Major depression occurs in 6–14% of medical in-patients, Reference Katon and Schulberg1 with even higher percentages in specific populations such as people with type 1 or type 2 diabetes Reference Lustman, Griffith, Freedland, Kissel and Clouse2 or cancer. Reference Hotopf, Chidgey, Addington-Hall and Ly3 Irrespective of the underlying somatic disease, depression always dramatically decreases quality of life. In medical and surgical in-patients, depressive symptoms are associated with longer hospital stays, higher rates of rehospitalisation and increased hospital utilisation. Reference Saravay, Pollack, Steinberg, Weinschel and Habert4,Reference Henk, Katzelnick, Kobak, Greist and Jefferson5 In out-patients with chronic medical conditions such as diabetes and heart disease, comorbid depressive disorder has been associated with increased medical consumption, amplification of somatic symptoms and disability, poor self-care and adherence to treatment, and increased morbidity and mortality. Reference Katon and Sullivan6 Although both pharmacological and psychological therapy are effective for depressive disorder in the general population, 7 studies in individuals with somatic diseases have yielded mixed results. Reference Berkman, Blumenthal, Burg, Carney, Catellier and Cowan8,Reference Yohannes, Connolly and Baldwin9 As antidepressant medication may interfere with somatic diseases or their treatment, leading to high drop-out rates, Reference Himelhoch, Medoff and Oyeniyi10 psychological treatments might be more suitable in this population. Therefore, it is not surprising that in the past decades a considerable number of studies have focused on the psychological treatment of depression in people with a somatic disease. Reference Himelhoch, Medoff and Oyeniyi10–Reference Meyer and Mark16 Recent meta-analyses on psychological treatment for depression in individuals with somatic diseases, however, were limited for the following reasons. First, previous meta-analyses focused on the treatment of depressive symptoms in people with specific somatic diseases such as cancer, rheumatoid arthritis or multiple sclerosis. However, as depressive symptoms occur in a variety of somatic diseases, this approach limits our knowledge about the generalisability of cognitive–behavioural therapy (CBT). Secondly, most meta-analyses included a wide range of psychological interventions, Reference Himelhoch, Medoff and Oyeniyi10,Reference Astin, Beckner, Soeken, Hochberg and Berman12–Reference Meyer and Mark16 which might have diluted the effect of CBT, and CBT has been shown by previous research to be one of the most effective interventions for depression. 17,18 Finally, none of these meta-analyses applied the diagnostic criteria of depressive disorder as an inclusion criterion.
We conducted a meta-analysis of the effectiveness of CBT for depression in people with an underlying somatic disease. As we hypothesised that we would find higher efficacy in people who met predefined criteria for depression, we conducted separate meta-analyses for studies restricted to participants with depressive disorder and from those with depressive symptoms.
Method
Identification of studies
To identify relevant studies, we conducted a search of the Cochrane Central Register of Controlled Trials, PubMed and PsycINFO up to October 2008. We used the text keywords depressive disorder, depression, major depressive disorder or depressive sympto* combined with the keywords psychotherapy, cognitive therapy, behavi* therapy or CBT. We did not include any terms related to underlying somatic diseases to prevent the exclusion of any possible relevant study. This search strategy was repeated using the MESH-terms depressive disorder (exploded), depression (exploded), depressive disorder, major (exploded) AND psychotherapy (exploded), limited to humans, clinical trial or randomised controlled trial. Articles were retrieved for further assessment if the title or abstract suggested that a CBT (broadly defined) was used in a population with a somatic disease. Subsequently, the search was extended by a manual search of the reference lists of all resulting randomised controlled trials (RCTs).
Inclusion criteria
Manuscripts were included if they met the following criteria:
-
(a) use of a randomised controlled research design;
-
(b) inclusion of participants with an underlying somatic disease;
-
(c) inclusion of a treatment arm with CBT, defined as a protocol-based psychological treatment including at least cognitive restructuring and behavioural activation procedures for depression for a minimum of six sessions. We also included problem-solving therapy and cognitive–behavioural stress management on the prerequisite that these interventions met our definition of CBT;
-
(d) results for depressive symptoms were presented separately for each treatment arm at pre- and post-treatment;
-
(e) use of a valid outcome measure (validated self-report questionnaire or clinical interview).
The first two authors (M.W.B. and R.C.O.V.) independently checked the inclusion and exclusion criteria of the identified studies. Disagreement over inclusion was resolved through discussion. When no consensus could be reached, a third investigator (A.E.S.) decided. Excluded were outcome studies without a randomised controlled design (e.g. open trials, case series and case reports), review papers, studies examining depression associated with unexplained somatic symptoms (e.g. fibromyalgia, chronic fatigue syndrome, irritable bowel syndrome, chronic pain), studies of individuals aged 18 years and under, studies in which the CBT could not be distinguished from other elements of the intervention (e.g. depression care management), studies including people with dementia or severe cognitive impairment, and studies limited to a specific psychiatric disorder with depressive symptoms as a second outcome measure. The language of publication was not an exclusion criteria.
Data extraction, selection procedure and quality assessment
The first two authors subsequently coded the selected studies separately on a coding form consisting of the following items: year of publication, total number of patients included, number of completers and number who dropped out, underlying somatic disease, setting (in- or out-patient clinic), age, gender, type of CBT intervention, delivery of treatment (face-to-face, telephone, internet), individual or group treatment, number of sessions, type of control condition (classified as either treatment as usual, a waiting list or other psychotherapy), outcome measures, whether depressive symptoms were the primary or secondary outcome measure, completer or intent-to-treat analysis, the results on post-treatment measures of interest (see Statistical analysis), and finally the psychiatric diagnostic criteria used at the time of inclusion (depressive disorder or depressive symptoms). Depressive disorder was defined as meeting DSM–IV 19 criteria or scoring above a predefined cut-off level on a screening questionnaire for depression. Depressive symptoms were used to indicate the dimensional score on a self-report questionnaire for depressive symptoms. Discrepancies in the two coding forms were resolved by discussion between both authors (M.W.B. and R.C.O.V.), and when no consensus could be reached the third author decided (A.E.S.).
When data on means, standard deviations or number of participants in the experimental or control groups at the end of the treatment were missing, we contacted the trial authors. Where standard deviations were not available from trial authors, they were calculated from t-tests, confidence intervals or standard errors, where reported in articles. Reference Deeks20,Reference Deeks21 If these additional data were not available, the study data were not included in the meta-analysis.
The methodological quality of the included studies was assessed using the Amsterdam–Maastricht consensus list ranging from 0 (poor quality) to 19 (excellent quality). The Amsterdam–Maastricht consensus list is accepted by the Cochrane Review Group Reference van Tulder, Assendelft, Koes and Bouter22 and has been used previously in systematic reviews of RCTs in psychiatric disorders. Reference van Boeijen, van Balkom, van Oppen, Blankenstein, Cherpanath and van Dyck23,Reference Oude Voshaar, Couvée, Van Balkom, Mulder and Zitman24 It covers the Chalmers criteria usually applied in the assessment of study quality. Reference van Tulder, Assendelft, Koes and Bouter22,Reference Chalmers, Smith, Blackburn, Silverman, Schroeder and Reitman25
Statistical analysis
For the main analyses, studies were subdivided in studies restricted to participants with depressive disorder according to predefined criteria and studies of participants with depressive symptoms. The primary outcome measure for both categories was the severity of depressive symptoms. We a priori decided to use the Beck Depression Inventory (BDI) Reference Beck, Ward, Mendelson, Mock and Erbaugh26 or Beck Depression Inventory–II (BDI–II) Reference Beck, Steer and Brown27 as the primary outcome measure and, if not available (in order of preference), the Hamilton Depression Rating Scale (HDRS) Reference Hamilton28 or the Center for Epidemiological Studies – Depression Scale (CES–D). Reference Radloff29 If a separate measure of depressive symptoms was lacking, a subscale of a validated generic psychiatric instrument was used (in order of preference): the depression subscale of the Hospital Anxiety and Depression Scale (HADS), Reference Zigmond and Snaith30 the Profile of Mood Symptoms (POMS), Reference McNair, Lorr and Drappelman31 the Symptom Checklist 90-item version (SCL–90–R) Reference Derogatis32 or the Impact of Rheumatic Diseases on Health and Lifestyle (IRGL). Reference Huiskes, Kraaimaat and Bijlsma33
Data were analysed with Review Manager (RevMan) version 5.0.17 for Windows. At the moment, Cochrane methodology is widely advised and accepted as the preferred method in meta-analytic studies. We used the standardised mean difference (SMD) as the summary statistic in our meta-analysis, which expresses the size of the treatment effect in each trial relative to the variability observed in that trial, enabling us to pool different scales assessing one outcome measure. The SMD thus reflects the difference in the mean outcome between groups divided by the standard deviation of outcomes among participants. We applied the chi-squared test for heterogeneity. Since the chi-squared test has low power to assess heterogeneity where a small number of participants or trials are included, the P-value was conservatively set at 0.10. Reference Higgins and Green34 We used a fixed-effects model if homogeneity was found and a random-effects model if not (although the latter model slightly compromised the statistical power of our analysis). Summary statistics were based on intention-to-treat data and when missing, on available case analyses. It is generally assumed to be more realistic to include papers based on their completer data than excluding papers by not providing intention-to-treat data, on the prerequisite that potential difference between studies will be discussed and taken into account as a source of heterogeneity. Reference Higgins and Green34
The following subgroup analyses were performed to examine whether specific characteristics of the studies were related to the effect sizes:
-
(a) type of CBT condition, classified as classic CBT, problem-solving therapy and cognitive–behavioural stress management;
-
(b) control condition, classified as treatment as usual, waiting-list control condition and other psychotherapy;
-
(c) treatment delivery in group versus individual format.
Sensitivity analyses were undertaken to assess whether the quality of the studies was related to the magnitude of the observed effect sizes. Studies were limited to those of higher quality as determined by risk of: drop-out rate lower than 20%, studies providing intention-to-treat data, outcome assessment by validated questionnaire (excluding subscales) or whether depressive symptoms was the primary or secondary outcome measure. Publication bias was explored by preparing funnel plots for all outcome measures.
Results
Search strategy
The study extraction and selection process as recommended by the Quality of Reporting of Meta-analyses (QUOROM) statement Reference Moher, Cook, Eastwood, Olkin, Rennie and Stroup35 is shown in Fig. 1. The initial search yielded 1361 reference titles in PubMed, 2677 in PsycINFO and 604 in the Cochrane Library of which 305 titles were identified as having a possible relevance to the treatment of depression in people with an underlying somatic disease by M.W.B. or R.C.O.V. After screening of the abstracts and the full text, we excluded 266 papers. Of the 39 articles of interest, 13 papers did not provide sufficient information for the meta-analyses and we contacted the corresponding author for this. Of the 13 authors contacted, Reference Lustman, Griffith, Freedland, Kissel and Clouse2,Reference Tan and Bruni36–Reference Doering, Cross, Vredevoe, Martínez-Maza and Cowan47 3 authors responded to our request and were included. Reference Edelman, Bell and Kidman42,Reference Lincoln and Flannaghan44,Reference Given, Given, Rahbar, Jeon, McCorkle and Cimprich45 Two authors claimed that the relevant study data were not available anymore Reference Tan and Bruni36,Reference Mulder, Emmelkamp, Antoni, Mulder, Sandfort and de Vries38 and 8 authors did not respond to our request, leaving 29 studies for the meta-analysis. Reference Edelman, Bell and Kidman42,Reference Lincoln and Flannaghan44,Reference Given, Given, Rahbar, Jeon, McCorkle and Cimprich45,Reference Larcombe and Wilson48–Reference Safren, O'Cleirig, Tan, Raminani, Reilly and Otto73
Study quality
Online Tables DS1 and DS2 list the scores for methodological quality of the studies as assessed with the Amsterdam–Maastricht consensus list. The sum score (range 0–19) is taken to reflect study quality but as neither participants nor therapists can be masked to a psychological treatment condition, the maximum score in psychotherapy studies is 17. Interrater discrepancies were limited to a one-point difference in eight studies and a two-point difference in two studies. The broad range from 8 to 16 indicates a high variation of study quality, which emphasises the need for the (planned) sensitivity analyses to evaluate the effect of the most important issues of study quality.

Fig. 1 Flow diagram of randomised controlled trials (RCTs) included and excluded in meta-analysis. CBT, cognitive–behavioural therapy.
Study characteristics
The study characteristics are summarised in Table 1 for studies restricted to individuals with depressive disorder and Table 2 for studies of individuals with depressive symptoms. The 13 trials that included participants with depressive disorder Reference Lincoln and Flannaghan44,Reference Larcombe and Wilson48,Reference Kelly, Murphy, Bahr, Kalichman, Morgan and Stevenson51,Reference Evans and Connis52,Reference Markowitz, Kocsis, Fishman, Spielman, Jacobsberg and Frances55,Reference Mohr, Likosky, Bertagnolli, Goodkin, van der Wende and Dwyer57,Reference Mohr, Boudewyn, Goodkin, Bostrom and Epstein58,Reference Nezu, Nezu, Felgoise, McClure and Houts63,Reference Mohr, Hart, Julian, Catledge, Honos-Webb and Vella66,Reference Savard, Simard, Giguère, Ivers, Morin and Maunsell68,Reference Gellis, McGinty, Horowitz, Bruce and Misener69,Reference Kunik, Veazy, Cully, Souchek, Graham and Hopko72,Reference Safren, O'Cleirig, Tan, Raminani, Reilly and Otto73 comprised a total of 1139 people with a mean age of 51 years. These studies all had treatment of depression as their primary goal. In total, 252 participants (22%) dropped out prematurely; 112 (21%) in the CBT condition and 140 (23%) in the control condition (χ2 = 0.62, d.f. = 1, P = 0.43).
Table 1 Study characteristics of participants with depressive disorder
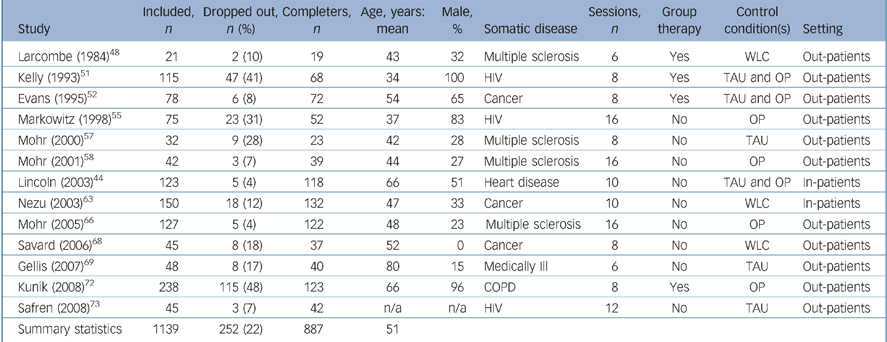
| Study | Included, n | Dropped out, n (%) | Completers, n | Age, years: mean | Male, % | Somatic disease | Sessions, n | Group therapy | Control condition(s) | Setting |
|---|---|---|---|---|---|---|---|---|---|---|
| Larcombe (1984) Reference Larcombe and Wilson48 | 21 | 2 (10) | 19 | 43 | 32 | Multiple sclerosis | 6 | Yes | WLC | Out-patients |
| Kelly (1993) Reference Kelly, Murphy, Bahr, Kalichman, Morgan and Stevenson51 | 115 | 47 (41) | 68 | 34 | 100 | HIV | 8 | Yes | TAU and OP | Out-patients |
| Evans (1995) Reference Evans and Connis52 | 78 | 6 (8) | 72 | 54 | 65 | Cancer | 8 | Yes | TAU and OP | Out-patients |
| Markowitz (1998) Reference Markowitz, Kocsis, Fishman, Spielman, Jacobsberg and Frances55 | 75 | 23 (31) | 52 | 37 | 83 | HIV | 16 | No | OP | Out-patients |
| Mohr (2000) Reference Mohr, Likosky, Bertagnolli, Goodkin, van der Wende and Dwyer57 | 32 | 9 (28) | 23 | 42 | 28 | Multiple sclerosis | 8 | No | TAU | Out-patients |
| Mohr (2001) Reference Mohr, Boudewyn, Goodkin, Bostrom and Epstein58 | 42 | 3 (7) | 39 | 44 | 27 | Multiple sclerosis | 16 | No | OP | Out-patients |
| Lincoln (2003) Reference Lincoln and Flannaghan44 | 123 | 5 (4) | 118 | 66 | 51 | Heart disease | 10 | No | TAU and OP | In-patients |
| Nezu (2003) Reference Nezu, Nezu, Felgoise, McClure and Houts63 | 150 | 18 (12) | 132 | 47 | 33 | Cancer | 10 | No | WLC | In-patients |
| Mohr (2005) Reference Mohr, Hart, Julian, Catledge, Honos-Webb and Vella66 | 127 | 5 (4) | 122 | 48 | 23 | Multiple sclerosis | 16 | No | OP | Out-patients |
| Savard (2006) Reference Savard, Simard, Giguère, Ivers, Morin and Maunsell68 | 45 | 8 (18) | 37 | 52 | 0 | Cancer | 8 | No | WLC | Out-patients |
| Gellis (2007) Reference Gellis, McGinty, Horowitz, Bruce and Misener69 | 48 | 8 (17) | 40 | 80 | 15 | Medically Ill | 6 | No | TAU | Out-patients |
| Kunik (2008) Reference Kunik, Veazy, Cully, Souchek, Graham and Hopko72 | 238 | 115 (48) | 123 | 66 | 96 | COPD | 8 | Yes | OP | Out-patients |
| Safren (2008) Reference Safren, O'Cleirig, Tan, Raminani, Reilly and Otto73 | 45 | 3 (7) | 42 | n/a | n/a | HIV | 12 | No | TAU | Out-patients |
| Summary statistics | 1139 | 252 (22) | 887 | 51 |
Table 2 Study characteristics of participants with depressive symptoms
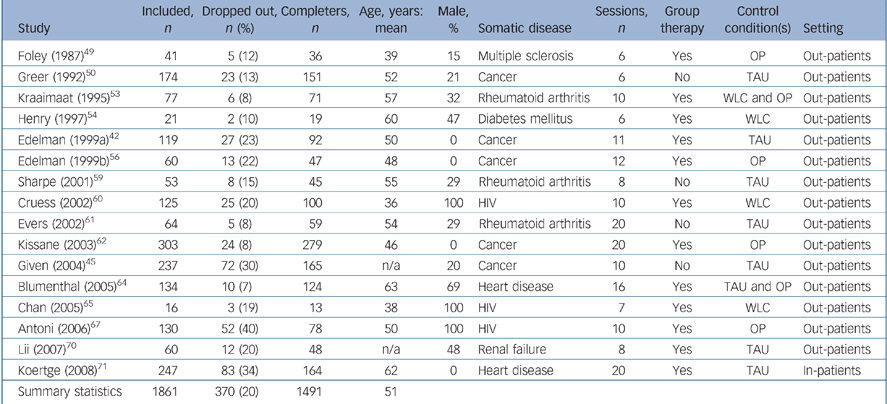
| Study | Included, n | Dropped out, n (%) | Completers, n | Age, years: mean | Male, % | Somatic disease | Sessions, n | Group therapy | Control condition(s) | Setting |
|---|---|---|---|---|---|---|---|---|---|---|
| Foley (1987) Reference Foley, Bedell, LaRocca, Scheinberg and Reznikoff49 | 41 | 5 (12) | 36 | 39 | 15 | Multiple sclerosis | 6 | Yes | OP | Out-patients |
| Greer (1992) Reference Greer, Moorey, Baruch, Watson, Robertson and Mason50 | 174 | 23 (13) | 151 | 52 | 21 | Cancer | 6 | No | TAU | Out-patients |
| Kraaimaat (1995) Reference Kraaimaat, Brons, Geenen and Bijlsma53 | 77 | 6 (8) | 71 | 57 | 32 | Rheumatoid arthritis | 10 | Yes | WLC and OP | Out-patients |
| Henry (1997) Reference Henry, Wilson, Bruce, Chisholm and Rawling54 | 21 | 2 (10) | 19 | 60 | 47 | Diabetes mellitus | 6 | Yes | WLC | Out-patients |
| Edelman (1999a) Reference Edelman, Bell and Kidman42 | 119 | 27 (23) | 92 | 50 | 0 | Cancer | 11 | Yes | TAU | Out-patients |
| Edelman (1999b) Reference Edelman, Bell and Kidman56 | 60 | 13 (22) | 47 | 48 | 0 | Cancer | 12 | Yes | OP | Out-patients |
| Sharpe (2001) Reference Sharpe, Sensky, Timberlake, Ryan, Brewin and Allard59 | 53 | 8 (15) | 45 | 55 | 29 | Rheumatoid arthritis | 8 | No | TAU | Out-patients |
| Cruess (2002) Reference Cruess, Antoni, Hayes, Penedo, Ironson and Fletcher60 | 125 | 25 (20) | 100 | 36 | 100 | HIV | 10 | Yes | WLC | Out-patients |
| Evers (2002) Reference Evers, Kraaimaat, van Riel and de Jong61 | 64 | 5 (8) | 59 | 54 | 29 | Rheumatoid arthritis | 20 | No | TAU | Out-patients |
| Kissane (2003) Reference Kissane, Bloch, Smith, Miach, Clarke and Ikin62 | 303 | 24 (8) | 279 | 46 | 0 | Cancer | 20 | Yes | OP | Out-patients |
| Given (2004) Reference Given, Given, Rahbar, Jeon, McCorkle and Cimprich45 | 237 | 72 (30) | 165 | n/a | 20 | Cancer | 10 | No | TAU | Out-patients |
| Blumenthal (2005) Reference Blumenthal, Sherwood, Babyak, Watkins, Waugh and Georgiades64 | 134 | 10 (7) | 124 | 63 | 69 | Heart disease | 16 | Yes | TAU and OP | Out-patients |
| Chan (2005) Reference Chan, Kong, Leung, Au, Li and Chung65 | 16 | 3 (19) | 13 | 38 | 100 | HIV | 7 | Yes | WLC | Out-patients |
| Antoni (2006) Reference Antoni, Carrico, Duran, Spitzer, Penedo and Ironson67 | 130 | 52 (40) | 78 | 50 | 100 | HIV | 10 | Yes | OP | Out-patients |
| Lii (2007) Reference Lii, Tsay and Wang70 | 60 | 12 (20) | 48 | n/a | 48 | Renal failure | 8 | Yes | TAU | Out-patients |
| Koertge (2008) Reference Koertge, Janszky, Sundin, Blom, Georgiades and László71 | 247 | 83 (34) | 164 | 62 | 0 | Heart disease | 20 | Yes | TAU | In-patients |
| Summary statistics | 1861 | 370 (20) | 1491 | 51 |
The 16 trials including participants with depressive symptoms Reference Edelman, Bell and Kidman42,Reference Given, Given, Rahbar, Jeon, McCorkle and Cimprich45,Reference Foley, Bedell, LaRocca, Scheinberg and Reznikoff49,Reference Greer, Moorey, Baruch, Watson, Robertson and Mason50,Reference Kraaimaat, Brons, Geenen and Bijlsma53,Reference Henry, Wilson, Bruce, Chisholm and Rawling54,Reference Edelman, Bell and Kidman56,Reference Sharpe, Sensky, Timberlake, Ryan, Brewin and Allard59–Reference Kissane, Bloch, Smith, Miach, Clarke and Ikin62,Reference Blumenthal, Sherwood, Babyak, Watkins, Waugh and Georgiades64,Reference Chan, Kong, Leung, Au, Li and Chung65,Reference Antoni, Carrico, Duran, Spitzer, Penedo and Ironson67,Reference Lii, Tsay and Wang70,Reference Koertge, Janszky, Sundin, Blom, Georgiades and László71 included 1861 people with a mean age of 51 years. A total of 370 participants (20%) dropped out before the outcome assessment; 192 (21%) in the CBT condition and 178 (19%) in the control condition (χ2 = 0.93, d.f. = 1, P = 0.34). In six (38%) of these studies the treatment of depressive symptoms was the primary goal Reference Edelman, Bell and Kidman42,Reference Given, Given, Rahbar, Jeon, McCorkle and Cimprich45,Reference Edelman, Bell and Kidman56,Reference Cruess, Antoni, Hayes, Penedo, Ironson and Fletcher60,Reference Lii, Tsay and Wang70,Reference Koertge, Janszky, Sundin, Blom, Georgiades and László71 and in 10 (62%) a secondary aim. Reference Foley, Bedell, LaRocca, Scheinberg and Reznikoff49,Reference Greer, Moorey, Baruch, Watson, Robertson and Mason50,Reference Kraaimaat, Brons, Geenen and Bijlsma53,Reference Henry, Wilson, Bruce, Chisholm and Rawling54,Reference Sharpe, Sensky, Timberlake, Ryan, Brewin and Allard59,Reference Evers, Kraaimaat, van Riel and de Jong61,Reference Kissane, Bloch, Smith, Miach, Clarke and Ikin62,Reference Blumenthal, Sherwood, Babyak, Watkins, Waugh and Georgiades64,Reference Chan, Kong, Leung, Au, Li and Chung65,Reference Antoni, Carrico, Duran, Spitzer, Penedo and Ironson67
Underlying somatic diseases in the different studies were: cancer (n = 8), HIV infection (n = 6), multiple sclerosis (n = 5), rheumatoid arthritis (n = 3), vascular disease (n = 3), diabetes mellitus (n = 1), chronic obstructive pulmonary disease (n = 1), chronic renal failure (n = 1) and various somatic diseases (n = 1).
Six studies Reference Lincoln and Flannaghan44,Reference Kelly, Murphy, Bahr, Kalichman, Morgan and Stevenson51–Reference Kraaimaat, Brons, Geenen and Bijlsma53,Reference Markowitz, Kocsis, Fishman, Spielman, Jacobsberg and Frances55,Reference Blumenthal, Sherwood, Babyak, Watkins, Waugh and Georgiades64 compared an active treatment with two control conditions (e.g. an attention control, as well as a standard care group). We combined data from these control conditions and compared this with data for the treatment group. In the subgroup analysis of the control condition we used both control conditions separately. One study Reference Nezu, Nezu, Felgoise, McClure and Houts63 compared two active treatments (problem-solving therapy and problem-solving therapy with a significant other) with a waiting-list control condition. In this case we combined the two treatment conditions and compared this with the control condition.
Most studies evaluated a classic CBT intervention, five studies evaluated cognitive–behavioural stress management, Reference Henry, Wilson, Bruce, Chisholm and Rawling54,Reference Cruess, Antoni, Hayes, Penedo, Ironson and Fletcher60,Reference Blumenthal, Sherwood, Babyak, Watkins, Waugh and Georgiades64,Reference Antoni, Carrico, Duran, Spitzer, Penedo and Ironson67,Reference Koertge, Janszky, Sundin, Blom, Georgiades and László71 and two studies problem-solving therapy Reference Nezu, Nezu, Felgoise, McClure and Houts63,Reference Gellis, McGinty, Horowitz, Bruce and Misener69 (all studies fulfilling our inclusion criteria for CBT). The number of treatment sessions ranged from 6 to 20 weeks, delivered either in a group format (16 studies) or individually (13 studies). In two studies, the intervention was delivered exclusively by telephone. Reference Mohr, Likosky, Bertagnolli, Goodkin, van der Wende and Dwyer57,Reference Mohr, Hart, Julian, Catledge, Honos-Webb and Vella66
Meta-analyses
The results for the primary outcome variable are presented in Fig. 2 for studies of individuals with depressive disorder and Fig. 3 for studies of individuals with depressive symptoms. Because of significant heterogeneity in the studies restricted to participants with depressive disorder (χ2 = 170.12, d.f. = 12, P<0.001, I 2 = 93%), we used a random-effects model. We found a significant pooled standardised mean difference of –0.83 (95% CI –1.36 to –0.31) in favour of the CBT condition (Z = 3.13, P = 0.002). Inspection of the data showed that the heterogeneity of results could not be explained by the underlying somatic disease, but was caused by three positive outliers. Reference Larcombe and Wilson48,Reference Nezu, Nezu, Felgoise, McClure and Houts63,Reference Gellis, McGinty, Horowitz, Bruce and Misener69 Removal of these studies led to more homogeneous results, but reduced the effect size by –0.64 yielding a still significant pooled SMD of –0.19 (95% CI –0.33 to –0.05, Z = 2.69, P = 0.007).
The studies of individuals with depressive symptoms yielded a smaller but still significant pooled SMD of –0.16 (95% CI –0.27 to –0.06) in favour of the CBT condition (Z = 3.19, P = 0.001).
The funnel plots of both categories of papers were not suggestive of publication bias. The effect size was neither correlated with publication year nor with the quality score (all P>0.22). A strong correlation was found between publication year and quality score among studies restricted to participants with depressive disorder, showing an increase of study quality over the years (Spearman's rho = 0.67, P = 0.013). Based on Cochrane methodology, differences in quality score are addressed in sensitivity analyses as planned (see below) instead of excluding low-quality studies. We did not expect to find studies delivering CBT by telephone. This finding urged us to perform a post hoc subgroup analyses excluding these two studies, which yielded a higher effect size of –0.92 (95% CI –1.55 to –0.29) in favour of the CBT condition (Z = 2.86, P = 0.004) using a random-effects model.
Furthermore, the effect size was significantly correlated with the number of CBT sessions provided in the studies of participants with depressive symptoms (Spearman's rho = 0.53, P = 0.033), but not in the studies of participants with depressive disorder. In the studies of people with depressive symptoms, a post hoc subgroup analysis showed that the effect size in studies providing less than ten CBT sessions was –0.39 (95% CI –0.61 to –0.16) in favour of the CBT condition (Z = 3.34, P = 0.00008), compared with –0.11 (95% CI –0.22 to 0.00, Z = 1.89, P = 0.06) in studies providing ten or more sessions.

Fig. 2 Forrest plot of standard mean difference (with 95% CI) of effect of cognitive–behavioural therapy on depressive symptoms in studies including participants with depressive disorder, with overall effect (black diamond), based on meta-analysis. Std, standard.

Fig. 3 Forrest plot of standard mean difference (with 95% CI) of effect of cognitive–behavioural therapy on depressive symptoms in studies of participants with depressive symptoms, with overall effect (black diamond), based on meta-analysis. Std, standard.
Planned subgroup analyses
Type of CBT
Meta-analyses of studies that applied classic CBT achieved significant overall effects. For studies restricted to participants with depressive disorder, the SMD was –0.34 (95% CI –0.60 to –0.09, Z = 2.64, P = 0.008) using a random-effects model and for studies of participants with depressive symptoms the SMD was –0.31 (95% CI –0.46 to –0.16, Z = 4.10, P<0.001).
Two small studies evaluating problem-solving therapy yielded a pooled SMD of –2.86 (95% CI –4.11 to –1.61, Z = 4.49, P<0.001) based on a total of 172 participants. Five studies evaluating cognitive–behavioural stress management yielded a non-significant SMD of –0.12 (95% CI –0.30 to 0.05).
Control condition
Of the studies restricted to participants with depressive disorder, those using a waiting list or a treatment as usual care control condition had a significant pooled effect size of –2.38 (95% CI –4.41 to –0.36, Z = 2.31, P = 0.02) and –0.72 (95% CI –1.21 to –0.24, Z = 2.94, P = 0.003) respectively. A random-effects model was used, because there was considerable heterogeneity. The studies using other psychotherapy as a control group yielded a non-significant pooled SMD of –0.06 (95% CI –0.23 to 0.10, Z = 0.76, P = 0.45).
For studies of participants with depressive symptoms, only studies comparing CBT with treatment as usual yielded a significant pooled SMD of –0.28 (95% CI –0.41 to –0.15) in favour of the CBT condition (Z = 4.20, P<0.001), whereas no differences were found comparing CBT versus a waiting-list control condition: SMD = –0.13 (95% CI –0.44 to 0.17, Z = 0.87, P = 0.38); or versus other psychotherapies: SMD = 0.04 (95% CI –0.15 to 0.22, Z = 0.37, P = 0.71).
Treatment delivery in group versus individual format
Only individual treatment delivery led to a significant effect in favour of CBT for both studies of participants with depressive disorder and depressive symptoms. The pooled SMD was –0.91 (95 % CI –1.65 to –0.17, Z = 2.41, P = 0.02) and –0.30 (95% CI –0.50 to –0.11, Z = 3.10, P = 0.002) respectively.
Sensitivity analyses
Drop-out rate ≥20%
Sensitivity analyses were performed excluding studies that had a drop-out rate higher or equal to 20%. Reference Edelman, Bell and Kidman42,Reference Given, Given, Rahbar, Jeon, McCorkle and Cimprich45,Reference Kelly, Murphy, Bahr, Kalichman, Morgan and Stevenson51,Reference Markowitz, Kocsis, Fishman, Spielman, Jacobsberg and Frances55–Reference Mohr, Likosky, Bertagnolli, Goodkin, van der Wende and Dwyer57,Reference Cruess, Antoni, Hayes, Penedo, Ironson and Fletcher60,Reference Antoni, Carrico, Duran, Spitzer, Penedo and Ironson67,Reference Lii, Tsay and Wang70–Reference Kunik, Veazy, Cully, Souchek, Graham and Hopko72 In the studies restricted to individuals with depressive disorder, the pooled SMD was –1.18 (95 % CI –1.96 to –0.41) in favour of the CBT condition (Z = 2.99, P = 0.003) using a random-effects model. A non-significant pooled SMD of –0.11 (95% CI –0.25 to –0.03, Z = 1.54, P = 0.12) was found in studies of individuals with depressive symptoms.
Intent-to-treat data
After excluding studies that did not provide intention-to-treat data Reference Edelman, Bell and Kidman42,Reference Lincoln and Flannaghan44,Reference Given, Given, Rahbar, Jeon, McCorkle and Cimprich45,Reference Larcombe and Wilson48–Reference Henry, Wilson, Bruce, Chisholm and Rawling54,Reference Edelman, Bell and Kidman56,Reference Mohr, Boudewyn, Goodkin, Bostrom and Epstein58–Reference Evers, Kraaimaat, van Riel and de Jong61,Reference Nezu, Nezu, Felgoise, McClure and Houts63,Reference Chan, Kong, Leung, Au, Li and Chung65–Reference Koertge, Janszky, Sundin, Blom, Georgiades and László71 a non-significant pooled SMD was found of –0.11 (95% CI –0.31 to 0.09, Z = 1.05, P = 0.29) for studies restricted to individuals with depressive disorder, as well as for the studies of individuals with depressive symptoms: SMD = 0.03 (95% CI –0.16 to 0.22, Z = 0.27, P = 0.79).
Outcome assessment with a depression subscale
After excluding studies that only measured depression with a subscale of a (generic) outcome measure, Reference Edelman, Bell and Kidman42,Reference Greer, Moorey, Baruch, Watson, Robertson and Mason50,Reference Kraaimaat, Brons, Geenen and Bijlsma53,Reference Edelman, Bell and Kidman56,Reference Mohr, Likosky, Bertagnolli, Goodkin, van der Wende and Dwyer57,Reference Sharpe, Sensky, Timberlake, Ryan, Brewin and Allard59,Reference Kissane, Bloch, Smith, Miach, Clarke and Ikin62 a significant pooled SMD of –0.86 (95% CI –1.41 to –0.30) was found in favour of the CBT condition (Z = 3.03, P = 0.002) in studies of participants with depressive disorder. The studies of participants with depressive symptoms yielded a significant SMD of –0.25 (95% CI –0.38 to –0.11) in favour of the CBT condition (Z = 3.48, P<0.001).
Depressive symptoms as primary or secondary outcome measure
Excluding the 10 studies with depressive symptoms as a secondary outcome measure, Reference Foley, Bedell, LaRocca, Scheinberg and Reznikoff49,Reference Greer, Moorey, Baruch, Watson, Robertson and Mason50,Reference Kraaimaat, Brons, Geenen and Bijlsma53,Reference Henry, Wilson, Bruce, Chisholm and Rawling54,Reference Sharpe, Sensky, Timberlake, Ryan, Brewin and Allard59,Reference Evers, Kraaimaat, van Riel and de Jong61,Reference Kissane, Bloch, Smith, Miach, Clarke and Ikin62,Reference Blumenthal, Sherwood, Babyak, Watkins, Waugh and Georgiades64,Reference Chan, Kong, Leung, Au, Li and Chung65,Reference Antoni, Carrico, Duran, Spitzer, Penedo and Ironson67 the pooled SMD was –0.23 (95% CI –0.39 to –0.07, Z = 2.76, P = 0.006).
Discussion
This meta-analysis of 29 RCTs investigated the effect of CBT on depressive symptoms in 1247 people with a somatic disease and 1338 controls. Our two main meta-analyses of CBT both showed that CBT is effective in treating depressive symptoms in people with a variety of somatic diseases. As expected, the pooled results of studies restricted to individuals with depressive disorder had a stronger significant effect (–0.83) than those including people with depressive symptoms (–0.16). However, the lower the level of depressive symptoms at baseline, the less likely treatment will result in a significant reduction of these symptoms. So, the difference between both meta-analyses may in fact reflect a floor effect.
Reported effect-sizes in meta-analyses of CBT for depressive symptoms or general distress in people with a specific underlying somatic disease are 0.37 for individuals with HIV infection, Reference Himelhoch, Medoff and Oyeniyi10 0.36–0.44 for people with cancer, Reference Sheard and Maguire15,Reference Akechi, Okuyama, Onishi, Morita and Furukawa74 0.29 for those with rheumatoid arthritis, Reference Astin, Beckner, Soeken, Hochberg and Berman12 and 0.31 for those with breast cancer. Reference Tatrow and Montgomery13 The similarity of effect sizes in different populations is suggestive of a general underlying mechanism for depressive symptoms in people with a somatic disease. Coping style might be an important mediating mechanism, Reference Caplette-Gingras and Savard75,Reference MacMahon and Lip76 as somatically ill individuals with a higher perceived level of control, higher self-efficacy and active coping styles are better adjusted to the somatic illness Reference Dunkel-Schetter, Feinstein, Taylor and Falke77,Reference Hirai, Suzuki, Tsuneto, Ikenega, Hosaka and Kashiwagi78 and are less depressed. Reference Hirai, Suzuki, Tsuneto, Ikenega, Hosaka and Kashiwagi78,Reference Ranchor, Sanderman, Steptoe, Wardle, Miedema and Ormel79 The reported effect sizes are all in between our effect sizes of –0.83 for studies including participants with depressive disorder and –0.16 for studies including those with depressive symptoms. Pooling of all studies in our meta-analysis yields a significant overall effect size of –0.49 (data not shown), which is in line with these previous meta-analyses and emphasises the relevance of looking specifically at the definition of depression at baseline.
By performing a comprehensive review and meta-analysis of CBT for the treatment of depressive symptoms in people with a somatic disease, we did not restrict our inclusion criteria to studies which included only individuals with a diagnosis of depression according to the diagnostic criteria of the DSM. In contrast to previous meta-analyses on depressive symptoms in people with a specific somatic disease, we a priori decided to perform separately meta-analyses for studies using predefined criteria for depressive disorder at baseline and those studies including participants with somatic diseases with depressive symptoms in general (in these studies, authors generally assume that a somatic disease is stress-provoking and thus all patients may benefit from CBT).
Furthermore, we included different types of CBT for the following reasons. First, we considered the content of the psychotherapy more important than the name given to it by the study authors. In this paper, CBT was defined as all psychotherapies that included both cognitive restructuring and behavioural activation. Second, we intended to perform a comprehensive review and meta-analysis on this important topic, thereby precluding the emergence of different smaller meta-analyses confined to specific subtypes of CBT. The first subgroup analyses on subtypes of CBT, for instance, might have been published as separate papers. In our opinion nobody would gain by this strategy. Problem-solving therapy is generally seen as a derivative of CBT. Reference Hawton, Kirk, Hawton, Salkovskis, Kirk and Clark80 Although we identified only two studies that evaluated problem-solving therapy in this population, the pooled effect size was very large, which may suggest the high value of problem-solving therapy for this particular patient group. In patients with a somatic disease (and thus more handicaps or limitations), the more pragmatic approach of problem-solving therapy may be particularly favourable. Exclusion of these studies would have led to a less informative paper.
Subgroup analyses of CBT versus the different control conditions showed that CBT was superior to treatment as usual as well as a waiting-list control condition. Cognitive–behavioural therapy, however, was not superior to studies using a control condition with another psychological therapy, which consisted mainly of supportive-expressive therapies. Therefore, the effects we found might be caused by non-specific therapeutic effects that are not exclusive for CBT. A recent meta-analysis on the comparison of seven kinds of psychological therapies for depressive disorder in general also found similar effect sizes for the different modalities (CBT, non-directive supportive treatment, behavioural activation treatment, psychodynamic treatment, problem-solving therapy, interpersonal psychotherapy and social skills training). Reference Cuijpers, van Straten, Andersson and van Oppen81 Interestingly, only individual CBT yielded a significant effect size in studies of both participants with depressive disorder and depressive symptoms. This finding is in contrast with CBT for depression in general. Reference McDermut, Miller and Brown82 Individual therapy might be more suitable than group therapy for people with depression with a comorbid somatic disorder.
Methodological considerations
The study has some limitations. First, the methodological quality of the included studies was highly variable. The sensitivity analyses showed that the results were partly biased by methodological shortcomings, but the overall conclusions seem valid. For example, exclusion of studies with a drop-out rate of 20% or more led to an increased effect size in studies restricted to individuals with depressive disorder (from –0.83 to –1.18), whereas in studies of participants with depressive symptoms the effect size didn't change very much (–0.17 v. –0.13). Second, analysing only studies providing intent-to-treat data reduced the effect size in the studies of people with depressive disorder from –0.83 to a non-significant –0.11. This was also the case in studies of people with depressive symptoms. Third, neither exclusion of studies that did not use a specific instrument for assessing depression, nor exclusion of studies that used depressive symptoms as a secondary outcome measure influenced the effect size. In addition to the high variability of the quality, we did find heterogeneity among studies restricted to participants with depressive disorder, caused by three studies with extreme positive results. After removal of these outliers the results remained significant. As a last limitation, we should mention that we could not control for the stage of the underlying disease.
Implications
Cognitive–behavioural therapy significantly reduces depressive symptoms in people with an underlying somatic disease. The effect size, however, was clearly dependent on the severity of depressive symptoms at the time of inclusion in the study, with less of an effect in individuals with a lower level of depressive symptoms. A significant gap in knowledge, that might contribute to this difference, is the lack of studies that address which components of CBT might be most helpful in this population and by which personality characteristics or coping styles these effects are mediated. Currently, we cannot conclude that the positive effects are specific for CBT-oriented interventions. However, the findings of our meta-analysis support the effectiveness of CBT and therefore as the treatment of choice in people with underlying somatic diseases. The results also suggest that individual treatment might be more effective than group therapy in somatically ill people with depressive disorder. This hypothesis, however, should be further examined in future research. In addition to the need to focus more specifically on which CBT-components are most efficacious, further research should also include long-term follow-up data with respect to both depressive symptoms and the prognosis of the underlying somatic disease.








eLetters
No eLetters have been published for this article.