The introduction of atypical antipsychotics has substantially changed the treatment of schizophrenia. Although atypical antipsychotics have dramatically reduced the frequency of acute extrapyramidal symptoms, substantial weight gain is common with many of these medications (Reference Allison, Mentore and HeoAllison et al, 1999). Estimates of mean weight gain associated with atypical antipsychotics have varied greatly and are confounded by the extent of previous antipsychotic treatment (Reference Ganguli, Brar and AyrtonGanguli et al, 2001) and the statistical methodology used to estimate weight gain from clinical trials with a significant withdrawal rate (Reference Allison and CaseyAllison & Casey, 2001). Typically, such trials estimate weight gain on an intent-to-treat basis using the last-observation-carried-forward (LOCF) approach. Estimating weight gain from observed cases and study completers provides complementary perspectives. In this study, we investigated the extent and time course of olanzapine- and haloperidol-associated weight gain in the treatment of first-episode psychosis, the clinical correlates of weight gain and the association of weight gain with treatment response and adherence.
METHOD
This analysis was based on the data collected as part of a multicentre randomised double-blind clinical trial. The design of this trial, as well as efficacy and safety results, have been reported previously (Reference Lieberman, Tollefson and TohenLieberman et al, 2003). The study was carried out between March 1997 and July 2001 at 14 academic medical centres in North America and Europe.
Participants
Study participants met DSM–IV criteria for schizophrenia, schizophreniform disorder or schizoaffective disorder (American Psychiatric Association, 1994) and were between 16 and 40 years of age. All had experienced psychotic symptoms for at least 1 month but no more than 60 months, met predefined criteria for being at least moderately ill, and were determined to be in clinical need of an antipsychotic medication. All participants or their authorised legal representative provided written informed consent for this study after the procedures had been fully explained. The appropriate institutional review boards approved the study.
Individuals were excluded from participating if they met any of the study exclusion criteria (Reference Lieberman, Tollefson and TohenLieberman et al, 2003). They were excluded if they had received prior antipsychotic treatment for more than 16 cumulative weeks, had ever received clozapine, or were currently in need of treatment with anticonvulsants, antidepressants, benzodiazepines (except for treatment of agitation and extrapyramidal symptoms), or other psychotropic medications.
Study design and procedures
Participants were randomly assigned to olanzapine or haloperidol under double-blind conditions. In the first 6 weeks of the study, doses could be titrated in the range of 5–10 mg/day (olanzapine) and 2–6 mg/day (haloperidol); for the second 6 weeks of the study, doses could be further adjusted in the range of 5–20 mg/day (olanzapine) and 2–20 mg/day (haloperidol). Antidepressants and mood stabilisers could not be used during the first 12 weeks of the study. Following the 12-week acute treatment period, fluoxetine 10–60 mg/day could be prescribed for individuals meeting DSM–IV criteria for major depressive disorder. Lithium carbonate or valproate could be added if they failed to respond to fluoxetine or if they developed mania or a mixed affective state.
Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS; Reference Kay, Opler and FiszbeinKay et al, 1992) as the primary efficacy variable. The PANSS was administered at study entry, on the day of randomisation, weekly for the first 6 weeks, every 2 weeks for the next 6 weeks and then monthly for the remainder of the study. Body weight was measured at each of these visits. Non-fasting serum glucose and cholesterol levels were measured on the day of study entry, on the day of randomisation, at 12 weeks, 6 months, 12 months, 18 months and 24 months.
Statistical analyses
We identified the primary outcome to test treatment group differences as the event of ‘clinically significant weight gain’, defined as ≥7% increase in weight (kg) from baseline; this definition is consistent with the US Food and Drug Administration guidelines (Reference Sachs and GuilleSachs & Guille, 1999). The primary analysis was able to use the intent-to-treat population of all randomised participants and compared the Kaplan–Meier survival curves of the two treatments using the log-rank test. A Cox regression elaborated the findings by modelling the treatment effects jointly with other potentially important covariates selected from baseline body mass index (BMI), age, gender, ethnicity, smoking status, premorbid functioning, diagnosis, age at onset, duration of untreated illness and history of previous antipsychotic treatment (backward model selection, P<0.05 to stay).
The descriptive statistics for amount of weight gained were plotted using data for LOCF, observed cases and completers to examine the effect of early withdrawal on weight gain estimates. A more sophisticated approach was taken to model growth curves of BMI change in the first 12 weeks using a random coefficient mixed model, and to identify significant clinical predictors (as described above) for the course of BMI change. The correlation of weight gain and clinical outcome (as assessed by PANSS total score) was calculated at each time point and summarised using mixed models with repeated measures. A hierarchy of Cox regression models was carried out to assess the difference in study retention between the two treatment groups, as well as the contribution of symptom improvement and weight gain to withdrawal from the study. Only the primary analysis on treatment effect was tested at a two-sided α level of 0.05. The supplemental exploratory analysis was undertaken to expand our understanding of clinical aspects of weight gain, and no adjustment for multiple comparisons was considered necessary. The statistical analysis was performed using SAS version 8.01 for Windows (SAS Institute, Cary, North Carolina, USA).
RESULTS
In total, 263 individuals entered the study (olanzapine, n=131; haloperidol, n=132). (Figure 1 illustrates the progress of participants through the trial.) Table 1 summarises baseline demographic and clinical features of the sample. The acute-phase mean modal doses were 9.1 mg/day of olanzapine and 4.4 mg/day of haloperidol.
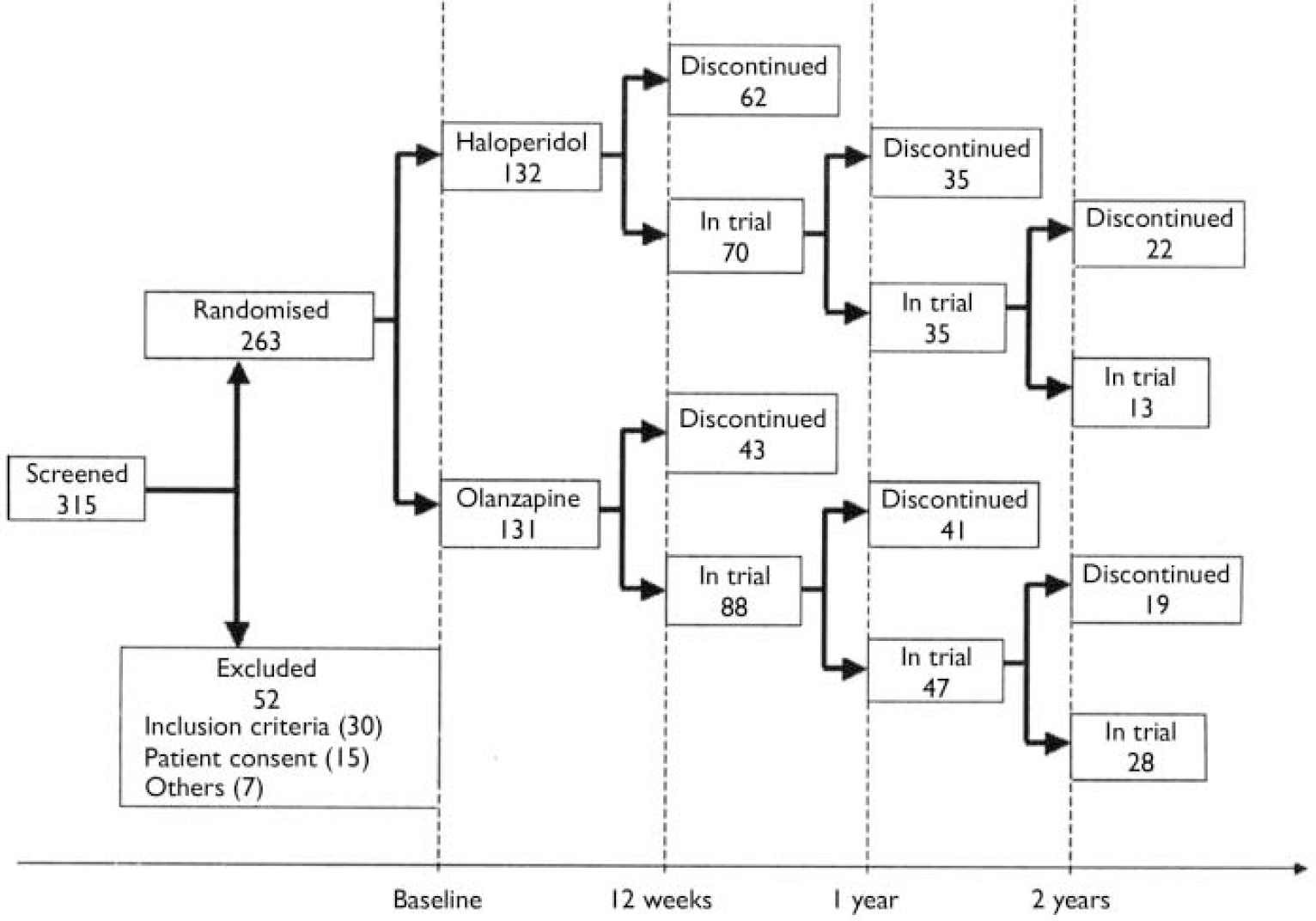
Fig. 1 CONSORT diagram with number of participants in trial or discontinued at 12 weeks, 1 year and 2 years.
Table 1 Participants’ demographic and clinical characteristics at baseline
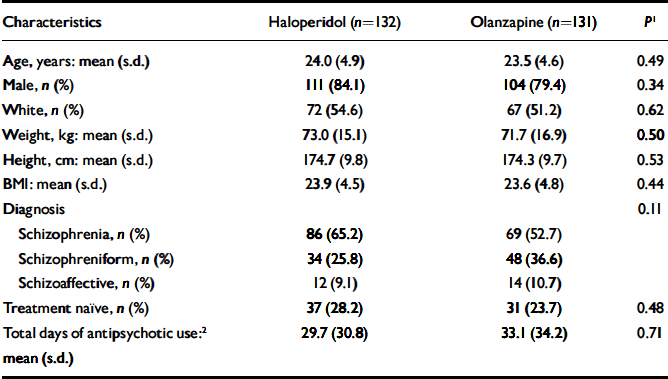
| Characteristics | Haloperidol (n=132) | Olanzapine (n=131) | P 1 |
|---|---|---|---|
| Age, years: mean (s.d.) | 24.0 (4.9) | 23.5 (4.6) | 0.49 |
| Male, n (%) | 111 (84.1) | 104 (79.4) | 0.34 |
| White, n (%) | 72 (54.6) | 67 (51.2) | 0.62 |
| Weight, kg: mean (s.d.) | 73.0 (15.1) | 71.7 (16.9) | 0.50 |
| Height, cm: mean (s.d.) | 174.7 (9.8) | 174.3 (9.7) | 0.53 |
| BMI: mean (s.d.) | 23.9 (4.5) | 23.6 (4.8) | 0.44 |
| Diagnosis | 0.11 | ||
| Schizophrenia, n (%) | 86 (65.2) | 69 (52.7) | |
| Schizophreniform, n (%) | 34 (25.8) | 48 (36.6) | |
| Schizoaffective, n (%) | 12 (9.1) | 14 (10.7) | |
| Treatment naïve, n (%) | 37 (28.2) | 31 (23.7) | 0.48 |
| Total days of antipsychotic use:2 mean (s.d.) | 29.7 (30.8) | 33.1 (34.2) | 0.71 |
Time to clinically significant weight gain
Among olanzapine-treated participants, 95 met the clinically significant weight gain criterion by the end of the 2-year study; one individual remaining on treatment and 35 who had discontinued treatment did not meet this criterion. Among haloperidol-treated participants, the numbers were 51, 3 and 78, respectively. Figure 2 shows the percentage of participants who were expected to have clinically significant weight gain at each time point, adjusting for withdrawal rates as calculated by Kaplan–Meier estimates. Olanzapine-treated participants met the clinically significant weight gain criterion at a significantly faster rate than haloperidol-treated individuals (χ2(1)=67.9, P<0.0001; log-rank test). The median treatment time before meeting the clinically significant weight gain criterion was 5 weeks for the olanzapine-treated group (95% CI 4–6) and 28 weeks (95% CI 16–40) for the haloperidol-treated group. Results from the Cox regression model confirmed the treatment-group differences in the likelihood of developing clinically significant weight gain. The hazard was more than five times greater for olanzapine-treated participants than for those treated with haloperidol (hazard ratio=5.19, χ2(1)=66.3, P<0.0001). Participants with higher baseline weight took longer to gain 57% of their baseline body weight, representing a 12% reduction in hazard (χ2(1)=20.9, P<0.0001). In addition, Black participants and those from minority ethnic groups demonstrated a significantly faster rate of clinically significant weight gain (hazard ratio 1.58, χ2 = (1)=6.28, P=0.01). The effects of baseline weight and ethnicity on the likelihood of developing clinically significant weight gain were not related to treatment group, as indicated by the non-significant interactions between these variables and treatment group (P>0.23).
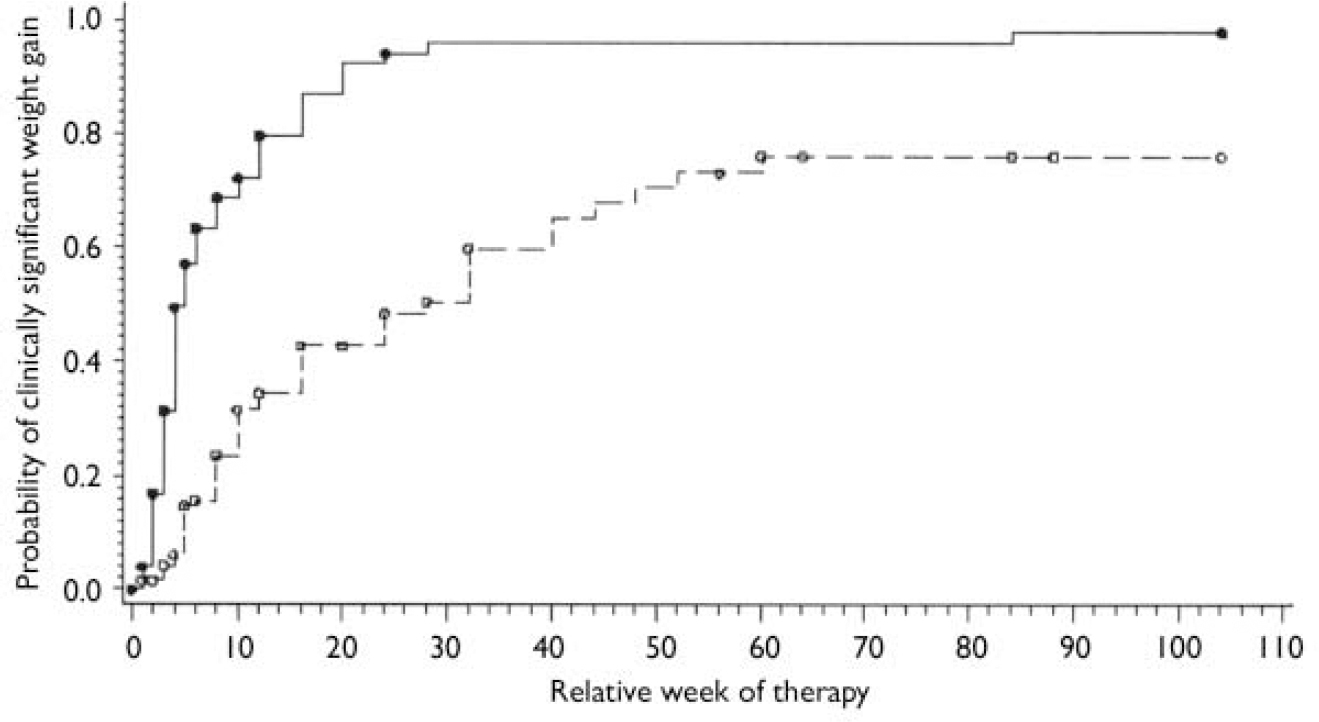
Fig. 2 Kaplan-Meier estimates for probability of clinically significant weight gain for participants who have received antipsychotic treatment for a certain period (olanzapine, n=131; haloperidol, n=132). The circles on the curves indicate censors (i.e. individuals who discontinued the trial at the time without experiencing clinically significant weight gain). - -, Haloperidol; ○, haloperidol censoring;—, olanzapine; •, olanzapine censoring.
Descriptive statistics for the time course of weight gain
Figure 3a presents mean changes in weight assessed by LOCF analysis. Individuals in both treatment groups gained weight rapidly in the first 12 weeks of treatment. The pace of weight gain tapered gradually thereafter and stabilised after 1 year. In the olanzapine-treated group, the mean weight gain estimated using LOCF was 7.3 kg (s.d.=6.1) at 12 weeks, 10.2 kg (s.d.=9.6) at 1 year and 10.2 kg (s.d.=10.1) at 2 years. In the haloperidol-treated group, the corresponding results were 2.6 kg (s.d.=4.5), 4.2 kg (s.d.=7.0) and 4.0 kg (s.d.=7.3).

Fig. 3 Mean weight change for modified intent-to-treat samples in olanzapine and haloperidol treatment groups. (a) Mean weight change (kg) among participants with at least one post-baseline weight measure included (two people in the haloperidol group had missing baseline weight; valid last-observation-carried-forward (LOCF) n=131 for olanzapine). Missing values after the first follow-up were filled in using the LOCF approach. ![]() , Haloperidol;
, Haloperidol; ![]() , olanzapine. (b)Mean weight change (kg) among observed cases at each visit in olanzapine andhaloperidol treatment groups. The patterns presented are from gradually decreasing sample sizes, owing to early withdrawal.
, olanzapine. (b)Mean weight change (kg) among observed cases at each visit in olanzapine andhaloperidol treatment groups. The patterns presented are from gradually decreasing sample sizes, owing to early withdrawal. ![]() , Haloperidol;
, Haloperidol; ![]() , olanzapine. (c) Mean weight change (kg) among the 12-week, 1-year and 2-year completers in olanzapine and haloperidol treatment groups (at 12 weeks: olanzapine, n=92; haloperidol, n=79; at 1 year: olanzapine, n=51; haloperidol, n=39; at 2 years: olanzapine, n=31; haloperidol, n=16).
, olanzapine. (c) Mean weight change (kg) among the 12-week, 1-year and 2-year completers in olanzapine and haloperidol treatment groups (at 12 weeks: olanzapine, n=92; haloperidol, n=79; at 1 year: olanzapine, n=51; haloperidol, n=39; at 2 years: olanzapine, n=31; haloperidol, n=16). ![]() , Haloperidol 12-week completers;
, Haloperidol 12-week completers; ![]() , haloperidol 1-year completers;
, haloperidol 1-year completers; ![]() , haloperidol 2-year completers;
, haloperidol 2-year completers; ![]() , olanzapine, 12-week completers;
, olanzapine, 12-week completers; ![]() , olanzapine 1 year completers;
, olanzapine 1 year completers; ![]() , olanzapine 2-year completers.
, olanzapine 2-year completers.
Figure 3b shows mean changes in weight assessed among observed-cases. Compared with the LOCF plots, the observed-cases plots show a longer course of steady weight gain: up to 36 weeks in the haloperidol group and 60 weeks in the olanzapine group. The overall weight gain was also greater when assessed using the observed-cases data. In the olanzapine-treated group, the observed mean weight gain was 9.2 kg (s.d.=5.3) after 12 weeks, 15.5 kg (s.d.=9.6) after 1 year and 15.4 kg (s.d.=10.0) after 2 years. For the haloperidol-treated group, corresponding results were 3.7 kg (s.d.=4.9), 7.1 kg (s.d.=6.7) and 7.5 kg (s.d.=9.2).
Figure 3c shows the time course for weight gain among olanzapine- and haloperidol-treated participants who completed 12 weeks, 1 year and 2 years of treatment. The time course for weight gain for the three completer groups is remarkably similar and is similar to the results of the observed-cases analysis.
The BMI change during the study follows a pattern similar to that for weight gain (kg) when analysed using LOCF, observed cases and completers. Last-observation-carried-forward analysis showed that in the olanzapine group the mean BMI increased from 23.6 (s.d.=4.8) at baseline to 26.0 (s.d.=5.1) at 12 weeks, 27.0 (s.d.=5.6) at 1 year and 27.0 (s.d.=5.6) at 2 years, compared with an increase from 23.9 (s.d.=4.5) to 24.8 (s.d.=4.5), 25.3 (s.d.=5.0) and 25.3 (s.d.=5.1) in the haloperidol group. However, according to analysis of observed cases, BMI increased from a mean of 23.6 (s.d.=4.8) at baseline to 26.4 (s.d.=4.6) at 12 weeks, 28.8 (s.d.=4.5) at 1 year and 28.3 (s.d.=4.0) at 2 years in the olanzapine group and from 23.9 (s.d.=4.5) to 24.8 (s.d.=4.1), 26.2 (s.d.=4.3) and 26.6 (s.d.=4.4) in the haloperidol group.
Growth curve modelling for clinical predictors of time course for weight gain
Overall, the time course of weight gain from baseline could be best described as an increasing binomial curve, whose rate of increase stabilised gradually over time. Body mass index change was not predicted by age, gender, smoking status, diagnosis, premorbid functioning, age at onset, duration of illness or history of previous antipsychotic treatment. Individuals with higher pre-treatment BMI had larger BMI increases in the first week of treatment but followed the same rate of weight gain as the others thereafter. Olanzapine-treated participants gained weight significantly faster than haloperidol-treated individuals but seemed to have greater propensity to stabilise over time, as indicated by both the significantly larger positive linear slope and larger negative quadratic slope in the growth curve models on BMI. The model-estimated linear slopes were 0.14 BMI units/week for haloperidol v. 0.41 BMI units/week for olanzapine (s.e.=0.03, t(1394)=6.5, P<0.0001); the estimated quadratic slopes were -0.005 BMI units/week2 for haloperidol v. -0.02 BMI units/week2 for olanzapine (s.e.=0.002, t(1396)= 4.22, P<0.0001). Black participants and those from minority ethnic groups gained weight faster than White participants and maintained the high rate of weight gain longer. The effects of baseline BMI and ethnicity on the BMI increase rate did not differ significantly between the olanzapine- and haloperidol-treated groups (P>0.40).
Laboratory correlates of weight gain
Body mass index changes did not significantly correlate with changes in non-fasting glucose levels. The correlation coefficients ranged from -0.21 to 0.18, with none significant for either treatment group at the 12-week, 6-month, 12-month, 18-month and 24-month points. However, change in BMI did significantly correlate with changes in non-fasting cholesterol levels. The Pearson correlations between BMI and cholesterol changes were 0.53 (P<0.001), 0.41 (P<0.01), 0.54 (P<0.001), 0.64 (P<0.01) and 0.29 (P=0.29) for the haloperidol group at the 12-week, 6-month, 12-month, 18-month and 24-month points, respectively; and 0.17 (P=0.11), 0.25 (P<0.05), 0.30 (P<0.05), 0.27 (P=0.17) and 0.54 (P<0.01) for the olanzapine group.
Weight gain and clinical response
Overall, we found evidence that higher weight gain was associated with greater symptom improvement before week 12, but the association was diminished thereafter. At weeks 1 and 6, a modest correlation was observed (r=0.21, P=0.02) between weight gain and clinical improvement in both treatment groups; after 12 weeks, this association could no longer be detected. The mixed model with repeated measures confirmed this observation. Changes in BMI were significantly associated with clinical improvement for both olanzapine- and haloperidol-treated participants (F(1,3364)=50.05, P<0.001). The association was significantly reduced at later stages of treatment (F(31,3364)=2.12, P<0.001).
Weight gain and study retention
In separate Cox regression models, olanzapine treatment, higher BMI increase and greater symptom improvement each predicted a significantly smaller hazard of early withdrawal from the study (see models 1–3, Table 2). When modelled jointly (see model 4, Table 2), reduction in the PANSS total score remained the most significant predictor of better study retention. With every 10 points of PANSS improvement, the hazard rate of early study discontinuation was reduced by 29%; even after controlling for weight gain and drug effect, the reduction remained at 28%. Greater BMI increase also reduced the hazard ratio for study discontinuation by 11%, but the effect was reduced to 7% after the effects of drug and PANSS improvement were taken into account.
Table 2 Cox regression models for effects of weight gain and clinical improvement on study discontinuation1
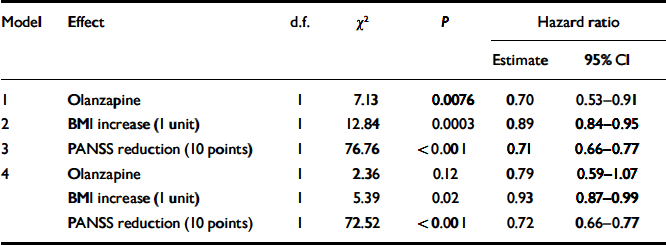
| Model | Effect | d.f. | χ2 | P | Hazard ratio | |
|---|---|---|---|---|---|---|
| Estimate | 95% CI | |||||
| 1 | Olanzapine | 1 | 7.13 | 0.0076 | 0.70 | 0.53–0.91 |
| 2 | BMI increase (1 unit) | 1 | 12.84 | 0.0003 | 0.89 | 0.84–0.95 |
| 3 | PANSS reduction (10 points) | 1 | 76.76 | < 0.001 | 0.71 | 0.66–0.77 |
| 4 | Olanzapine | 1 | 2.36 | 0.12 | 0.79 | 0.59–1.07 |
| BMI increase (1 unit) | 1 | 5.39 | 0.02 | 0.93 | 0.87–0.99 | |
| PANSS reduction (10 points) | 1 | 72.52 | < 0.001 | 0.72 | 0.66–0.77 | |
DISCUSSION
Weight gain estimates
This study is unique in examining the weight gain associated with antipsychotic treatment in a large randomised double-blind study of people with first-episode psychosis and very limited previous exposure to antipsychotic medication. Previous estimates of mean olanzapine-associated weight gain have ranged from 2.2 kg over 4 months to 10.0 kg over 7 months (Reference Beasley, Hamilton and CrawfordBeasley et al, 1997; Reference Tollefson, Beasley and TamuraTollefson et al, 1997; Reference Allison, Mentore and HeoAllison et al, 1999; Reference Gupta, Droney and Al-SamarraiGupta et al, 1999; Reference Sheitman, Bird and BinzSheitman et al, 1999; Reference Ganguli, Brar and AyrtonGanguli et al, 2001; Reference Kinon, Basson and GilmoreKinon et al, 2001). Estimates of weight gain in this sample varied greatly depending on the method used: LOCF resulted in lower estimates of weight gain among both olanzapine- and haloperidol-treated participants, whereas the analysis of observed cases and completers yielded higher estimates. Application of the observed-cases methodology has demonstrated that olanzapine was associated with a mean 2-year weight gain of 15.4 kg, whereas haloperidol-associated mean weight gain was 7.5 kg. These estimates are higher than previously reported for these two drugs and raise questions about what degree of weight gain would be observed for other antipsychotics if they were examined using a similar study design and analysis methods (Reference Allison, Mentore and HeoAllison et al, 1999).
The magnitude of weight gain observed is of clinical concern; Table 3 uses criteria established by the US National Heart, Lung, and Blood Institute to describe the number and percentage of participants at each time point of the study who would be categorised as of normal weight (BMI<25), overweight (BMI≤5<30) or obese (BMI≥30). After 1 year of treatment, 36.7% of olanzapine-treated participants and 20.5% of haloperidol-treated participants had BMIs in the obese range; in addition, 40.8% of the olanzapine-treated group and 35.9% of the haloperidol-treated group had BMIs in the overweight range.
Table 3 Number and percentage of individuals in the two treatment groups according to weight category
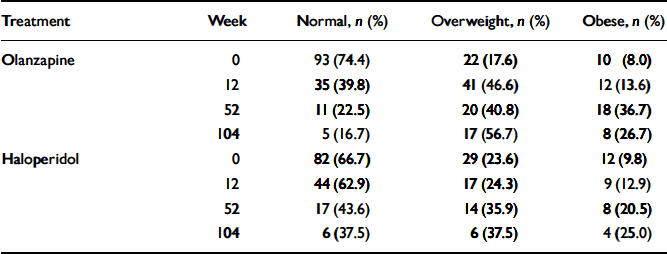
| Treatment | Week | Normal, n (%) | Overweight, n (%) | Obese, n (%) |
|---|---|---|---|---|
| Olanzapine | 0 | 93 (74.4) | 22 (17.6) | 10 (8.0) |
| 12 | 35 (39.8) | 41 (46.6) | 12 (13.6) | |
| 52 | 11 (22.5) | 20 (40.8) | 18 (36.7) | |
| 104 | 5 (16.7) | 17 (56.7) | 8 (26.7) | |
| Haloperidol | 0 | 82 (66.7) | 29 (23.6) | 12 (9.8) |
| 12 | 44 (62.9) | 17 (24.3) | 9 (12.9) | |
| 52 | 17 (43.6) | 14 (35.9) | 8 (20.5) | |
| 104 | 6 (37.5) | 6 (37.5) | 4 (25.0) |
Clinical predictors of weight gain
There has been much interest in the possibility that weight gain associated with antipsychotics may be related to a range of clinical parameters (for review see Reference Blin and MicallefBlin & Micallef, 2001). To date, such correlations have been mainly studied among people treated with clozapine. Clozapine-associated weight gain has been reported to be more likely in people who started treatment at a lower weight, in women, in younger people, in those treated with higher doses of clozapine and in people receiving antipsychotic treatment for the first time (Reference Blin and MicallefBlin & Micallef, 2001). In this study, we were unable to identify predictors of the amount of weight gained other than treatment group. However, our data clearly demonstrate that virtually all of the participants treated with olanzapine and approximately three-quarters of those treated with haloperidol were predicted to gain >7% of their baseline body weight after 1 year of treatment.
Metabolic correlates of weight gain
Elevations in glucose levels have been described in people receiving some atypical and some typical antipsychotics (Reference WirshingWirshing, 2001; Reference Newcomer, Haupt and FucetolaNewcomer et al, 2002; Reference Lindenmayer, Czobor and VolavkaLindenmayer et al, 2003). Increases in cholesterol and triglyceride levels have also been described in individuals receiving clozapine and olanzapine (Reference WirshingWirshing, 2001; Reference Lindenmayer, Czobor and VolavkaLindenmayer et al, 2003). In our study, weight change was not significantly correlated with changes in non-fasting glucose levels, but was significantly correlated with increases in non-fasting cholesterol levels at some time points for both treatment groups. Given the extent of the weight gain in both treatment groups, it would clearly be important to assess fasting glucose and complete lipid profiles in future clinical trials of antipsychotic medications for first-episode psychosis to further evaluate the extent to which there is a significant correlation between weight gain and changes in glucose and lipid levels.
Weight gain and clinical outcome
Some recent publications have also observed that weight gain associated with atypical antipsychotics may correlate with clinical outcomes, although findings have been inconsistent (Reference Blin and MicallefBlin & Micallef, 2001). We were able to demonstrate the previously reported association between weight gain and clinical response. However, our analysis found that this association was present only in the early weeks of treatment and that even at these points it was small, accounting for only 4% of the variance in clinical improvement observed in both treatment groups at weeks 1 and 6. After 12 weeks, weight gain did not account for any variance in clinical improvement in either treatment group.
There are a number of possible explanations for this finding. Use of LOCF data for studying this association is problematic because participants who withdraw early may experience both little clinical improvement and little weight gain, whereas those who remain in the study for longer periods are expected to have both greater clinical improvement and higher weight gain. Use of LOCF data may therefore lead to a spurious association between weight gain and clinical response. Why weight gain and clinical response may be associated only in the early weeks of treatment is not clear. This may in part be due to individual pharmacokinetic parameters: people whose absorption and metabolism of antipsychotics resulted in relatively higher plasma drug concentrations very early in the study might be expected to have greater symptom improvement and possibly higher weight gain, whereas those with lower plasma drug concentrations in the early weeks may have neither. A small correlation could thus be generated early in treatment and be expected to disappear once doses have been adjusted to provide therapeutic drug concentrations for all participants. However, this presumes a relationship between weight gain and drug dose/concentrations that has not yet been established. It is possible that other mechanisms may account for the small and transient association between weight gain and clinical response.
Weight gain and study adherence
The weight gain associated with antipsychotic medications, particularly some atypical antipsychotics, is of concern both because of the potential health consequences associated with weight gain and because weight gain may affect the long-term adherence to these medications. In this clinical trial, olanzapine-treated participants were significantly more likely to complete the 2-year trial despite their higher mean weight gain; haloperidol-treated participants were significantly more likely to withdraw because of adverse events and lack of efficacy (Reference Lieberman, Tollefson and TohenLieberman et al, 2003). Furthermore, BMI increases were associated with higher study retention during the first 12 weeks of treatment for both treatment groups. Our data do not support the view that weight gain contributes to non-adherence in the short term. Rather, they suggest that for younger people with first-episode psychosis the degree of clinical improvement may be the best predictor of adherence to medication in the short term regardless of adverse events, including weight gain. After controlling for the effect of clinical improvement, BMI increase (but not treatment group) remained a significant predictor of study retention. Although it is not known what features of olanzapine explain the higher study retention associated with this treatment, it may be that this is mediated through a mechanism that also contributes to weight gain.
In summary, we have demonstrated that the weight gain associated with extended treatment of a first-episode psychosis with either olanzapine or haloperidol is greater than has been previously estimated. Our results highlight the importance of evaluating weight gain in clinical trials using data derived from observed cases in addition to LOCF procedures. The observed weight gain was significantly greater for olanzapine-treated participants, but no other clinical factors were predictive of the amount of weight gained. Our results suggest that weight gain is associated with clinical improvement but in a minor and transient way, which is unlikely to be of clinical significance. Nor is it clear whether weight gain adversely affects short-term treatment adherence. However, it is clear that the extent of weight gain observed justifies additional study of the potential health consequences that may be experienced by young people who are being treated for a first-episode of psychosis.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ The weight gain associated with the extended treatment of a first episode of psychosis with either olanzapine or haloperidol is greater than has been previously estimated.
-
▪ Olanzapine leads to a significantly greater average weight gain than haloperidol.
-
▪ Clinical features were not predictive of weight gain with either drug, suggesting that all patients receiving these medications for a first episode of psychosis are at high risk of significant weight gain. The degree of clinical improvement experienced with extended treatment does not appear to be meaningfully associated with the amount of weight gained.
LIMITATIONS
-
▪ The accuracy of our estimates of weight gain and the investigation of its clinical correlates may have been limited by the large percentage of participants who withdrew prior to completing the study.
-
▪ The patient sample involved individuals experiencing a first episode of psychosis who were willing to participate in a randomised double-blind clinical trial. The results may not be generalisable to other groups with psychotic disorders or those receiving antipsychotic medication for other illnesses.
-
▪ This study deals only with two antipsychotic medications, one typical and the other atypical. Further research is required to determine how the findings of this study are relevant to treatment with other antipsychotic medications.
Acknowledgements
The HGDH Study Group includes Drs Jeffrey A. Lieberman, Diana O. Perkins, Robert Hamer, Department of Psychiatry, University of North Carolina School of Medicine, North Carolina; Dr Charles B. Nemeroff, Department of Psychiatry, Emory University School of Medicine, Georgia; Drs Bruce Cohen, Franca Centhorrino, McLean Hospital, Harvard Medical School, Massachusetts; Drs Gary Tollefson,Todd Sanger, Mauricio Tohen, Lilly Research Laboratories and McLean Hospital, Belmont, Massachusetts; Dr Joseph P. McEvoy, John Umstead Hospital, North Carolina; Dr John Kuldau, Department of Psychiatry, University of Florida, Florida; Dr Alan I. Green, Dartmouth Medical School, New Hampshire; Dr Anthony J. Rothschild, Jayendra K. Patel, Department of Psychiatry, University of Massachusetts Medical Center, Massachusetts; Dr Raquel E. Gur, Department of Psychiatry, University of Pennsylvania Medical Center, Pennsylvania, USA; Drs Robert B. Zipursky, Zafiris J. Daskulakis, Department of Psychiatry,University of Toronto Faculty of Medicine, Canada; Dr Stephen M. Strakowski, Department of Psychiatry, University of Cincinnati, Ohio; Dr Ira Glick, Department of Psychiatry, Stanford University School of Medicine, California; Dr John De Quardo, Department of Psychiatry,University of Michigan Medical Center, Missouri, USA; Professor Dr R. S. Kahn, University Hospital Utrecht, The Netherlands; Professor Tonmoy Sharma, Clinical Neuroscience Research Centre, Kent; Professor Robin Murray, Institute of Psychiatry, London,UK.









eLetters
No eLetters have been published for this article.