another study of alcoholism reported no significant interaction between smoking status and μ opioid receptor availability in patients and controls (Reference Heinz, Riemold and WraseHeinz et al, 2005).
Limitations
Although this study was appropriately powered to detect the measured effect in a PET study of this nature, it may have been underpowered to determine associations with clinical variables, especially craving. The studies reporting an association between craving and opioid receptor levels had dependent groups of 10, 17 and 25 respectively (Reference Zubieta, Gorelick and StaufferZubieta et al, 1996; Reference Gorelick, Kim and BencherifGorelick et al, 2005; Reference Heinz, Riemold and WraseHeinz et al, 2005). However, the participants in our study were craving at similar levels and with a wide range of craving scores, making it likely that any association should have been apparent.
Implications
We have reported a significant widespread increase in brain opioid receptor availability in people with opioid dependence during early abstinence from methadone. Together with previous evidence, we argue that this reflects an increase after cessation of methadone rather than a chronic change. If this is the case, it could give us a crucial insight into the mechanisms that underlie opioid craving. Although clinically we know that substitution treatment is effective we do not know whether prolonged agonist exposure permanently alters brain neurochemistry and whether these changes hamper recovery. Furthermore, since such an increase in opioid receptors has also been shown in alcohol and cocaine dependence, this argues for a fundamental role of the opioid system in addiction, or at least in the early abstinence syndrome. The contribution of this to clinical states and treatment outcomes has yet to be fully characterised.
Acknowledgements
This work was funded by a programme grant from the Medical Research Council, UK, and by Avon and Wiltshire Partnership NHS Trust research and development funding. Radiotracers and scanners were supplied by Hammersmith Imanet, GE Healthcare.






eLetters
No eLetters have been published for this article.