Convergent evidence suggests a link between cannabis use and psychosis. Reference Moore, Zammit, Lingford-Hughes, Barnes, Jones, Burke and Lewis1 However, cannabis comprises a combination of cannabinoids and these different constituents may have distinct effects, not all of which are detrimental to mental health. The main component of smoked cannabis is Δ9-tetrahydrocannabinol (Δ9-THC), which is thought to be responsible for the majority of the psychotomimetic effects of the drug: it has been shown to elevate levels of anxiety and psychotic symptoms in healthy individuals. Reference D'Souza, Perry, MacDougall, Ammerman, Cooper, Wu, Braley, Gueorguieva and Krystal2 In contrast, cannabidiol (CBD), another major constituent of some strains of cannabis, has been found to be anxiolytic and to have antipsychotic properties, Reference Zuardi, Crippa, Hallak, Moreira and Guimaraes3,Reference Leweke, Schneider, Radwan, Schmidt and Enrich4 and may be neuroprotective in humans. Reference Hermann, Sartorius, Welzel, Walter, Skopp and Ende5 The ratio of these two compounds in smoked cannabis varies – there are higher levels of Δ9-THC in ‘skunk’ or genetically modified strains of the plant. Reference Ross, Mehmedic, Murphy and Elsohly6 Cannabis users are often unaware of the ratio of CBD to Δ9-THC because CBD has no psychotomimetic effect in humans. Reference Zuardi, Crippa, Hallak, Moreira and Guimaraes3 Elevated levels of psychosis proneness and delusions have been found in people who use cannabis regularly. Reference Nunn, Rizza and Peters7 Despite suggestions about the different psychological properties of these two constituents of smoked cannabis, no prior research has examined the link between psychosis proneness and delusions and the CBD/Δ9-THC ratio in those who use cannabis. This study aimed to use hair analytic techniques to examine levels of Δ9-THC and CBD, and relate these objective indices of cannabis use to measures of psychosis proneness and delusional thinking.
Method
Our sample consisted of 140 individuals who were taking part in an ongoing longitudinal study, Reference Morgan, Muetzelfeldt and Curran8 which involved groups categorised as current and former ketamine users, other drug users and non-users. Inspection of the hair analysis results from the sample revealed that 54 individuals screened positive for cannabis. Confirmatory screens showed both CBD and Δ9-THC in the hair of 26 of these individuals but Δ9-THC alone in the hair of 20 others. Only individuals who showed evidence of Δ9-THC–carboxylic acid in hair as well as Δ9-THC were included, because this indicated actual consumption rather than passive use. The remaining 8 people who screened positive showed evidence of CBD alone in their hair samples, but they were excluded from this study as the group size was too small for statistical analysis. Rather than correlating the exact levels of CBD and Δ9-THC with symptoms – as concentrations have been shown to be vulnerable to other factors (e.g. hair washing or dyeing) Reference Skopp, Strohbeck-Kuehner, Mann and Hermann9 – we divided the sample into three groups: those with Δ9-THC only in hair (‘THC only’: n=20; mean age 26.1 years, s.d.=6.21; 13 males); those with Δ9-THC and CBD in hair (‘THC+CBD’: n=27; mean age 27.8 years, s.d.=6.26; 21 males); and those with no cannabinoids in hair (n=85; mean age 26.7 years, s.d.=6.59; 58 males).
Hair analysis was performed by gas chromatography/mass spectrometry (GC/MS). Following addition of THC-d3 (Tricho-Tech, UK; www.tricho-tech.co.uk) as the internal standard, 50 mg of the powdered hair was dissolved in 1 ml of 0.1 mol/l sodium hydroxide (100°C for 30 min). The solution was adjusted to a pH of 5.5 with 1.0 mol/l hydrochloric acid, and the Δ9-THC and CBD were extracted into n-hexane and quantified by GC/MS after silyation. The lower limit of detection was 0.025 ng/mg for both Δ9-THC and CBD.
The short form of the Oxford Liverpool Inventory of Life Experiences (OLIFE) questionnaire was used to assess psychosis proneness. Reference Mason, Linney and Claridge10 This measure yields four factors: unusual experiences (an analogue of positive symptoms in schizophrenia, including hallucinations and delusions); cognitive disorganisation (roughly corresponding to thought disorder); introvertive anhedonia (negative symptoms such as social withdrawal); and impulsive non-conformity (relates to behavioural impulsivity).
Peter's Delusion Inventory (PDI) was used to index delusional thinking. Reference Peters, Joseph and Garety11
Results
One-way analysis of variance (ANOVA) showed that there was no significant difference in age or in drug use (other than cannabis) reported in the three groups. Chi-squared tests revealed no difference in gender. The mean CBD level in the THC+CBD group was 0.15 ng/mg (s.d.=0.27). A Mann–Whitney U-test (as variance was heterogeneous) found no significant difference in the mean level of Δ9-THC in the THC only group (0.17 ng/mg, s.d.=0.07) and the THC+CBD group (0.19 ng/mg, s.d.=0.33).
Subjective estimates of cannabis use in these two groups did not differ in days per month of use (THC only, mean=19.4 days, s.d.=10.0; THC+CBD, mean=21.1 days, s.d.=10.1); age at which the participant became a regular user (THC only, mean=16.5 years, s.d.=3.07; THC+CBD, mean=5.48 years, s.d.=4.69) or days since last use (THC only, mean=3.89 days, s.d.=8.56; THC + CBD, mean=2.67 days, s.d.=3.96). There was a significant difference in number of days taken to smoke 3.5 g of cannabis (the standard quantity in which cannabis is sold in the UK, used as a more reliable indicator than amount smoked per session), with the THC only group taking longer (mean=10.2 days, s.d.=8.61) to smoke 3.5 g than the THC+CBD group (mean=5.0 days, s.d.=6.03); F (1,46)=5.59, P=0.023. Subjective estimates of cannabis use were not correlated with levels of Δ9-THC and CBD obtained from hair analysis.
Psychosis proneness
One-way ANOVA yielded significant differences between the three groups in scores on the OLIFE factor of unusual experiences: F (2,129)=12.86, P<0.001 (Fig. 1). Post hoc Scheffe's test showed that this was due to greater scores in the THC only group compared with the no cannabinoid group (P<0.001) and the THC+CBD group (P=0.021). Significant differences also emerged for the factor of introvertive anhedonia (F (2,129)=7.45, P=0.001), with significantly lower scores in the THC+CBD group compared with the no cannabinoid group (P=0.001) and the THC only group (P=0.035).
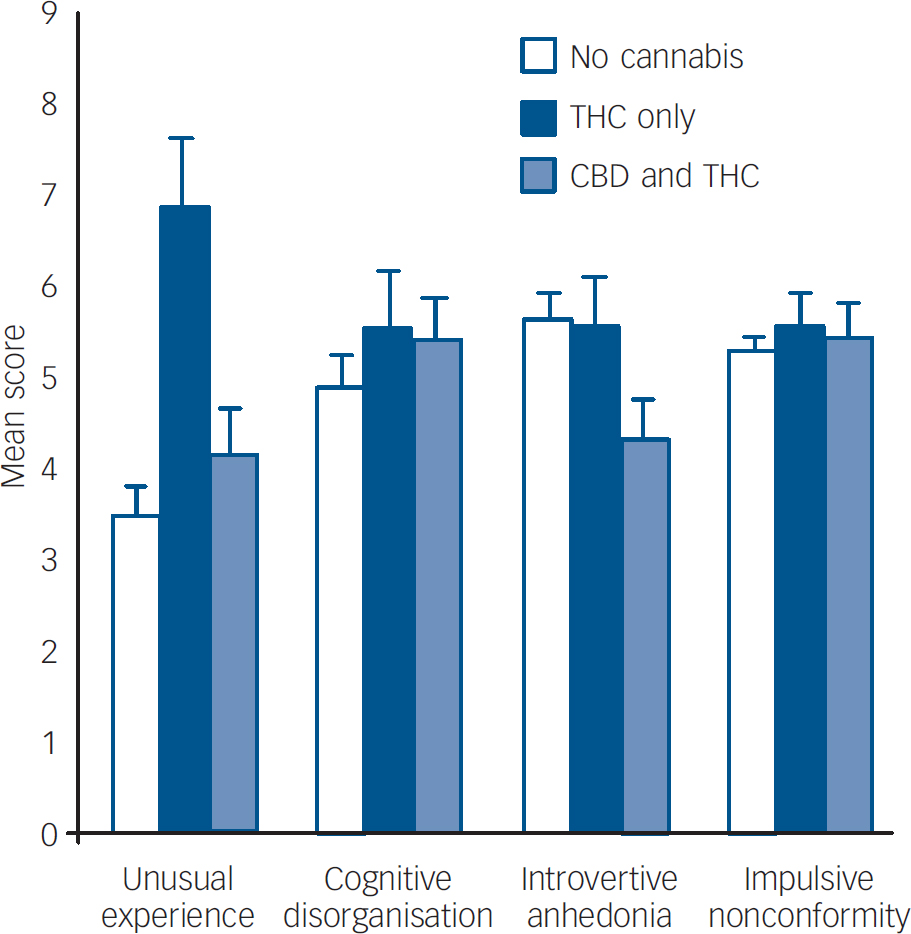
Fig. 1 Scores on the Oxford Liverpool Inventory of Life Experiences factors categorised by cannabis group. CBD, cannabidiol; THC, Δ9-tetrahydrocannabinol.
Delusional thinking
One-way ANOVA revealed significant group differences in scores on the PDI: F (2,129)=5.90, P=0.004. Compared with the no cannabinoid group (mean score 5.48, s.d.=3.58) there were significantly higher scores in the THC only group (mean score 8.15, s.d.=3.16; P=0.012) and a trend for greater scores in the THC+CBD group (mean score 7.22, s.d.=3.23; P=0.088).
Discussion
Our results show higher levels of unusual experiences – an analogue of hallucinations and delusions – in individuals who had evidence of only Δ9-THC in their hair compared with those with both Δ9-THC and CBD, and those with no cannabinoid. There were also greater levels of delusions in this THC only group compared with individuals who showed no evidence of cannabinoids in their hair, with a similar trend in the THC+CBD group. The THC+CBD group reported less anhedonia than the other two groups. This study is the first to demonstrate that hair analytic techniques can be used to define subsets of cannabis users. The implications of these findings are that people who smoke different strains of cannabis manifest different psychological symptoms.
These preliminary findings may support previous work showing the antipsychotic properties of CBD in the laboratory. Reference Zuardi, Crippa, Hallak, Moreira and Guimaraes3,Reference Leweke, Schneider, Radwan, Schmidt and Enrich4 Moreover, this suggests that smoking strains of cannabis containing CBD in addition to Δ9-THC may be protective against the psychotic-like symptoms induced by Δ9-THC alone. This is further evident from the findings that participants with both Δ9-THC and CBD in their hair had significantly less anhedonia than the other groups in this study. However, another potential explanation of the results of our study is that pre-existing differences in psychosis proneness between people who use cannabis may draw them to smoke different strains of the drug. In spite of this, the former explanation seems more plausible in light of the absence of differences in any other recreational drug use between these groups, and the emerging evidence of neuroprotective effects of CBD. A further limitation of this research is that the mechanisms by which cannabinoids are incorporated into hair are not well understood, and thus we cannot directly infer the ratio of CBD to Δ9-THC. Despite this, our study highlights the importance of distinguishing between different cannabinoids and has implications in the debate over the link between cannabis use and psychosis.
Acknowledgements
H.V.C. and C.J.A.M. were supported by a grant from the Economic and Social Research Council (RES-000-23-0945).




eLetters
No eLetters have been published for this article.