Benzodiazepines are extensively used to treat anxiety and sleep disorders, as adjuvant therapy in patients with depression and as muscle relaxants. 1 Although these drugs are considered effective and safe in the short term, their long-term use is associated with adverse health outcomes, including tolerance and dependence, increased risk of motor vehicle accidents, Reference Thomas2,Reference Smink, Egberts, Lusthof, Uges and de Gier3 falls and hip fractures in the elderly, Reference Leipzig, Cumming and Tinetti4,Reference Wang, Bohn, Glynn, Mogun and Avorn5 and cognitive and memory impairment. Reference Glass, Lanctot, Herrmann, Sproule and Busto6,Reference Barker, Greenwood, Jackson and Crowe7 More recently, long-term benzodiazepine use has been associated with dementia and with increased global mortality. Reference Billioti de Gage, Begaud, Bazin, Verdoux, Dartigues and Peres8-Reference Kripke, Langer and Kline10 Although international clinical guidelines and medical authorities in many countries recommend limiting the duration of benzodiazepine treatment to only a few weeks, 1 the prevalence of long-term use remains widespread. These agents are regularly used by over 5% of the populations of Spain, France and Italy, but by fewer than 2% of individuals in Germany and the UK. Reference Bejarano Romero, Pinol Moreso, Mora Gilabert, Claver Luque, Brull Lopez and Basora Gallisa11,Reference Ohayon and Lader12 Rates are higher in elderly people, Reference Lader13 who are particularly vulnerable to their adverse effects. The magnitude of this problem has become a health concern in most European countries and many efforts have been made to develop strategies to reduce the extent of benzodiazepine usage.
Since three of four benzodiazepine prescriptions are written by general practitioners (GPs), Reference Vicens, Fiol, Llobera, Campoamor, Mateu and Alegret14,Reference Lader, Tylee and Donoghue15 patient withdrawal in the primary care setting is an important goal. Approaches to discontinuation have ranged from simple to more complex interventions. Simple interventions include GPs sending a letter; Reference Cormack, Sweeney, Hughes-Jones and Foot16-Reference Ten Wolde, Dijkstra, van Empelen, van den Hout, Neven and Zitman19 brief advice by GPs and pharmacists; Reference Bashir, King and Ashworth20,Reference Van de Steeg-van Gompel, Wensing and De Smet21 and educational approaches by GPs based on gradual dose reduction. Reference Vicens, Fiol, Llobera, Campoamor, Mateu and Alegret14,Reference Oude Voshaar, Gorgels, Mol, van Balkom, van de Lisdonk and Breteler22 More complex interventions include cognitive-behavioural therapy (CBT) conducted by psychologists and GPs, Reference Oude Voshaar, Gorgels, Mol, van Balkom, van de Lisdonk and Breteler22-Reference Morin, Bastien, Guay, Radouco-Thomas, Leblanc and Vallieres24 and alternative pharmacotherapy. Reference Denis, Fatseas, Lavie and Auriacombe25 Benzodiazepine discontinuation does not cause psychological distress, Reference Heather, Bowie, Ashton, McAvoy, Spencer and Brodie18,Reference Bashir, King and Ashworth20 and may improve aspects of cognition. Reference Barker, Greenwood, Jackson and Crowe26 General practitioners usually have limited time for consultations with each patient and often encounter difficulties in managing withdrawal. Therefore, efforts should be made to develop feasible, evidence-based, effective and expeditious interventions that can be easily implemented in primary care. This study assessed two interventions: a structured educational intervention with gradual tapering backed up by fortnightly follow-up visits (SIF) and the same structured educational intervention supported by written instruction rather than follow-up visits (SIW), requiring less GP involvement. The aim was to compare the effectiveness of these two interventions with that of usual care on the discontinuation of long-term benzodiazepine use in primary care patients, delivered at the level of the GP. We also attempted to determine the effectiveness of each intervention relative to patient characteristics. Cluster randomisation was used to avoid potential cross-contamination bias which could lead to more intensive management in the usual care group.
Method
A three-arm, parallel, multicentre, cluster-randomised trial was carried out in three regions of Spain (the Balearic Islands, Catalonia and the Valencian community) between June 2010 and March 2012. The trial was registered with Current Controlled Trials (ISRCTN13024375) and a detailed research protocol has been published elsewhere. Reference Vicens, Socias, Mateu, Leiva, Bejarano and Sempere27 The study was approved by the research ethics committees of the Balearic Islands (Mallorca), the Primary Care Research Institute Jordi Gol (Barcelona) and the Valencia Health Agency. Study design, procedures and reporting followed guidance from the CONSORT statement on cluster-randomised controlled trials. Reference Campbell, Piaggio, Elbourne and Altman28
Participants and recruitment
Participating GPs were selected from 21 primary care centres in the three regions and were included if they were able to commit to taking part until completion. Patients eligible for the trial were aged 18-80 years and had been taking benzodiazepines daily for at least 6 months. Exclusion criteria were psychotic or personality disorder, or current treatment by a psychiatrist; severe anxiety, depressive disorder or severe medical illness including dementia and epilepsy as clinically assessed by the GP, or in cases where they considered that stopping benzodiazepine might be harmful; alcohol or illicit drug misuse; patient in residential care or terminally ill; inability to read and speak Spanish; or unwillingness to provide informed consent.
A list of patients taking benzodiazepines for more than 6 months was obtained from the computerised prescription database of each participating GP, and 30 patients were randomly chosen by the coordinating centre. Pharmaceutical categories included anxiolytics, hypnotics and sedatives (World Health Organization Anatomical, Therapeutic and Chemical classification system codes N05BA, N05CD, N05CF and M03BX07). Each GP had to systematically assess the eligibility of the patients from the random list in order to recruit 8 patients in a 4-week period. To avoid cluster heterogeneity and postrandomisation selection bias, GPs were randomised following patient enrolment. The reasons for GP and patient non-eligibility and non-participation are summarised in Fig. 1. Patients were chosen in October and November 2010. The mean interval between inclusion and baseline visit was about 4-6 weeks. The GPs collected patients’ sociodemographic, benzodiazepine-related and clinical characteristics.
Sample size
To detect a difference in the proportion of patients who had discontinued benzodiazepine treatment at 12 months of at least 20% and 15% in the SIF and SIW groups respectively, and assuming 25% patient loss to follow-up, an individually randomised study would require 129 patients per arm. To maintain homogeneity within each cluster we decided that each GP should recruit the same number of patients. We considered that 8 patients per GP could be easily included and followed. To account for the clustering effects from randomised GPs, with a moderate intracluster correlation coefficient of 0.04 and a cluster size of 8 patients, the number of patients required was multiplied by 1.28, corresponding to the cluster design effect. Reference Campbell, Grimshaw and Steen29 Thus, the final sample for each group consisted of 165 patients. Because each GP had to recruit 8 patients, at least 21 GPs per arm were needed (63 in total).
Randomisation
Each region enrolled 25-30 GPs and once all they had been selected and their patients included in the trial, GPs in each of the three regions were randomised 1:1:1 to one of the three study arms using a computer-generated block randomisation in blocks of 6 GPs. Randomisation and concealment was centralised through a single coordinating centre and the sequence was concealed from both patients and GPs until interventions were assigned.
Masking
Owing to study procedures, patients and GPs could not be masked to their random allocation. The main outcome was externally evaluated through personal interviews by psychologists not involved in the study and masked to patient allocation. The statistician and data-entry staff were also unaware of patient allocation.
Interventions
General practitioners assigned to the three groups attended an hour-long workshop explaining the study protocol and providing training in filling out the case report form. Practitioners assigned to the SIF and SIW groups attended a supplementary 3 h workshop on structured interviews, individualised patient information and training in managing benzodiazepine discontinuation and optimal gradual dose reduction. In addition, GPs assigned to the SIF group attended a brief 30 min workshop to standardise the dose-reduction follow-up visits. Training was provided by researchers with extensive experience in the management of benzodiazepine withdrawal. The SIF and SIW interventions were both based on a structured educational interview and GP-tailored stepped benzodiazepine dose reduction. These two interventions differed only in the follow-up. The content of the educational interview was structured and included four key points:
-
(a) information on benzodiazepine dependence, abstinence and withdrawal symptoms;
-
(b) the risks of long-term use, memory and cognitive impairment, accidents and falls;
-
(c) reassurance about reducing medication;
-
(d) a self-help leaflet to improve sleep quality if patients were taking benzodiazepines for insomnia.
The tailored gradual taper consisted of a 10-25% reduction in the daily dose of the benzodiazepine every 2-3 weeks. 1 To facilitate withdrawal, GPs were allowed to switch from a benzodiazepine with a short half-life to one with a longer half-life (diazepam). After the first intervention visit patients in the SIF group were scheduled for follow-up appointments with their GPs every 2-3 weeks until the end of the dose reduction. The GPs reinforced education, reassured patients regarding withdrawal symptoms and obtained patient agreement for the next step in dose reduction. Patients in the SIW group received written instructions reinforcing educational information at their first and only contact with their GP, along with a tailored gradual dose reduction until benzodiazepine cessation. No follow-up visit was scheduled, although patients could spontaneously request an appointment with their GP when needed. Patients allocated to the control group received routine care; their GPs could provide brief advice but did not receive any specific recommendation about the management of long-term benzodiazepine use from the study trainers.
Primary and secondary outcomes
The primary outcome was benzodiazepine discontinuation at 12 months, assessed in a personal interview and defined as self-declared non-consumption or consumption of fewer than four doses in the previous month. Consumption was reviewed and confirmed by prescription claims in the clinical records. A priori proposed variables for subgroup analysis were age, gender, short benzodiazepine half-life, more than 24 months taking benzodiazepine, dose higher than 10 mg diazepam equivalents, anxiety or depression, insomnia, alcohol consumption and benzodiazepine dependence, rated as described below. Secondary outcomes were benzodiazepine discontinuation at 6 months and safety outcomes measured at 6 months and 12 months, including changes in anxiety and depression symptoms, changes in sleep satisfaction, alcohol consumption and withdrawal symptoms.
Measures
Anxiety and depression symptoms were measured using the Hospital Anxiety and Depression Scale (HADS), Reference Zigmond and Snaith30,Reference Quintana, Padierna, Esteban, Arostegui, Bilbao and Ruiz31 a 14-item, four-point Likert scale (range 0-3 according to the severity of symptoms) validated for general hospital patients and patients in primary care, and cross-culturally validated in Spanish. Reference Quintana, Padierna, Esteban, Arostegui, Bilbao and Ruiz31 Sleep satisfaction and insomnia were assessed through two subscales of the Oviedo Sleep Questionnaire. Reference Bobes, Gonzalez, Saiz, Bascaran, Iglesias and Fernandez32 The sleep satisfaction subscale is measured with a seven-point Likert scale ranging from 1 (not satisfied) to 7 (very satisfied). Benzodiazepine dependence was rated with the Severity of Dependence Scale (SDS), a five-item questionnaire; Reference De las Cuevas, Sanz, de la Fuente, Padilla and Berenguer33 each of the five items is scored on a four-point scale (0-3), and a total score above 7 was considered to indicate benzodiazepine dependence. Changes in alcohol intake during follow-up were self-reported and measured in standard alcohol units per week (1 unit equals 10 g alcohol). Reference Saunders, Aasland, Amundsen and Grant34 Patients were also asked about adverse withdrawal effects, using a list of the most frequent symptoms including tremor, irritability, anxiety, insomnia and seizures, and the severity of these symptoms was scored as none, mild, moderate or severe. The GPs were asked to report any serious adverse event during the follow-up period to the trial coordinating centre. Our study protocol was approved by the Primary Care Research Committee and the Mallorca Ethical Committee of Clinical Research.
Statistical analysis
Primary and secondary outcomes were analysed at patient level on an intention-to-treat basis adjusted for data clustering. Categorical baseline variables were compared using a two-sided, cluster-adjusted chi-squared test. Continuous baseline variables and analysis of withdrawal symptoms, anxiety, depression, sleep satisfaction and alcohol intake and between-group analysis of anxiety, depression, sleep satisfaction and alcohol intake at 6 months and 12 months were compared using Somers’ D rank statistics. Within-group analysis was carried out by paired-sample Somers’ D rank test. Median differences between groups and within groups were calculated by Hodges-Lehmann and von Mises estimates for clustering data. Hodges-Lehmann and von Mises are robust and non-parametric estimators of the median differences between two populations and two related populations respectively. A score of 0 on the Hodges-Lehmann estimator or on the von Mises estimator respectively means no between-group difference or no difference between the same group on two different visits. A negative score means a reduction (of anxiety, depression, sleep satisfaction or alcohol consumption) in the second group compared with the first group or a decrease during the latest visit compared with the previous visit, whereas a positive score represents an increase. Categorical variables are reported as numbers and percentages, and continuous variables as medians with interquartile range (IQR). The estimated relative risks (RRs) of patients in each group who discontinued benzodiazepine treatment at 12 months were adjusted for cluster by means of a log link in a binomial distribution of a robust generalised estimating equation and an exchangeable correlation structure. Absolute risk reduction and number needed to treat (NNT) were also calculated from estimated RR. Intraclass correlation coefficient was calculated by one-way analysis of variance. Subgroup analysis was carried out through a statistically significant interaction term of the proposed variables and the treatment efficacy. We estimated the RRs of those subgroups in which the interaction term was statistically significant. No relevant data were missing, with only 35 of 532 (6.6%) patients missing the final evaluation, although prescription data were available from the clinical records of 26. Thus, data were unavailable for only 9 of 532 (1.7%) patients; based on an intention-to-treat analysis they were considered as still taking benzodiazepines. Statistical significance was set at 5%. Stata version 11.0 for Windows 2000 was used for analyses.
Results
Of the 98 GPs working in 21 primary care centres who were asked to participate in the trial (Fig. 1), 23 declined and 75 entered the study. Of 1564 patients assessed for eligibility by their GPs, 961 were excluded based on the study criteria, 61 could not be contacted and 10 refused to participate. Informed consent was obtained from 532 patients, who were enrolled in the study. The GPs and their clusters of patients were randomly assigned to one of the three study arms: 26 GPs (191 patients) to the structured intervention with follow-up visits (SIF), 24 GPs (168 patients) to the structured intervention with written instructions (SIW) and 25 GPs (173 patients) to the control group. None of the 75 GPs left the study during follow-up and 35 patients (6.6%) were lost to follow-up at 12 months. Final data were available from 523 of 532 (98.3%) patients.
Baseline data
Characteristics of the GPs
The characteristics of the GPs (Table 1) differed in the three study groups; those in the control group were more likely to be male and tended to be slightly older and with less experience in benzodiazepine withdrawal than those in the intervention groups.
Patient characteristics
At baseline the patients’ median age was 64 years (IQR 55-72) and 72% were women (Table 2). Insomnia (68%), anxiety (65%) or both were the main reasons for the initial prescription of benzodiazepines, of which 73.8% were prescribed by the patient’s GP. Median treatment duration was 60 months (IQR 24-120), ranging from 6 months to 480 months. The most frequently prescribed drugs were lorazepam (32.3%), alprazolam (17.7%), lormetazepam (15.2%) and zolpidem (13.9%). A total of 84.8% of these patients were taking a short half-life benzodiazepine (half-life shorter than 24 h) and 28.9% were taking doses higher than the equivalent of 10 mg diazepam. Dependence based on SDS score was observed in 36.6% of patients.
Outcomes
Primary outcome
Efficacy results are shown in Table 3. At 12 months, 76 of 168 (45.2%) patients in the SIW group and 86 of 191 (45.0%) patients in the SIF group had discontinued benzodiazepine use compared with 26 of 173 (15.0%) in the control group. After adjusting for cluster, the RRs for benzodiazepine discontinuation were 3.01 (95% CI 2.03-4.46, P<0.0001) for SIW and 3 (95% CI 2.04-4.40, P<0.0001) for SIF. There was no statistically significant difference in efficacy between the SIF and SIW groups (RR = 1.00, 95% CI 0.78-1.28, P = 0.984). Withdrawal at 12 months did not differ by gender, age, short or long half-life benzodiazepine use, depression (HADS score), insomnia (Oviedo questionnaire) or degree of dependence (SDS). We observed statistically significant differences in the interaction term and treatment efficacy for benzodiazepine dosage and anxiety. The discontinuation rate at 12 months was significantly greater for patients taking less than 10 mg v. more than 10 mg diazepam equivalent in each of the study groups: control group, 25/118 (21.2%) v. 1/55 (1.8%); SIW, 65/122 (53.3%) v. 11/46 (23.9%); SIF, 69/138 (50%) v. 17/53 (32%). Similarly, the discontinuation rate at 12 months was greater for less anxious patients, as assessed by the HADS anxiety scale: control group, 25/137 (18.2%) v. 1/33 (3%); SIW, 65/120 (54.2%) v. 11/41 (26.8%); SIF, 63/135 (46.7%) v. 23/49 (46.9%). Relative to the control group, the absolute risk reduction was 30.2% for the SIW group and 30.0% for the SIF group. The NNT for the SIW and SIF groups was 4 (95% CI 3-5). Consequently, four patients had to receive either of the proposed interventions within a year to achieve one benzodiazepine-free patient when compared with patients receiving usual care. The intraclass correlation coefficient for benzodiazepine discontinuation at 12 months was 0.03.

Fig. 1 Study profile. Thirty-five patients (6.6%) were lost to the study and data were unavailable for 9 (1.7%). GP, general practitioner; SIF, intervention group with follow-up visits; SIW, intervention group with written instructions.
Table 1 Characteristics of participating general practitioners
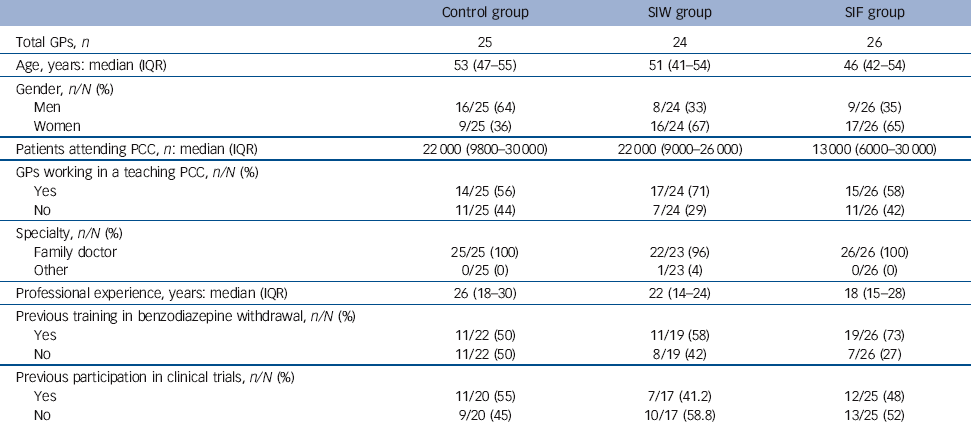
| Control group | SIW group | SIF group | |
|---|---|---|---|
| Total GPs, n | 25 | 24 | 26 |
| Age, years: median (IQR) | 53 (47-55) | 51 (41-54) | 46 (42-54) |
| Gender, n/N (%) | |||
| Men | 16/25 (64) | 8/24 (33) | 9/26 (35) |
| Women | 9/25 (36) | 16/24 (67) | 17/26 (65) |
| Patients attending PCC, n: median (IQR) | 22 000 (9800-30 000) | 22 000 (9000-26 000) | 13 000 (6000-30 000) |
| GPs working in a teaching PCC, n/N (%) | |||
| Yes | 14/25 (56) | 17/24 (71) | 15/26 (58) |
| No | 11/25 (44) | 7/24 (29) | 11/26 (42) |
| Specialty, n/N (%) | |||
| Family doctor | 25/25 (100) | 22/23 (96) | 26/26 (100) |
| Other | 0/25 (0) | 1/23 (4) | 0/26 (0) |
| Professional experience, years: median (IQR) | 26 (18-30) | 22 (14-24) | 18 (15-28) |
| Previous training in benzodiazepine withdrawal, n/N (%) | |||
| Yes | 11/22 (50) | 11/19 (58) | 19/26 (73) |
| No | 11/22 (50) | 8/19 (42) | 7/26 (27) |
| Previous participation in clinical trials, n/N (%) | |||
| Yes | 11/20 (55) | 7/17 (41.2) | 12/25 (48) |
| No | 9/20 (45) | 10/17 (58.8) | 13/25 (52) |
GP, general practitioner; IQR, interquartile range; PCC, primary care centre; SIF, intervention group with follow-up visits; SIW, intervention group with written instructions.
Table 2 Patients’ characteristics at baseline
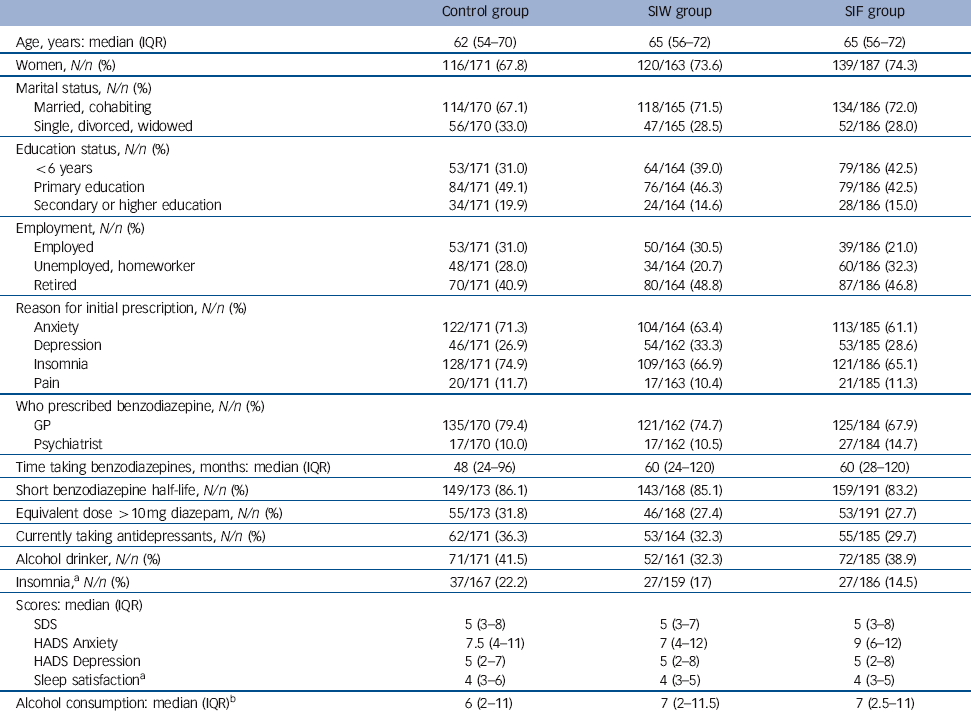
| Control group | SIW group | SIF group | |
|---|---|---|---|
| Age, years: median (IQR) | 62 (54-70) | 65 (56-72) | 65 (56-72) |
| Women, N/n (%) | 116/171 (67.8) | 120/163 (73.6) | 139/187 (74.3) |
| Marital status, N/n (%) | |||
| Married, cohabiting | 114/170 (67.1) | 118/165 (71.5) | 134/186 (72.0) |
| Single, divorced, widowed | 56/170 (33.0) | 47/165 (28.5) | 52/186 (28.0) |
| Education status, N/n (%) | |||
| <6 years | 53/171 (31.0) | 64/164 (39.0) | 79/186 (42.5) |
| Primary education | 84/171 (49.1) | 76/164 (46.3) | 79/186 (42.5) |
| Secondary or higher education | 34/171 (19.9) | 24/164 (14.6) | 28/186 (15.0) |
| Employment, N/n (%) | |||
| Employed | 53/171 (31.0) | 50/164 (30.5) | 39/186 (21.0) |
| Unemployed, homeworker | 48/171 (28.0) | 34/164 (20.7) | 60/186 (32.3) |
| Retired | 70/171 (40.9) | 80/164 (48.8) | 87/186 (46.8) |
| Reason for initial prescription, N/n (%) | |||
| Anxiety | 122/171 (71.3) | 104/164 (63.4) | 113/185 (61.1) |
| Depression | 46/171 (26.9) | 54/162 (33.3) | 53/185 (28.6) |
| Insomnia | 128/171 (74.9) | 109/163 (66.9) | 121/186 (65.1) |
| Pain | 20/171 (11.7) | 17/163 (10.4) | 21/185 (11.3) |
| Who prescribed benzodiazepine, N/n (%) | |||
| GP | 135/170 (79.4) | 121/162 (74.7) | 125/184 (67.9) |
| Psychiatrist | 17/170 (10.0) | 17/162 (10.5) | 27/184 (14.7) |
| Time taking benzodiazepines, months: median (IQR) | 48 (24-96) | 60 (24-120) | 60 (28-120) |
| Short benzodiazepine half-life, N/n (%) | 149/173 (86.1) | 143/168 (85.1) | 159/191 (83.2) |
| Equivalent dose >10 mg diazepam, N/n (%) | 55/173 (31.8) | 46/168 (27.4) | 53/191 (27.7) |
| Currently taking antidepressants, N/n (%) | 62/171 (36.3) | 53/164 (32.3) | 55/185 (29.7) |
| Alcohol drinker, N/n (%) | 71/171 (41.5) | 52/161 (32.3) | 72/185 (38.9) |
| Insomnia,Footnote a N/n (%) | 37/167 (22.2) | 27/159 (17) | 27/186 (14.5) |
| Scores: median (IQR) | |||
| SDS | 5 (3-8) | 5 (3-7) | 5 (3-8) |
| HADS Anxiety | 7.5 (4-11) | 7 (4-12) | 9 (6-12) |
| HADS Depression | 5 (2-7) | 5 (2-8) | 5 (2-8) |
| Sleep satisfactionFootnote a | 4 (3-6) | 4 (3-5) | 4 (3-5) |
| Alcohol consumption: median (IQR)Footnote b | 6 (2-11) | 7 (2-11.5) | 7 (2.5-11) |
GP, general practitioner; HADS, Hospital Anxiety and Depression Scale; IQR, interquartile range; SDS, Severity Dependence Scale; SIF; intervention group with follow-up visits; SIW, intervention group with written instructions.
a. Oviedo Sleep Questionnaire.
b. Standard alcohol units per week among drinkers.
Table 3 Comparison of benzodiazepine discontinuation between the control and intervention groups after 6 months and 12 months of follow-up
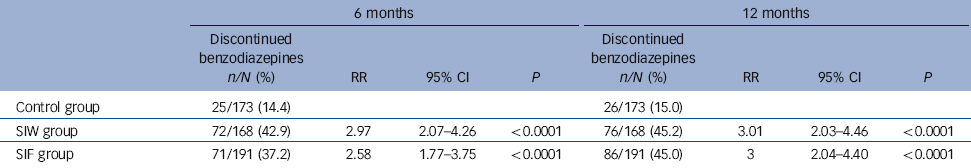
| 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Discontinued benzodiazepines n/N (%) |
RR | 95% CI | P | Discontinued benzodiazepines n/N (%) |
RR | 95% CI | P | |
| Control group | 25/173 (14.4) | 26/173 (15.0) | ||||||
| SIW group | 72/168 (42.9) | 2.97 | 2.07-4.26 | <0.0001 | 76/168 (45.2) | 3.01 | 2.03-4.46 | <0.0001 |
| SIF group | 71/191 (37.2) | 2.58 | 1.77-3.75 | <0.0001 | 86/191 (45.0) | 3 | 2.04-4.40 | <0.0001 |
RR, relative risk; SIF, intervention group with follow-up visits; SIW, intervention group with written instructions.
Secondary outcomes
The RRs of the interventions at 6 months were 2.97 (95% CI 2.07-4.26, P<0.0001) for SIW and 2.58 (95% CI 1.77-3.75, P<0.0001) for SIF. Safety outcomes are shown in Tables 4 and 5. We found no increase in HADS scores, sleep dissatisfaction or alcohol consumption compared with baseline, with all groups improving slightly in these parameters over time. Between-group analysis showed no increase in anxiety, depression or alcohol intake. At 12 months, sleep satisfaction was significantly higher in the SIF group than in the SIW group. Although within-group analysis showed no difference from baseline in the SIW group, statistically significant improvements in sleep satisfaction at 6 months and 12 months were observed in the SIF group. The most frequently reported withdrawal symptoms during benzodiazepine discontinuation at 6 months were insomnia, anxiety and irritability, with higher incidences in both intervention groups. At 12 months, however, there was no statistically significant difference. Anti-depressant treatment was initiated during the 12 months of follow-up in 82 (15.9%) patients: 20/161 (12.4%) in the SIW group, 39/187 (20.9%) in the SIF group and 23/169 (13.6%) in the control group. To facilitate withdrawal 76 (14.7%) patients were switched to a longer-acting benzodiazepine: 21/161 (13%) in the SIW group, 42/187 (22.5%) in the SIF group and 13/168 (7.7%) in the control group.
The mean number of GP visits per patient was 4.6 (s.d. = 3.5) in the SIF group compared with 1.2 (s.d. = 2.0) in the SIW group and 0.7 (s.d. = 2.1) in the control group. The average duration of the structured intervention with the GP in both intervention arms was approximately 20 min. Follow-up visits by patients in the SIF group and spontaneous visits by patients in the SIW and control groups lasted approximately 12 min.
Adverse events
Any serious adverse event such as admission to hospital, a life-threatening event, significant disability/incapacity or death related to the intervention was reported by the GPs during the study. Two adverse events were reported: one patient had a stroke and died before the baseline intervention visit, and another patient in the SIW group attempted suicide by taking an overdose of benzodiazepines 4 months after the intervention, following a stressful event. Prior to the overdose he was not taking benzodiazepines and the clinical diagnosis was adjustment disorder with depressive mood. Eventually he recovered.
Discussion
We found that a structured intervention by a GP along with stepped-dose reduction, with or without follow-up visits, was up to three times more effective than routine care in discontinuing long-term benzodiazepine use in patients without severe comorbidity at whom this intervention was targeted. Both interventions had similar efficacy but the approach without follow-up visits required less involvement by and fewer visits to the GP. This can be particularly relevant for busy public primary care services. The interventions can be considered safe as they did not increase patient anxiety, depression levels, dissatisfaction with sleep quality or alcohol consumption. Slight improvements in anxiety and depression symptoms were observed in both intervention groups at 6 months and 12 months. A higher proportion of patients in the SIF group than in the other two groups commenced antidepressant treatment, although at baseline the percentages of patients with depression were equivalent in the three groups. Regular contact with a GP may have increased the possibility of additional pharmacological treatment. Subgroup analysis showed that patients who might have been expected to experience more withdrawal difficulties, such as those with higher anxiety levels and those taking higher benzodiazepine doses, were even more effectively helped by receiving an intervention, especially the one including follow-up visits. Thus in this subgroup, greater GP involvement resulted in higher rates of successful benzodiazepine withdrawal.
As most patients were benzodiazepine-free before the 6-month evaluation, reported withdrawal symptoms at 6 months were more frequent in both intervention groups than in the control group, as expected. Nevertheless, because these symptoms were mostly mild to moderate, these differences did not persist to 12 months. Two major adverse events were reported during the study: one was unrelated to the benzodiazepine withdrawal, but in the other the person attempted suicide following a stressful event after he had discontinued benzodiazepines. It is possible that the benzodiazepine cessation contributed to this event by increasing the individual’s vulnerability to stressful conditions, but this seems unlikely when some studies find a sixfold increased risk of attempted suicide in patients currently taking benzodiazepines but not in discontinuation programmes. Reference Neutel and Patten35 After a critical revision of harm in benzodiazepine discontinuation trials, we did not find any previously reported serious adverse event.
Strengths and limitations
One of the strengths of our research is that all GPs completed the study and most patients were successfully followed up until the end of the trial, with final data unavailable for only nine patients. The large patient sample size and large number of participating GPs, together with the small average cluster size and small number of patients lost to follow-up, provided this study with sufficient power to detect small differences between groups. However, as only 34% of eligible patients were included, a limitation of the study is that our results are applicable only to patients in primary care with no major depressive or anxiety disorder, not currently receiving psychiatric treatment and free from severe medical conditions, as patients with these conditions were excluded. Future research should consider including a broader patient range, with joint decisions by patient and psychiatrist about the adequacy of the discontinuation, and the long-term effectiveness of the interventions should be evaluated as well their implementation into practice.
Table 4 Intervention safety outcomes
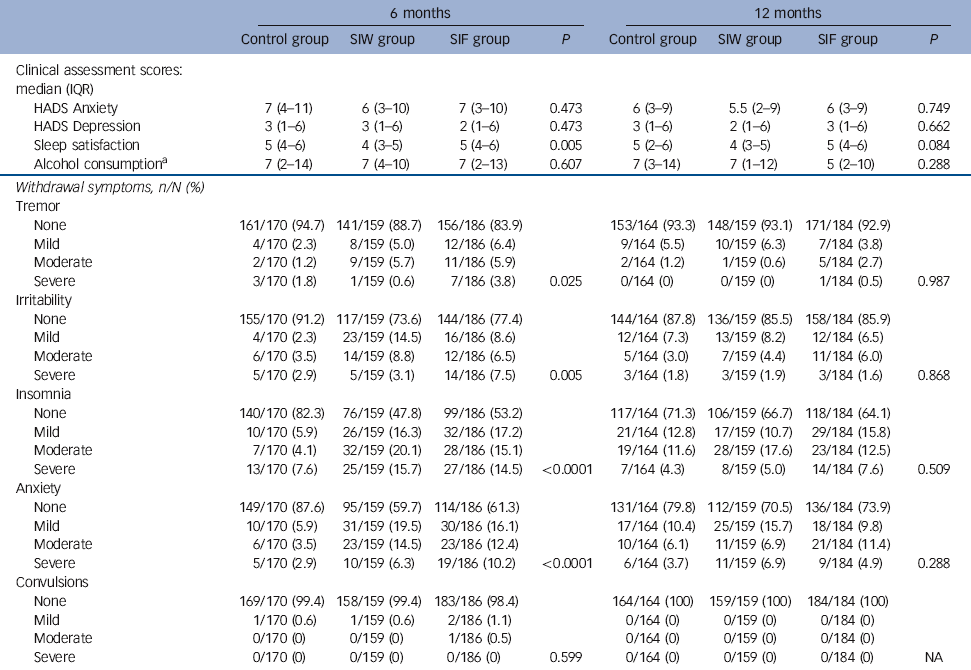
| 6 months | 12 months | |||||||
|---|---|---|---|---|---|---|---|---|
| Control group | SIW group | SIF group | P | Control group | SIW group | SIF group | P | |
| Clinical assessment scores: median (IQR) | ||||||||
| HADS Anxiety | 7 (4-11) | 6 (3-10) | 7 (3-10) | 0.473 | 6 (3-9) | 5.5 (2-9) | 6 (3-9) | 0.749 |
| HADS Depression | 3 (1-6) | 3 (1-6) | 2 (1-6) | 0.473 | 3 (1-6) | 2 (1-6) | 3 (1-6) | 0.662 |
| Sleep satisfaction | 5 (4-6) | 4 (3-5) | 5 (4-6) | 0.005 | 5 (2-6) | 4 (3-5) | 5 (4-6) | 0.084 |
| Alcohol consumptionFootnote a | 7 (2-14) | 7 (4-10) | 7 (2-13) | 0.607 | 7 (3-14) | 7 (1-12) | 5 (2-10) | 0.288 |
| Withdrawal symptoms, n/N (%) | ||||||||
| Tremor | ||||||||
| None | 161/170 (94.7) | 141/159 (88.7) | 156/186 (83.9) | 153/164 (93.3) | 148/159 (93.1) | 171/184 (92.9) | ||
| Mild | 4/170 (2.3) | 8/159 (5.0) | 12/186 (6.4) | 9/164 (5.5) | 10/159 (6.3) | 7/184 (3.8) | ||
| Moderate | 2/170 (1.2) | 9/159 (5.7) | 11/186 (5.9) | 2/164 (1.2) | 1/159 (0.6) | 5/184 (2.7) | ||
| Severe | 3/170 (1.8) | 1/159 (0.6) | 7/186 (3.8) | 0.025 | 0/164 (0) | 0/159 (0) | 1/184 (0.5) | 0.987 |
| Irritability | ||||||||
| None | 155/170 (91.2) | 117/159 (73.6) | 144/186 (77.4) | 144/164 (87.8) | 136/159 (85.5) | 158/184 (85.9) | ||
| Mild | 4/170 (2.3) | 23/159 (14.5) | 16/186 (8.6) | 12/164 (7.3) | 13/159 (8.2) | 12/184 (6.5) | ||
| Moderate | 6/170 (3.5) | 14/159 (8.8) | 12/186 (6.5) | 5/164 (3.0) | 7/159 (4.4) | 11/184 (6.0) | ||
| Severe | 5/170 (2.9) | 5/159 (3.1) | 14/186 (7.5) | 0.005 | 3/164 (1.8) | 3/159 (1.9) | 3/184 (1.6) | 0.868 |
| Insomnia | ||||||||
| None | 140/170 (82.3) | 76/159 (47.8) | 99/186 (53.2) | 117/164 (71.3) | 106/159 (66.7) | 118/184 (64.1) | ||
| Mild | 10/170 (5.9) | 26/159 (16.3) | 32/186 (17.2) | 21/164 (12.8) | 17/159 (10.7) | 29/184 (15.8) | ||
| Moderate | 7/170 (4.1) | 32/159 (20.1) | 28/186 (15.1) | 19/164 (11.6) | 28/159 (17.6) | 23/184 (12.5) | ||
| Severe | 13/170 (7.6) | 25/159 (15.7) | 27/186 (14.5) | <0.0001 | 7/164 (4.3) | 8/159 (5.0) | 14/184 (7.6) | 0.509 |
| Anxiety | ||||||||
| None | 149/170 (87.6) | 95/159 (59.7) | 114/186 (61.3) | 131/164 (79.8) | 112/159 (70.5) | 136/184 (73.9) | ||
| Mild | 10/170 (5.9) | 31/159 (19.5) | 30/186 (16.1) | 17/164 (10.4) | 25/159 (15.7) | 18/184 (9.8) | ||
| Moderate | 6/170 (3.5) | 23/159 (14.5) | 23/186 (12.4) | 10/164 (6.1) | 11/159 (6.9) | 21/184 (11.4) | ||
| Severe | 5/170 (2.9) | 10/159 (6.3) | 19/186 (10.2) | <0.0001 | 6/164 (3.7) | 11/159 (6.9) | 9/184 (4.9) | 0.288 |
| Convulsions | ||||||||
| None | 169/170 (99.4) | 158/159 (99.4) | 183/186 (98.4) | 164/164 (100) | 159/159 (100) | 184/184 (100) | ||
| Mild | 1/170 (0.6) | 1/159 (0.6) | 2/186 (1.1) | 0/164 (0) | 0/159 (0) | 0/184 (0) | ||
| Moderate | 0/170 (0) | 0/159 (0) | 1/186 (0.5) | 0/164 (0) | 0/159 (0) | 0/184 (0) | ||
| Severe | 0/170 (0) | 0/159 (0) | 0/186 (0) | 0.599 | 0/164 (0) | 0/159 (0) | 0/184 (0) | NA |
HADS, Hospital Anxiety and Depression Scale; IQR, interquartile range; SIF, intervention group with follow-up visits; SIW, intervention group with written instructions.
a. Standard alcohol units per week among drinkers.
In general, GPs are responsible for most benzodiazepine treatments, and the high prevalence of long-term use suggests that patients do not willingly discontinue these drugs. Nevertheless, we observed that when GPs proposed that their patients participate in a withdrawal programme most accepted, with only ten patients refusing to take part in this study. In some cases long-term benzodiazepine use is due to a misleading prescription. About 30% of patients were initially prescribed a benzodiazepine for a depressive disorder. During the initial phases of antidepressant treatment, benzodiazepines are often prescribed as a coadjuvant if anxiety, agitation and/or insomnia are problematic. These patients should be treated with the benzodiazepine for no longer than 2 weeks to prevent the development of dependence. Once initiated, however, benzodiazepine treatment is often maintained for longer.
As with most clinical trials, the GPs who agreed to participate may not be representative of primary care physicians because they might have been more highly motivated or interested in the subject of the study. Indeed, the GPs participating in this study differed in gender distribution, with a higher percentage of men in the control group than in the SIW and SIF groups. However, after a thorough review of the randomisation scheme, we concluded that these differences were due to chance. Nevertheless, these differences may have influenced patient recruitment, as we observed that women were slightly more prone to recruit women, resulting in some differences in patient baseline characteristics and influencing the discontinuation rate in the control group. Indeed, the discontinuation rate in our control group was higher than in similar studies. Reference Vicens, Fiol, Llobera, Campoamor, Mateu and Alegret14,Reference Cormack, Sweeney, Hughes-Jones and Foot16,Reference Heather, Bowie, Ashton, McAvoy, Spencer and Brodie18,Reference Bashir, King and Ashworth20,Reference Gorgels, Oude Voshaar, Mol, van de Lisdonk, van Balkom and van den Hoogen36 Also, some of the GPs in the intervention and control groups were in the same practices, and may have shared information and strategies. Had this occurred, it might have led to more benzodiazepine discontinuations by GPs in the control group, which would have reduced the magnitude of the observed benefits. Although sample size was not calculated for subgroup analysis, our results indicate that the efficacy of intervention may vary according to some patient characteristics, such as higher benzodiazepine dose and patient anxiety. These finding suggest that specific patient characteristics should be taken into account when designing targeted interventions.
Comparison with other studies
Various controlled trials have assessed the efficacy of strategies designed to wean patients off long-term benzodiazepine use. However, these trials differed in complexity, methodological aspects, sample size and follow-up period. Brief interventions have included sending a letter to patients who are long-term benzodiazepine users suggesting they stop, Reference Cormack, Sweeney, Hughes-Jones and Foot16-Reference Ten Wolde, Dijkstra, van Empelen, van den Hout, Neven and Zitman19 resulting in discontinuation rates between 10% and 24% at 6-month follow-up; sending a letter and offering a short GP consultation, resulting in a cessation rate of 10% at 6 months; Reference Heather, Bowie, Ashton, McAvoy, Spencer and Brodie18 a brief advice intervention by a GP supplemented by a self-help booklet to reinforce information and give practical advice on stopping benzodiazepine use, resulting in a success rate of 18% at 6-month follow-up; Reference Bashir, King and Ashworth20 and finally, a brief GP intervention based on gradual dose reduction, resulting in 3-12 months discontinuation rates of 51% and 45% respectively. Reference Vicens, Fiol, Llobera, Campoamor, Mateu and Alegret14,Reference Oude Voshaar, Gorgels, Mol, van Balkom, van de Lisdonk and Breteler22 All of these interventions resulted in higher discontinuation rates than usual care. Reference Oude Voshaar, Couvée, van Balkom, Mulder and Zitman37,Reference Parr, Kavanagh, Cahill, Mitchell and McD Young38 Sending a letter is helpful and cost-effective, Reference Godfrey, Heather, Bowie, Brodie, Parrot and Ashton39 but its efficacy is exceeded by the GP approach with gradual dose reduction. Our SIF group had a discontinuation rate similar to those of previous studies, Reference Vicens, Fiol, Llobera, Campoamor, Mateu and Alegret14 although a structured educational intervention reinforced by written individualised gradual tapering had not yet been evaluated in a large randomised controlled trial. In another study, brief GP advice supplemented by a self-help booklet yielded a 20% cessation rate compared with 7% in the control group. Reference Bashir, King and Ashworth20
Table 5 Secondary outcomes of the interventions: between-group and within-group analyses
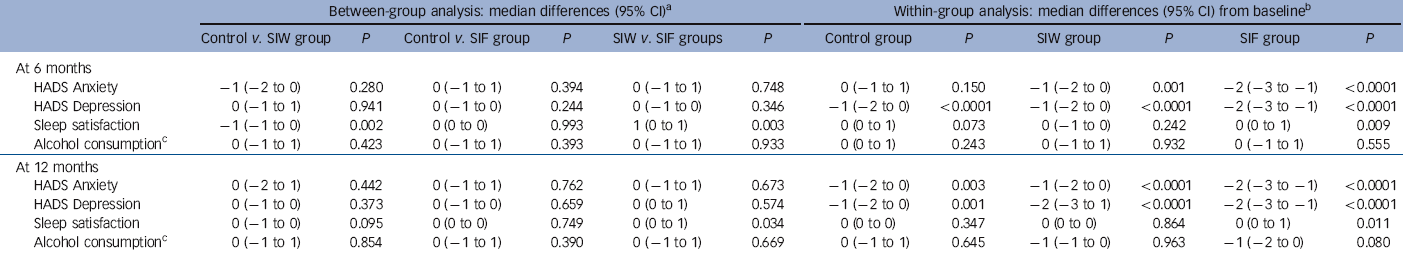
| Between-group analysis: median differences (95% CI)Footnote a | Within-group analysis: median differences (95% CI) from baselineFootnote b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control v. SIW group | P | Control v. SIF group | P | SIW v. SIF groups | P | Control group | P | SIW group | P | SIF group | P | |
| At 6 months | ||||||||||||
| HADS Anxiety | −1 (−2 to 0) | 0.280 | 0 (−1 to 1) | 0.394 | 0 (−1 to 1) | 0.748 | 0 (−1 to 1) | 0.150 | −1 (−2 to 0) | 0.001 | −2 (−3 to −1) | <0.0001 |
| HADS Depression | 0 (−1 to 1) | 0.941 | 0 (−1 to 0) | 0.244 | 0 (−1 to 0) | 0.346 | −1 (−2 to 0) | <0.0001 | −1 (−2 to 0) | <0.0001 | −2 (−3 to −1) | <0.0001 |
| Sleep satisfaction | −1 (−1 to 0) | 0.002 | 0 (0 to 0) | 0.993 | 1 (0 to 1) | 0.003 | 0 (0 to 1) | 0.073 | 0 (−1 to 0) | 0.242 | 0 (0 to 1) | 0.009 |
| Alcohol consumptionFootnote c | 0 (−1 to 1) | 0.423 | 0 (−1 to 1) | 0.393 | 0 (−1 to 1) | 0.933 | 0 (0 to 1) | 0.243 | 0 (−1 to 1) | 0.932 | 0 (−1 to 1) | 0.555 |
| At 12 months | ||||||||||||
| HADS Anxiety | 0 (−2 to 1) | 0.442 | 0 (−1 to 1) | 0.762 | 0 (−1 to 1) | 0.673 | −1 (−2 to 0) | 0.003 | −1 (−2 to 0) | <0.0001 | −2 (−3 to −1) | <0.0001 |
| HADS Depression | 0 (−1 to 0) | 0.373 | 0 (−1 to 0) | 0.659 | 0 (0 to 1) | 0.574 | −1 (−2 to 0) | 0.001 | −2 (−3 to 1) | <0.0001 | −2 (−3 to −1) | <0.0001 |
| Sleep satisfaction | 0 (−1 to 0) | 0.095 | 0 (0 to 0) | 0.749 | 0 (0 to 1) | 0.034 | 0 (0 to 0) | 0.347 | 0 (0 to 0) | 0.864 | 0 (0 to 1) | 0.011 |
| Alcohol consumptionFootnote c | 0 (−1 to 1) | 0.854 | 0 (−1 to 1) | 0.390 | 0 (−1 to 1) | 0.669 | 0 (−1 to 1) | 0.645 | −1 (−1 to 0) | 0.963 | −1 (−2 to 0) | 0.080 |
HADS, Hospital Anxiety and Depression Scale; SIF, intervention group with follow-up visits; SIW, intervention group with written instructions.
a. Hodges-Lehmann median difference.
b. Von Mises median difference, Somers’ D rank statistics P-values.
c. Standard alcohol units per week among drinkers.
More complex strategies include adding CBT or pharmacotherapy to gradual dose reduction. The results of studies on such strategies have been inconsistent. For example, adding CBT was of limited value in one trial, Reference Oude Voshaar, Gorgels, Mol, van Balkom, van de Lisdonk and Breteler22 but facilitated discontinuation in other trials. Reference Baillargeon, Landreville, Verreault, Beauchemin, Gregoire and Morin23,Reference Morin, Bastien, Guay, Radouco-Thomas, Leblanc and Vallieres24 Limited evidence is available on the use of adjuvant pharmacotherapy to assist withdrawal from benzodiazepines. Reference Denis, Fatseas, Lavie and Auriacombe25 We did not consider adding psychological therapy to gradual dose reduction because of the impracticality of this intervention in the Spanish health system primary care setting. However, it may be valuable to analyse whether psychological interventions might be appropriate for patients who fail first-line treatment and those who are psychologically distressed at baseline.
Brief approaches are a priority in primary care settings; efficacy and the time spent on health interventions are decisive factors behind GPs’ decisions on whether to implement these approaches. We found that GPs spent about 20 min on the structured interview with each patient in the SIF and SIW group and 12 min on each follow-up visit. They considered both interventions as feasible and easily accommodated them in their routine practice. Moreover, patients tend to accept stepped-dose benzodiazepine reduction when it is proposed by their GP. Since both interventions were effective in primary care, GPs may choose one or other depending on their working context (busy practices, heavy workload, time available per consultation), preferences and patients’ baseline characteristics. Indeed, we found that more intensive patient follow-up was more effective in patients taking higher doses of benzodiazepines and those with higher anxiety.
Implications of the study
A considerable percentage of the populations of most European countries are taking benzodiazepines on a long-term basis. If GPs implemented the brief interventions outlined here, these patients - especially the elderly ones - would benefit by reduced risks of their most prevalent adverse events such as dependence, falls, fractures and cognitive impairment.
Funding
The study was funded by the Carlos III Health Institute of the Ministry of Economy and Competitiveness (contract PS09/00947). The Ministry had no role in the design and conduct of the study, the collection, management, analysis or interpretation of the data, and the preparation, review, and approval of the manuscript or in the decision to submit for publication. The research was conducted independently from the funding body.
Acknowledgements
The authors would like to thank the participating general practitioners for recruitment, collecting data and delivering the interventions.








eLetters
No eLetters have been published for this article.