The development of clozapine over 50 years ago was seen as a major breakthrough in the treatment of schizophrenia. However, initial enthusiasm for its use was tempered by the risks of adverse drug reactions, notably a cluster of agranulocytosis cases detected in Finland in the 1970s. Reference Idanpaan-Heikkila, Alhava, Olkinuora and Palva1 This led to the drug's removal from the market in many jurisdictions. In spite of this, continued evidence of its effectiveness for psychotic symptoms led to its widespread reintroduction in the 1990s. Reference Kane, Honigfeld, Singer and Meltzer2,Reference Leucht, Cipriani, Spineli, Mavridis, Orey and Richter3 Clozapine is now generally reserved for people with treatment-refractory schizophrenia, usually defined as two failed trials of other antipsychotic medications of adequate dose and duration. Reference Lehman, Lieberman, Dixon, McGlashan, Miller and Perkins4–Reference Warnez and Alessi-Severini7 In spite of the value of clozapine in these situations there is still a reluctance to prescribe it, with the result that usage rates vary widely. Reference Forrester, Siskind, Winckel, Wheeler and Hollingworth8 This reluctance may mean long delays before people with treatment-refractory disorder are given clozapine, resulting in poorer outcomes and exposure to potentially hazardous antipsychotic polypharmacy. Reference Howes, Vergunst, Gee, McGuire, Kapur and Taylor9 One way to address this reluctance is to demonstrate the particular value of clozapine in treatment-refractory schizophrenia. However, most Cochrane reviews comparing clozapine with other antipsychotic medications have not focused on such patients. Furthermore, the sole meta-analysis that specifically examined the effectiveness of clozapine in this patient group included only comparisons with first-generation antipsychotic medications. Reference Moncrieff and Clozapine10 That review found clozapine to be superior but also noted that the effect was greatest in studies of shorter duration and studies funded by manufacturers of clozapine. Since that time there have been several further randomised controlled trials (RCTs) in people with treatment-refractory schizophrenia that, importantly, compared clozapine with second-generation antipsychotics. These may be more relevant to current practice. We therefore undertook a systematic review and meta-analysis of RCTs comparing clozapine with other antipsychotics for treatment-refractory schizophrenia. Outcomes were grouped into short-term and long-term categories for total, positive and negative psychotic symptoms, adverse drug reactions, study withdrawals and response to treatment. Additionally, we conducted sensitivity analyses for the effects of pharmaceutical industry funding, types of antipsychotic controls (first or second generation), dosage and initial psychosis score.
Method
The study was registered with the PROSPERO international database of prospectively registered systematic reviews (CRD42014013134). Reference Booth, Clarke, Dooley, Ghersi, Moher and Petticrew11 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement recommendations were followed for background, search strategy, methods, results, discussion and conclusions. Reference Moher, Liberati, Tetzlaff and Altman12
Eligibility criteria
We included all RCTs that compared people with treatment-refractory schizophrenia taking clozapine with those prescribed a first- or second-generation antipsychotic. Outcomes of interest included psychotic symptoms (total, positive and negative), adverse drug reactions, study withdrawal and response to treatment. Published data in all languages were included and translated into English.
Search strategy
We searched PubMed, EMBASE and the Cochrane Schizophrenia Group's Trials Register from inception to 6 February 2015. In the case of PubMed we used the following terms: (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials OR randomly OR trial) AND (clozapin* OR clozaril OR zaponex OR denzapin* or clopine). Experts in the field were contacted and asked about unpublished data.
Study selection
Studies were included if they were randomised and double- or rater-blinded. Diagnoses included schizophrenia, schizoaffective disorder or schizophreniform disorder. Participants had to have demonstrated a resistance to treatment as defined by a failure to respond to at least one trial (and preferably two) of a first- or second-generation antipsychotic of at least 6 weeks' duration at dosage equivalents greater than 600 mg chlorpromazine. Studies were included if they compared clozapine with any other first- or second-generation antipsychotic medication. Studies were excluded if there was extensive crossover between the clozapine and control groups. All identified studies were screened at the title and abstract level by two authors (L.M. and R.G.). Studies that met the inclusion criteria on title and abstract review, or that could not be excluded on the basis of information provided in the abstract, were reviewed at full-text level. Snowball searches of key papers and the included studies' reference lists were conducted. Narrative and systematic reviews, posters, conference abstracts, case reports, letters to editors and other articles that did not meet the inclusion criteria were cross-referenced for additional potential sources of RCTs. Attempts were made to contact first authors of included studies in cases where information was missing.
Data collection
Data extraction was conducted by two independent researchers (L.M. and R.G.). All discrepancies during each stage of study selection, data extraction and quality assessment were resolved by re-checking source papers. Extracted data were validated by D.S. Data analysis was conducted by two authors (D.S. and S.K.). We extracted data on the following: study duration, setting, diagnostic tool and type of interventions (e.g. control medication, first- or second-generation antipsychotic, mean age and standard deviation, number commenced in study arm, mean dose of clozapine or control medication, and dose of clozapine or control medication). Doses of clozapine and control medications were converted to chlorpromazine and olanzapine equivalents. Reference Andreasen, Pressler, Nopoulos, Miller and Ho13,Reference Leucht, Samara, Heres, Patel, Furukawa and Cipriani14 These were used in separate meta-analyses comparing dose equivalents for clozapine and the control medication for each study. Where data in studies were missing or unclear, attempts were made to contact the study's corresponding author.
Outcomes
Where possible, end-points were measured from commencement of intervention. Data from studies were divided into short term (less than 3 months) and long term (3 months or more). These time frames were selected after data were extracted, based on an approach used in a previous meta-analysis. Reference Wahlbeck, Cheine, Essali and Adams15 Where multiple outcome time points were reported in the same study, the data for the last outcome time point in each period (short or long term) were used. Analysis was also conducted for all time points, with the data for the last outcome time point in each study included.
The primary outcome was a change in overall psychotic symptoms as measured by the Brief Psychiatric Rating Scale (BPRS) or the Positive and Negative Syndrome Scale (PANSS). Reference Overall and Gorham16,Reference Kay, Fiszbein and Opler17 Where standard deviations for change in psychotic symptoms were not reported, the change score was calculated from baseline and end-point scores and the standard deviation imputed. Reference Higgins and Green18 Secondary outcomes included changes in positive and negative symptom scales. For changes in positive symptoms we used scores from the Scale for the Assessment of Positive Symptoms (SAPS), Reference Andreasen19 and the positive subscales of the PANSS and BPRS. For changes in negative symptoms, scores for the PANSS negative symptom subscale and the Scale for the Assessment of Negative Symptoms (SANS) were used. Reference Andreasen20 We also collected data on response, based on the definition by Kane et al of a 20% decrease in BPRS total score plus either a post-treatment Clinical Global Inventory (CGI) Scale rating of mildly ill (⩾3) or a post-treatment BPRS score of 35 or less. Reference Kane, Honigfeld, Singer and Meltzer2 We have noted where studies used other response criteria. Finally, we collected data on those leaving the study and the following adverse drug reactions: sialorrhoea, tachycardia, seizures, fever, dizziness, sedation, constipation, nausea or vomiting, insomnia, dry mouth, hypotension, headache and weight gain.
Study quality
We assessed the quality of included studies using the following criteria adapted from Cochrane Collaboration guidelines:
-
(a) adequate generation of allocation sequence;
-
(b) masking of allocation to conditions to participant and/or assessor;
-
(c) adequate random sequence generation;
-
(d) pre-specified primary outcome measures;
-
(e) appropriate reporting on missing data;
-
(f) use of intention-to-treat analysis;
-
(g) other sources of potential bias including pharmaceutical company funding.
Statistical analysis
We used Review Manager version 5.3 for Mac for the meta-analyses and Comprehensive Meta-Analysis version 3.3 for the meta-regression. We calculated the standardised mean difference (SMD) for continuous data that used different scales. We reported the risk ratio (RR) for any dichotomous outcome. Where possible, intention-to-treat analyses were used. We conducted sensitivity analyses for the effect of dosages, use of first- or second-generation control medications, pharmaceutical company sponsorship and community or hospital study settings. We used meta-regression to assess the effect of baseline psychosis score as a continuous variable. Reference Leucht, Rothe, Davis and Engel21 We assessed heterogeneity using the I 2 statistic, a measure that does not depend on the number of studies in the meta-analysis and hence has greater power to detect heterogeneity when the number of studies is small; it provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. An estimate of 50% or greater indicates possible heterogeneity, and scores of 75–100% indicate considerable heterogeneity. The I 2 estimate is calculated using the chi-squared statistic (Q) and its degrees of freedom. Reference Higgins and Green18 The random effects model was used for all the analyses as we could not definitely exclude between-study variation even in the absence of statistical heterogeneity, given the range of medications under review. We tested for publication bias using funnel plot asymmetry where low P values suggest publication bias. Reference Higgins and Green18
Results
A total of 2589 articles were identified in the initial search of the electronic databases. Of these, 2402 were excluded at the title and abstract level and a further 167 were excluded after review of the full text (see online Fig. DS1). One additional paper was included after a review of reference lists of key articles. Twenty-one papers were included for the review. The sum of enrolled patients was 1131 commenced on clozapine and 1233 on control medications with 801 clozapine and 799 control participants at final follow-up.
Study characteristics
Study quality was fair (see online Table DS1). Seventeen papers reported adequate allocation concealment, 18 were double-blind and 3 were blinded only to assessor. Adequate random sequence generation was reported in 18 papers. All papers reported the primary outcome measures and adequately described missing data. Seventeen papers reported intention-to-treat data. Three papers did not provide any information about the funding source. Eleven papers reported financial support from a pharmaceutical company (8 were funded by the manufacturer of the control medication, 2 by the manufacturer of clozapine and 1 by a manufacturer of both clozapine and the control medication). Among long-term studies, all pharmaceutical funding was from the manufacturers of control medications.
Twenty-one papers were included in the meta-analysis. Reference Kane, Honigfeld, Singer and Meltzer2,Reference Azorin, Spiegel, Remington, Vanelle, Pere and Giguere22–Reference Wang, Geng, Pan and Zhang41 They provided data on comparisons with 25 control groups, hereafter referred to as studies. In papers where clozapine was compared with more than one control medication, the number of participants in the clozapine group was divided by the number of control medications, rounded down to the nearest integer, and used as the number of clozapine participants in analyses comparing clozapine with each control medication. This was done to avoid double-counting the individuals taking clozapine. Papers were published between 1988 and 2009. Studies reported data at time points ranging from 6 weeks to 78 weeks. Seventeen papers reported data on 21 short-term studies. Nine papers reported data on 13 long-term studies.
Six papers had strict adherence to our definition of treatment-refractory schizophrenia. Five papers included participants with only one failed trial of an antipsychotic, three papers had trial durations less than 6 weeks or did not report on trial duration, and nine papers reported that previous antipsychotic trials had a chlorpromazine-equivalent dose of less than 600 mg or did not report a dose. Eleven papers included some participants who had left previous antipsychotic trials owing to treatment intolerance rather than specifically treatment failure (online Table DS1).
Control medications comprised first-generation antipsychotics including chlorpromazine and haloperidol and second-generation antipsychotics including olanzapine, risperidone, quetiapine and ziprasidone (Table DS1). There was no statistically significant difference between clozapine and control groups in terms of age or baseline psychosis score in any of the included studies.
Psychotic symptoms
Twenty short-term studies had usable data for change in total psychotic symptoms for 604 people given clozapine and 708 people given a control medication. The standardised mean difference (SMD) favoured clozapine (Fig. 1). Eleven long-term studies had usable data with 368 people given clozapine and 451 people given a control medication. In contrast to the short-term results, there was no statistically significant difference in SMD between the groups (Fig. 1). However, clozapine was associated with a greater improvement in score when all time frames were combined, using the last reported time point in each study (SMD = −0.29, 95% CI −0.49 to −0.09, P<0.005; 24 studies, n = 1858). Clozapine had a greater effect on positive symptoms with statistically superior outcomes at all time points (Fig. 2) compared with negative symptoms where benefits were only seen in the short term (Fig. 3).

Fig. 1 Change in total psychotic symptoms. SMD, standardised mean difference.

Fig. 2 Change in positive symptoms. SMD, standardised mean difference.

Fig. 3 Change in negative symptoms. SMD, standardised mean difference.
Sensitivity analyses
In studies that were restricted to in-patients or had not received pharmaceutical company funding, clozapine had significantly better outcomes in both the short and long term. For instance, the SMD for long-term studies without pharmaceutical company funding was −0.67 (95% CI −1.15 to −0.19, P = 0.006; 4 studies, n = 142). We also investigated the effect of dosing. Overall, patients on clozapine were given 228 mg less of chlorpromazine equivalents per day than controls (95% CI 188 to 267, P<0.0001) and 9.8 mg less of olanzapine equivalents per day (95% CI 8.4 to 11.1, P<0.0001). When studies were ranked on degree of equivalence of dose and the half with the greatest difference excluded, the SMD more strongly favoured clozapine in each time frame, becoming significantly superior in the long term (SMD = −0.42, 95% CI −0.85 to −0.01, P = 0.05; 6 studies, n = 201). When studies that had included any treatment-intolerant participants were excluded, there was no difference in the overall results, with clozapine showing superior outcomes in the short term but not in the long term. Similarly, a sensitivity analysis on whether the study was single-blinded made no difference to the results.
There was no difference in results for studies that used first- or second-generation comparator antipsychotics, with clozapine showing superior outcomes in the short term but not in the long term. We examined clozapine against specific comparator medications for which there were two or more studies in each time frame. Clozapine was significantly superior to olanzapine, haloperidol and chlorpromazine in the short term; however, there was no significant difference between clozapine and risperidone in the short or long term, nor against olanzapine in the long term.
Three short-term studies looked specifically at people under age 18 years. Reference Kumra, Frazier, Jacobsen, McKenna, Gordon and Lenane29,Reference Kumra, Kranzler, Gerbino-Rosen, Kester, Thomas and Kafantaris30,Reference Shaw, Sporn, Gogtay, Overman, Greenstein and Gochman37 When only these studies were included, the SMD more strongly favoured clozapine, whereas when the studies specifically looking at children and adolescents were excluded, the SMD less strongly favoured clozapine. Finally, we explored the effect of baseline mean psychosis score using meta-regression: higher scores predicted greater response for clozapine in the long term (regression coefficient 0.03, s.e. = 0.01, P = 0.0034, τ2 = 0.0008) but not the short term.
Response
Six studies defined response using the criteria outlined by Kane et al of a greater than 20% reduction in BPRS from baseline in the presence of a post-treatment CGI Scale score of 3 or less or a BPRS of 35 or less (online Table DS1). Reference Kane, Honigfeld, Singer and Meltzer2,Reference Azorin, Spiegel, Remington, Vanelle, Pere and Giguere22,Reference Bitter, Dossenbach, Brook, Feldman, Metcalfe and Gagiano23,Reference Hong, Chen, Chiu and Sim27,Reference Shaw, Sporn, Gogtay, Overman, Greenstein and Gochman37,Reference Tollefson, Birkett, Kiesler and Wood38 A further five studies defined response as an improvement on the BPRS or PANSS of greater than 20%. Reference Bondolfi, Dufour, Patris, May, Billeter and Eap24,Reference Kane, Marder, Schooler, Wirshing, Umbricht and Baker28,Reference Rosenheck, Cramer, Xu, Thomas, Henderson and Frisman35,Reference Sacchetti, Galluzzo, Valsecchi, Romeo, Gorini and Warrington36,Reference Wahlbeck, Cheine, Tuisku, Ahokas, Joffe and Rimon40 A final study defined response as a greater than 30% reduction in the BPRS plus a post-treatment CGI score of 2 or less. Reference Kumra, Kranzler, Gerbino-Rosen, Kester, Thomas and Kafantaris30 Eight short-term studies had usable data for 598 participants taking clozapine and 620 control group participants. People taking clozapine were significantly more likely to respond in the short term (risk ratio (RR) = 1.17, 95% CI 1.07 to 2.73, P = 0.03; 8 studies, n = 1218). The absolute risk reduction was 12.48% (95% CI 7.52 to 17.43). Based on this response rate, the number needed to treat was 9. Five long-term studies had usable data for 479 participants in the clozapine group and 489 in the control group, with no significant difference between clozapine and control. For all time frames combined, results just failed to reach statistical significance (RR = 1.31, 95% CI 0.98 to 1.70, P = 0.07; 11 studies, n = 1692).
Sensitivity analyses
There were insufficient studies to do meaningful sensitivity analyses for the short- and long-term periods separately. Sensitivity analyses are limited to the data for all time frames combined. Sensitivity analysis on strictness of criteria of response or equivalence of dose did not alter the absence of statistically significant difference between the groups. When only studies using first-generation antipsychotics as the comparator were included, clozapine was statistically significantly more likely to lead to a response (RR = 1.77, 95% CI 1.19 to 2.64, P = 0.008; 4 studies, n = 164). There was no statistically significant difference between the groups when only second-generation antipsychotics were included. When studies with pharmaceutical funding were excluded, response statistically significantly favoured clozapine (RR = 1.68, 95% CI 1.20 to 2.35, P = 0.002; 6 studies, n = 208).
Study completion
The number of participants at the completion of the study time frame was compared with the number of participants commencing the studies. For both the short- and long-term analyses there was no statistically significant difference between clozapine and control antipsychotics on study completion.
Adverse drug reactions
Meta-analyses on adverse reactions were conducted for any results reported by two or more studies. The data for the last time-point in each study was used. It was not feasible to separate adverse reactions into time groups as only two papers reported long-term data. Participants taking clozapine reported significantly greater rates of sialorrhoea, tachycardia, seizures, fever, dizziness, sedation, constipation, and nausea and vomiting (Table 1). The number needed to harm ranged from 4 for sialorrhoea to 19 for nausea and vomiting. Participants taking clozapine reported significantly lower rates of insomnia and dry mouth. There was no significant difference for hypotension, headache or weight gain.
Table 1 Adverse drug reactions
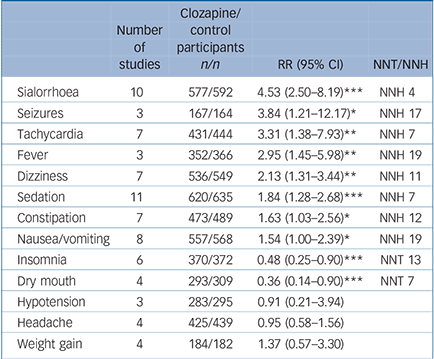
| Number of studies |
Clozapine/ control participants n/n |
RR (95% CI) | NNT/NNH | |
|---|---|---|---|---|
| Sialorrhoea | 10 | 577/592 | 4.53 (2.50–8.19)*** | NNH 4 |
| Seizures | 3 | 167/164 | 3.84 (1.21–12.17)* | NNH 17 |
| Tachycardia | 7 | 431/444 | 3.31 (1.38–7.93)** | NNH 7 |
| Fever | 3 | 352/366 | 2.95 (1.45–5.98)** | NNH 19 |
| Dizziness | 7 | 536/549 | 2.13 (1.31–3.44)** | NNH 11 |
| Sedation | 11 | 620/635 | 1.84 (1.28–2.68)*** | NNH 7 |
| Constipation | 7 | 473/489 | 1.63 (1.03–2.56)* | NNH 12 |
| Nausea/vomiting | 8 | 557/568 | 1.54 (1.00–2.39)* | NNH 19 |
| Insomnia | 6 | 370/372 | 0.48 (0.25–0.90)*** | NNT 13 |
| Dry mouth | 4 | 293/309 | 0.36 (0.14–0.90)*** | NNT 7 |
| Hypotension | 3 | 283/295 | 0.91 (0.21–3.94) | |
| Headache | 4 | 425/439 | 0.95 (0.58–1.56) | |
| Weight gain | 4 | 184/182 | 1.37 (0.57–3.30) | |
NNH, number needed to harm; NNT, number needed to treat; RR, risk ratio.
* P<0.05,
** P<0.01,
*** P<0.001.
Publication bias
There were sufficient short-term studies to test for publication bias for the primary outcome of change in total psychosis scores. The Egger's regression asymmetry test did not suggest publication bias (intercept −0.81, 90% CI −2.11 to 0.49, P = 0.420).
Discussion
This study is the first systematic review and meta-analysis to look specifically at the pharmacotherapy of treatment-refractory schizophrenia with clozapine compared with all antipsychotics, not solely first-generation agents. We were able to include 21 studies with 2364 participants. These included 14 papers (n = 1379) published since the data collection periods of the previous meta-analysis, Reference Moncrieff and Clozapine10 all of which used a second-generation antipsychotic as a comparator. We also used a tighter definition of treatment-refractory schizophrenia than previous meta-analyses, based on the criteria described by Kane et al. Reference Kane, Honigfeld, Singer and Meltzer2 We found that clozapine was superior to other antipsychotics in reducing positive psychotic symptoms in both the short and long term for people with treatment-refractory disorder. In contrast, clozapine was not superior for negative symptoms in the long term although it was in the short term. This, in turn, may explain the lack of any difference in both total psychotic symptoms and response in the long term. It is unclear why the long-term advantages of clozapine are restricted to positive symptoms. Of the two previous meta-analyses of the effect of study duration on outcome, one reported that clozapine was superior in long-term studies, Reference Wahlbeck, Cheine, Essali and Adams15 whereas the other reported that clozapine was superior in short-term studies. Reference Moncrieff and Clozapine10 Clozapine was particularly effective for more severe baseline symptoms.
The source of funding did appear to have an effect on our results. Studies without pharmaceutical industry funding favoured clozapine more strongly and were statistically significant for all time frames, whereas those with such support favoured the comparator medication. This is in contrast to earlier findings that pharmaceutical industry funding either did not alter rates of improvement, Reference Wahlbeck, Cheine, Essali and Adams15 or actually increased the likelihood of a study favouring clozapine. Reference Moncrieff and Clozapine10 One explanation is that the earlier meta-analyses included comparisons with first-generation antipsychotics that were funded by manufacturers of clozapine. In contrast, all but two of the additional 14 papers included in this review were comparisons with second-generation antipsychotics that were funded by the manufacturers of the second-generation agents. Pharmaceutical industry funding is a known source of systematic bias either through suppression of non-favourable results or inappropriate comparator medications. Reference Turner, Matthews, Linardatos, Tell and Rosenthal42,Reference Lexchin, Bero, Djulbegovic and Clark43
Sensitivity analyses for first- or second-generation comparator antipsychotic group did not appear to affect change in psychotic symptoms. With the exception of risperidone, clozapine was superior in the short term to individual second-generation antipsychotics but failed to reach statistical significance in long-term studies. However, this finding in the long term may be confounded by pharmaceutical industry funding of more recent comparisons with second-generation antipsychotics. When the five second-generation antipsychotic studies without pharmaceutical funding were examined, there was a trend favouring clozapine, but it failed to reach statistical significance. When dosage equivalents were compared, people taking clozapine were receiving significantly lower doses of medication. We attempted to validate this discrepancy using two different dosage equivalence formulas, and both demonstrated that clozapine doses were significantly lower than those of control group medication. It is therefore possible that lower clozapine doses may have biased the data against clozapine, although this is difficult to validate in the absence of reported serum clozapine levels in most of the included studies. Levels were reported in only three studies and ranged from 281 ng/ml to 715 ng/ml; Reference Bondolfi, Dufour, Patris, May, Billeter and Eap24,Reference Kumra, Kranzler, Gerbino-Rosen, Kester, Thomas and Kafantaris30,Reference Shaw, Sporn, Gogtay, Overman, Greenstein and Gochman37 clozapine is therapeutic at levels above 350 ng/ml. Reference Stark and Scott44,Reference Potkin, Bera, Gulasekaram, Costa, Hayes and Jin45 Reporting of serum clozapine levels in future RCTs would assist in confirming whether therapeutic doses were used.
Study limitations
There were several limitations of this study. Many of our results showed heterogeneity. Although we attempted to explore this further with sensitivity analyses and meta-regression as well as using a random effects model throughout to incorporate heterogeneity into our analysis, our results should still be treated with caution. As noted above, there are potential biases associated with pharmaceutical industry funding, class of control antipsychotic and comparative doses of clozapine and control medications. We attempted to address these by conducting sensitivity analyses and meta-regression. It is important to note the difficulty of masking in studies where clozapine is a comparator, given the significant adverse drug reactions associated with clozapine. It is possible that this may have systematically biased the overall results. Several studies included participants who had been intolerant to previous antipsychotic trials, as opposed to a strict definition of two or more failed adequate trials. A sensitivity analysis of studies with strict inclusion criteria did not alter the results. We were unable to report on relapse, as the included studies did not provide usable data on this variable. Although we attempted to locate unpublished findings, it is possible that there are unpublished data that we were unable to include.
Clinical implications
Our results suggest that clozapine should remain the treatment of choice for refractory schizophrenia, at least in the short term. Clozapine demonstrated superiority for positive symptoms across all time frames. Given the challenges associated with treating people with refractory disorder, our finding of a number needed to treat of 9 is moderately good. Reference Citrome and Ketter46 However, this must be balanced against numbers needed to harm that ranged from 4 for sialorrhoea to 19 for fever. In addition, if there is no meaningful improvement of symptoms or function at 6 months, our findings suggest clozapine should be stopped and consideration given to an antipsychotic with a more favourable adverse reaction profile. Pharmacological treatment should always be provided in concert with evidence-based psychosocial interventions. Reference Dixon, Dickerson, Bellack, Bennett, Dickinson and Goldberg47
Funding
D.S. is funded in part through an NHMRC ECF, APP1111136, 2016–2019.
Acknowledgements
We would like to thank Evelyn Ma, Dagmar Hlincikova and Oliver Freudenreich for their assistance in translating articles.






eLetters
No eLetters have been published for this article.