Perinatal depression, occurring during pregnancy or shortly after childbirth, is a common disorder that affects between 10 and 15% of women during this period.Reference Gelaye, Rondon, Araya and Williams1 In Nigeria reported prevalence rates range between 10 and 30%.Reference Aderibigbe, Gureje and Omigbodun2, Reference Uwakwe and Okonkwo3 Prenatal depression is associated with a greater risk of premature delivery, low birth weight of infants and greater risk of adverse obstetrical outcomes;Reference Bonari, Pinto, Ahn, Einarson, Steiner and Koren4, Reference Grote, Bridge, Gavin, Melville, Iyengar and Katon5 postnatal depression might interfere with mother–infant interactions and impair infants' cognitive and emotional development.Reference Field6, Reference Stein, Pearson, Goodman, Rapa, Rahman and McCallum7 Furthermore, mothers with perinatal depression are more likely to miss their infants' routine immunisation visits and delay help-seeking for potentially serious childhood illnesses.Reference Prince, Patel, Saxena, Maj, Maselko and Phillips8
Despite evidence suggesting that integration of the care of perinatal depression into routine maternal care is the most efficient way to bridge the treatment gap for the condition, it is estimated that less than 50% of perinatal depression cases are detected by primary healthcare professionals in routine clinical practice.Reference Hearn, Iliff, Jones, Kirby, Ormiston and Parr9 Systematic reviews of studies in high-income countries show that, in most instances, perinatal depression can be effectively managed with psychological and psychosocial interventions.Reference Dennis, Ross and Grigoriadis10 There is growing evidence from low- and middle-income countries (LMICs) that such interventions can be effectively implemented by trained and supervised non-physician primary healthcare workers, with benefit for both mothers and their children.Reference Clarke, King and Prost11, Reference Rahman, Fisher, Bower, Luchters, Tran and Yasamy12 In most LMICs – especially countries in sub-Saharan Africa where primary care providers are few and often burdened with a heavy workload – it is important to determine how intense such an intervention needs to be for it to be effective in bringing about remission from perinatal depression. The primary hypothesis of this study is that an intervention package consisting of primary care worker-administered problem-solving treatment delivered within a stepped-care approach will be more effective than enhanced care as usual at alleviating perinatal depression 6 months after childbirth.
Method
Study design
This study was a cluster randomised controlled trial in which the unit of randomisation was eligible and consenting primary maternal care clinics in selected local government areas, and the unit of analysis was individual women participants. A full description of the study methods has been published.Reference Gureje, Oladeji, Araya, Montgomery, Kola and Kirmayer13 The study was conducted in Oyo State in south-western Nigeria. Nine local government areas (four urban and five rural) were randomly selected for the study. Appropriate institutional ethics approval was obtained from the University of Ibadan/University College Hospital Ibadan Ethical Review Committee (approval number UI/EC/12/0278).
Participants
Participating clinics had to provide both antenatal and postnatal services. Participants were all consecutive pregnant women registering for antenatal care at the participating clinics. These women were approached for screening if they were aged between 16 and 45 years, with a foetal gestational age of between 16 and 28 weeks. We administered the Edinburgh Postnatal Depression Scale (EPDS) to those women who consented to be screened and spoke Yoruba, the language of the study.Reference Cox, Holden and Sagovsky14 Women who scored ≥12 on the EPDS were asked further questions, derived from the short version of the Composite International Diagnostic Interview (CIDI),Reference Kessler, Calabrese, Farley, Gruber, Jewell and Katon15 to confirm the presence of major depression according to DSM-IV (1994) criteria and the absence of psychotic symptoms and bipolar disorder. Women with major depression, who had no psychotic symptoms, were not actively suicidal, who provided signed informed consent and were going to be available in the study area up to 12 months after childbirth, were enrolled into the study. A full baseline assessment took place within 72 h of recruitment. Participants in both arms of the study were given their EPDS score and asked to give this information to the attending primary maternal care provider (PMCP) who would initiate treatment according to the arm of the study. Screening and recruitment took place at the maternal and child care clinics; baseline and other outcome assessments took place at participants' homes or other places of their choice.
Randomisation and masking
Clinics were randomised to deliver either a high intensity intervention or to enhance care as usual. Treatment was delivered individually to patients by primary care workers who provided routine antenatal and postnatal care to the women. Eligible and consenting maternal care clinics were stratified by local government area and allocated to the intervention or control arm by using a computer-generated random number sequence. Allocation was conducted by one of the authors (A.A.M.), using anonymous codes for clinics and local government areas provided by other members of the research team.
All outcome assessments were conducted in participants' homes by experienced research interviewers who had received 2 weeks training in trial procedure, were not involved in participants' recruitment and who were blind to the participants' treatment.
Procedures
High-intensity treatment
In the high-intensity treatment (HIT) arm, in addition to the enhanced usual care (see below), providers offered a stepped-care treatment, using a manualised psychological intervention package. We have previously provided details of the development and piloting of a stepped-care intervention for depression in primary care.Reference Oladeji, Kola, Abiona, Montgomery, Araya and Gureje16 A full description of the intervention package used in this study has been published (and is available from the authors on request).Reference Gureje, Oladeji, Araya, Montgomery, Kola and Kirmayer13 The core component was a locally adapted form of Problem Solving Treatment (PST) for Primary Care. In this intervention, the patient is guided through a step-by-step process of breaking down current psychosocial stressors and then exploring and trying out options for their resolution, which includes using personal resources and available social support.
The intervention, which commenced within 1 week of participant enrolment, was delivered in three steps determined by the participant's score on the EPDS, time since enrolment and gestational status. Step one comprised eight sessions of psychological interventions, delivered weekly in the antenatal period. Step two commenced 6 weeks after delivery, during the mother's routine postnatal visit. Depending on participants' EPDS scores (<12 or ≥12), providers delivered either four fortnightly top-up sessions of the PST or eight weekly intervention sessions. At the completion of step two, participants who still had EPDS scores of 12 or more proceeded to step three in which they were reassessed by the community physician with a view to initiate pharmacotherapy in addition to continuing with the psychological intervention or referral to a specialist service. Each session of the psychological intervention lasted approximately 30–45 min. Mothers in the HIT arm of the study also received parenting skills training, which included information on issues such as the importance of routine antenatal visits, adequate nutrition and rest, the care of and nutrition for the newborn and information on how to engage and stimulate the infant. All sessions were based on the individual.
The intervention was delivered by the PMCP who had received an initial 3 day training and a 2 day top-up training (1 month later) on the delivery of the intervention and had ongoing structured support and supervision from primary care physicians who, in turn, could consult with a psychiatrist when needed. The support, supervision and specialist consultation were provided via mobile phones except when face-to-face assessment was indicated. Participants also received automated mobile phone voice messages and calls from the PMCP to remind them of clinic appointments and homework related to the therapy session.
Enhanced care as usual (low-intensity treatment)
Following a recent government health policy that specified the World Health Organization Mental Health Gap Action Programme (mhGAP) as the pathway to scaling up mental healthcare in the country, participants in the comparator arm were offered enhanced usual care, which constituted the low-intensity treatment (LIT). The PMCP in the LIT arm received a 1.5 day training on the use of the mhGAP – Intervention Guide (mhGAP-IG) and were given copies of the mhGAP-IG as well as a manual describing the nature and standard treatment approaches for perinatal depression. The providers thus delivered the intervention to participants by using the basic specifications of mhGAP-IG. These basic specifications, as previously stated, consist of psychoeducation, addressing current psychosocial stressors and reactivation of social network. No structured sessions were stipulated and no stepped-care procedure was specified; the number/frequency of visits and content of the psychosocial interventions were at the discretion of the PMCP.
Outcomes
The primary outcome was remission from depression at 6 months postpartum, defined as an EPDS score of less than six (which, from our pilot experience, validly operationalises our protocol pre-specified outcome of no longer meeting DSM-IV criteria for major depression). Maternal secondary outcomes were: (a) depressive symptoms (as shown by EPDS scores over the follow-up period), (b) disability (measured with the World Health Organization Disability Assessment Scale [WHODAS])17 and parenting skills (measured with the Maternal Adjustment and Maternal Attitudes Questionnaire [MAMA]Reference Kumar, Robson and Smith18 and the Infant Toddler version of the Home Inventory for Measurement of the Environment),Reference Caldwell and Bradley19 and (c) experience of stigma (measured with the 12-item Discrimination and Stigma Scale [DISC-12]).Reference Lasalvia, Zoppei, Van Bortel, Bonetto, Cristofalo and Wahlbeck20 The EPDS, WHODAS and DISC-12 have been previously validated and used in Nigeria.Reference Uwakwe and Okonkwo3 Infant secondary outcomes consisted of (a) growth and health at 6 months (measured by height and weight, history of completed immunisation and history of any illness, including fevers and diarrhoea), (b) nutrition at 6 months (measured by history of breastfeeding), and (c) motor and cognitive development at 12 months (as assessed with the Bayley's Scale for Infant Development). We used the Client Service Receipt Inventory – Postnatal Depression version to collect service use data for the estimation of cost-effectiveness.Reference Chisholm, Conroy, Glangeaud-Freudenthal, Oates, Asten and Barry21 A full description of the measures, with evidence of previous use and their validation in our setting, was published earlier.Reference Gureje, Oladeji, Araya, Montgomery, Kola and Kirmayer13
We monitored fidelity and quality of care in the HIT arm by reviewing the clinical records of all participants, documenting contacts, sessions attended, and consultations and referrals to physicians and psychiatrists. The clinical records were designed to capture each step and structure of the PST. Independent assessments of quality of delivery of, and adherence to, the structure of the PST in the HIT arm were conducted by senior members of the research team on 18 PMCPs by direct observation. Either session two or five was rated on nine dimensions. These dimensions included quality of eye contact, appropriate probes relevant to the PST session, listening and use of cues and they were rated on a three-point scale of very good, good or poor. Of a total 180 ratings (from 20 sessions), 58% were rated very good, 32% good and 10% poor.
Evaluation of the process of care in the LIT arm was conducted by reviewing all case records of contacts to retrieve documented evidence of provider's attention to the participant's depression (either by treatment mentioned or clinical progress reported).
To enable an assessment of the relative cost-effectiveness of HIT versus LIT, primary study outcomes were linked to estimates of the service costs incurred in each arm. A service use questionnaire that had been previously piloted and used in the local context was administered alongside other measures at baseline and at 3, 6 and 12 months follow-up. Simplified costing templates and local data inputs were used to generate a set of unit costs and prices for all in-patient and out-patient service use components, as well as the cost of the interventions themselves.
Statistical analysis
Data from previous trials among women with perinatal or postnatal depression in Pakistan and ChileReference Rahman, Malik, Sikander, Roberts and Creed22, Reference Rojas, Fritsch, Solis, Jadresic, Castillo and González23 suggested a remission rate of about 50 and 75% in control and intervention arms, respectively. As the study compared a high-intensity intervention with enhanced care as usual, we conservatively sought to detect an absolute difference of 15% (40% remission in LIT and 55% remission in HIT groups, respectively) at 6 months after birth. An individually randomised trial requires 186 participants per arm for analysis to detect this difference with 80% power and 5% two-sided alpha. Using pilot study data, we estimated the intra-cluster correlation coefficient for the primary outcome to be 0.026, 85% collection of primary outcome data and a cluster size for analysis of 43. We therefore aimed to recruit 18 clinics and 916 individuals. Participant recruitment was slower than anticipated, so we recruited and randomised a further 11 clinics in January 2014, giving a total of 29 in the study. In August 2014, prior to the planned completion of participant recruitment, we undertook a formal review of sample size and found some assumptions in the original estimate were inaccurate: (a) there was an imbalance in the ratio of women recruited of around 1.9 in favour of the HIT arm, (b) there was variation in cluster size that was not ignorable when estimating the design effect, and (c) we examined the remission rate among the control (LIT) arm participants who had reached the primary follow-up and found this to be much higher than expected at around 84%. (We did not examine remission in the intervention arm or between-group effect.) We no longer considered an absolute difference of 15% to be plausible with such high remission in the low-intensity arm, and thus estimated sample size for a smaller difference of 11.5%. An individually randomised trial requires 258 participants for analysis with an allocation ratio of 1.9 and the same power and alpha as before. With both the projected mean cluster size for analysis and s.d. of around 24, the design effect is 2.2,24 giving a total of 670 participants to be recruited with 85% collection of primary outcome data. In essence, our study was fully powered (80%) to detect a difference of 11.5%.
Analyses were conducted by using Stata/SE 13.1 software (for Windows). We used appropriate descriptive statistics (χ2 test and t-test) to examine the balance between the arms at baseline and to describe outcomes at 6 and 12 months follow-up. In view of the high follow-up rate, the main approach to analysis was modified intention to treat at the individual level, that is, analysis according to randomised group regardless of adherence with allocation and without imputation of missing outcome data. We used multivariable mixed effects regression models (logistic or linear dependent on outcome type) with clinic and local government area included as random effects and baseline value of the outcome, if measured, as a covariate to estimate between-arm effects and 95% confidence intervals. For the primary outcome, we conducted sensitivity analyses with further adjustment for any variables displaying between-arm differences at baseline, and by multiple imputation of missing outcome data using chained equations. We conducted a per-protocol subgroup analysis of the primary outcome according to baseline severity of depression by including an interaction term between arm and baseline EPDS score (<16, ≥16) in the primary regression model. We conducted a pre-specified analysis of EPDS as a continuous outcome at 6 months and also over the 12 month postnatal follow-up period, using repeated measures analysis by including follow-up occasion (3, 6, 9, 12 months) as a random effect in the regression model. We analysed other secondary outcomes by using a similar approach as for the primary outcome.
For the economic analysis, mean service costs were computed for each study arm and then linked to primary outcomes through a series of multivariate regression analyses that controlled for baseline differences in cost as well as key demographic characteristics. Because of the skewness of cost data, the non-parametric bootstrapping technique was employed to generate confidence intervals around mean costs and cost-effectiveness ratios (N = 1000 replications were made).
The conduct of the study and its adherence to approved procedures were monitored by an independent Data Monitoring and Ethics Committee which reported to another independent body, the Trial Steering Committee. The trial is registered with the International Standard Randomised Controlled Trials Number Registry (http://www.isrctn.com/isrctn) under trial number ISRCTN60041127.
Results
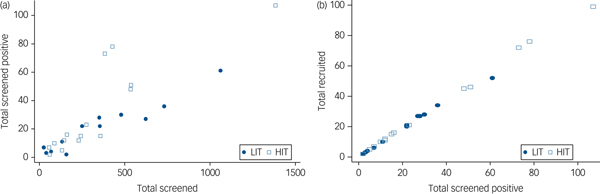
All 137 primary care clinics located in the nine local government areas were assessed for eligibility. Of the 49 providing comprehensive maternal care which were therefore eligible, 29 consented to participate and were randomised (Fig. 1). Data to further describe participating clinics prior to randomisation were not collected. A total of 9352 women were screened, of whom 727 (7.7%) scored at least 12 on the EPDS, and 686 were recruited. Although the proportions of women screening positive were similar in both arms at baseline, a higher proportion of women subsequently screened positive in the HIT arm (9.4 v. 5.8%) due primarily to two clinics with high positive screen rates (Fig. 2). Participant recruitment took place between 18 June 2013 and 14 October 2014, and the final 12 month postnatal follow-up was concluded on 11 December 2015. Follow-up was high at both 6 (85%) and 12 (79%) months follow-up and was similar in both arms. There were very few refusals at both time points. Most of those lost to follow-up had either moved to new addresses that could not be traced or were not available after multiple efforts (at least four attempts) were made to interview them. No demographic or clinical features were significantly associated with refusal to participate or with attrition.
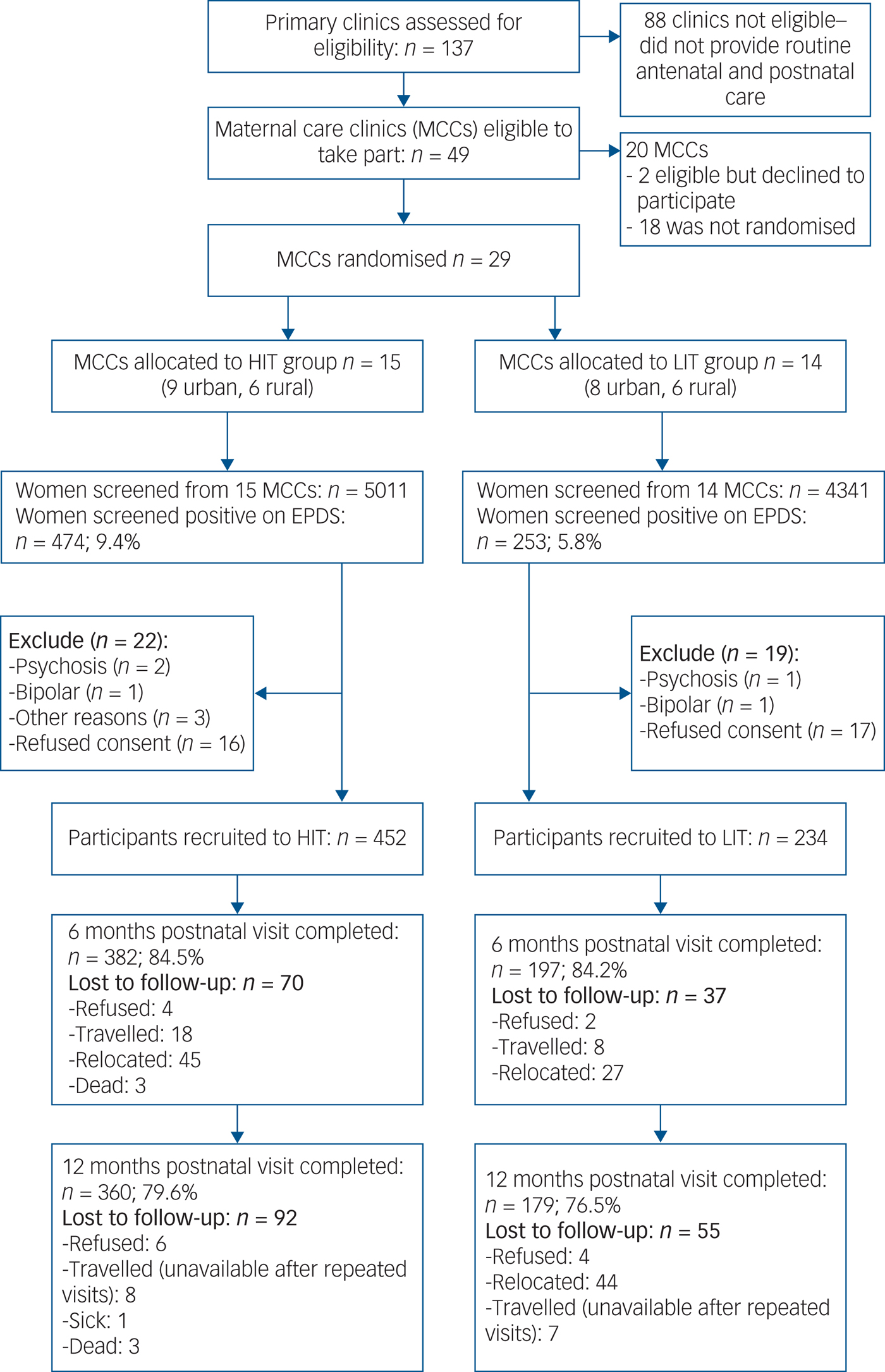
Fig. 1 Flow diagram of the recruitment and follow-up process. EPDS, Edinburgh Postnatal Depression Scale; HIT, high-intensity treatment; LIT, low-intensity treatment.
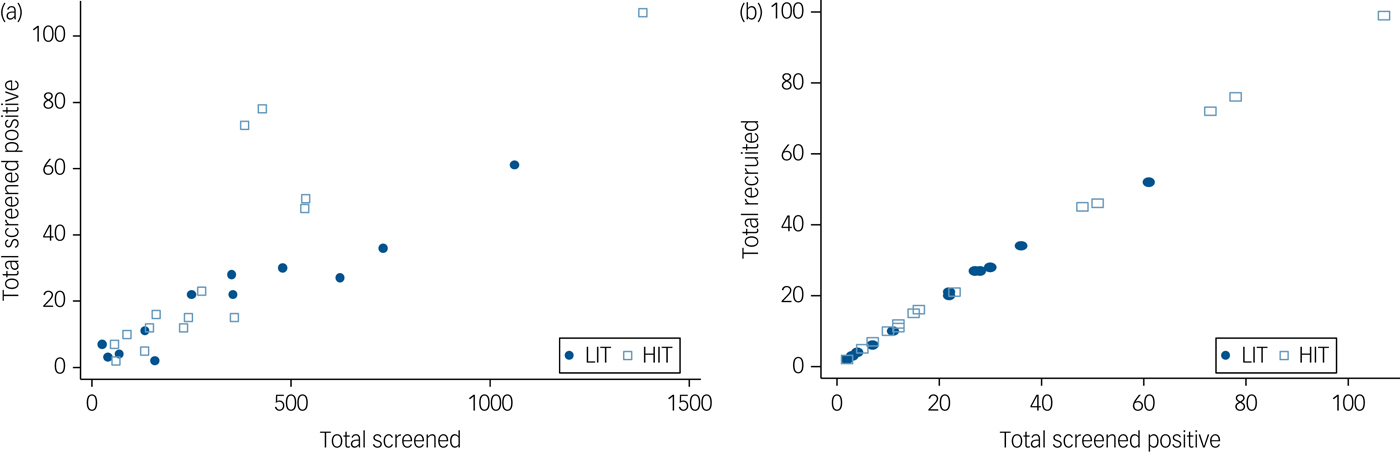
Fig. 2 Conversion from (a) screened to screened positive by clinic and (b) screened positive to recruited by clinic.
Table 1 shows the sociodemographic and clinical features of trial participants at baseline. This was a sample of young women with mean age under 25 years and about 11 years of education. About four in five were married, most were in the second trimester at time of recruitment and about 50% were primiparous, although this was slightly higher in the HIT group than the LIT group. The trial focused on people with moderate to severe perinatal depression. A mean score of 14 on the EPDS suggests that most indeed had moderate depression. Participants in both groups showed similar levels of adjustment to pregnancy and of disability, as indicated by MAMA and WHODAS scores, respectively. Importantly, even though rates of recruitment were different between trial arms, baseline features were very similar except for parity. Pregnancy outcomes were similar between the arms (Supplementary Table 1 available at https://doi.org/10.1192/bjp.2019.4). Over 90% of pregnancies resulted in a live birth, of which over 98% were singletons.
Table 1 Baseline demographic and clinical characteristics
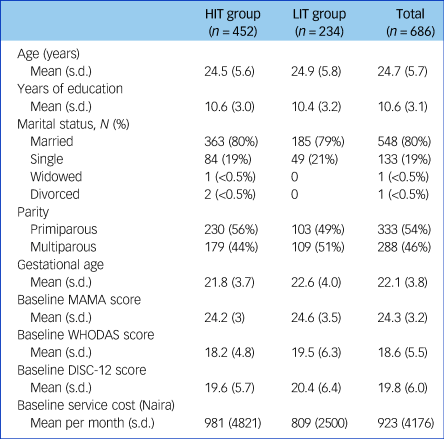
HIT, high-intensity treatment; LIT, low-intensity treatment; MAMA, Maternal Adjustment and Maternal Attitude questionnaire (contains 12 questions, each ranging from not at all to very much); WHODAS, World Health Organization Disability Assessment Scale (contains 12 questions, ranging from 1 [best] to 5 [worst] plus three additional questions about the number of days in the past month they had the difficulties); DISC-12, Discrimination and Stigma Scale (contains 16 questions, each ranging from not at all to a lot).
At the primary follow-up 6 months after childbirth, similar proportions of women in both arms had recovered from depression and there was no evidence of any between-group difference (adjusted risk difference of 4%; 95% CI −4.1%, 12.0%) (Table 2). Additional adjustment by baseline parity and multiple imputation for the 107 women who did not provide primary outcome data made no material difference to the results. There was some evidence that HIT was more effective than LIT among women who had higher EPDS scores at baseline (interaction odds ratio 2.29; 95% CI 1.01, 5.20; P = 0.047).
Table 2 Primary outcome, sensitivity and subgroup analyses
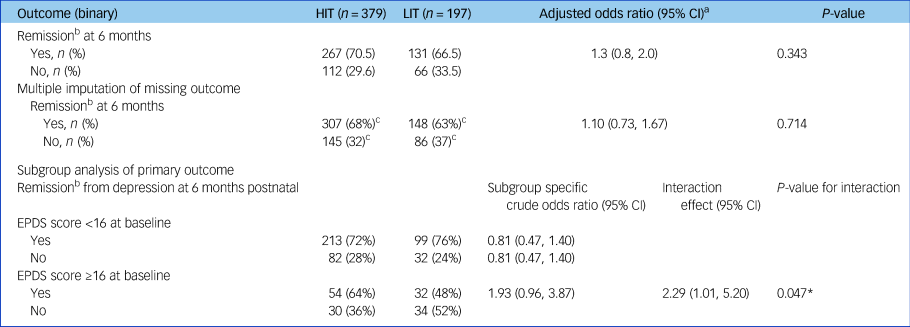
HIT, high-intensity treatment; LIT, low-intensity treatment; EPDS, Edinburgh Postnatal Depression Scale (contains 10 questions, each ranging 0 [best] to 3 [worst]).
a. Adjusted by baseline EPDS score, baseline parity, maternal care clinics and local government area to which participants belong. Maternal care clinics and local government areas are included as random effects.
b. Remission is defined as EPDS score lower than six.
c. Predicted totals from multiple imputation.
* P < 0.05.
The HIT arm had a significantly lower mean level of disability than the LIT arm at 6 months (adjusted mean difference −0.6; 95% CI −1.1, −0.0; P = 0.045) and lower mean EPDS score at 12 months (adjusted mean difference −0.9; 95% CI −1.7, −0.2; P = 0.012) (Table 3). Over the 12 month postnatal period, mean EPDS scores were lower in with HIT than with LIT. Mean scores at 3, 6, 9 and 12 months were 3.4, 3.7, 3.4 and 3.5 for HIT and 4.7, 4.5, 3.8 and 4.6 for LIT, respectively. The between-group difference averaged over all four follow-up time points was −0.8 (95% CI −1.3, −0.2; P = 0.007) in favour of HIT. There were no other significant differences in secondary maternal outcomes.
Table 3 Effect of intervention on secondary outcomes at 6 and 12 months
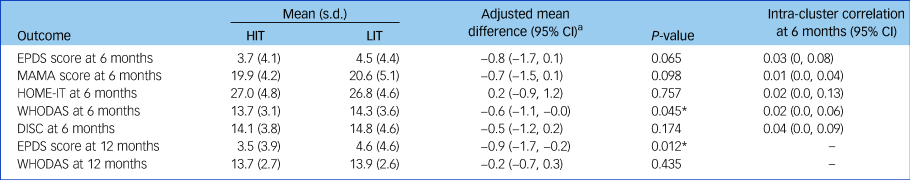
HIT, high-intensity treatment; LIT, low-intensity treatment; EPDS, Edinburgh Postnatal Depression Scale (contains 10 questions, each ranging 0 [best] to 3 [worst]); MAMA, Maternal Adjustment and Maternal Attitude questionnaire (contains 12 questions, each ranging from not at all to very much); HOME-IT, Infant Toddler version of the Home Inventory for Measurement of the Environment; WHODAS, World Health Organization Disability Assessment Scale (contains 12 questions, ranging from 1 [best] to 5 [worst] plus three additional questions about the number of days in the past month they had the difficulties); DISC, Discrimination and Stigma Scale (contains 16 questions, each ranging from not at all to a lot).
a. Adjusted by baseline EPDS score, maternal care clinics and local government area to which participants belong. Maternal care clinics and local government area are included as random effects.
* P < 0.05.
Infants of mothers in both arms were similar in weight, height and head circumference at 6 months (Supplementary Table 2). They were also similar regarding measures of cognitive and motor development. The proportions of infants who had been administered scheduled immunisations or who had experienced any physical illness were also similar across groups. However, mothers in the HIT arm were twice as likely to have complied with the recommendation to feed babies exclusively on breast milk as mothers in the LIT arm (19 v. 10%; odds ratio 2.17; 95% CI 1.27–3.73; P = 0.005).
Every participant in the HIT arm received at least one session of psychological intervention, about 90% received at least four sessions and 61% completed the initial prenatal eight treatment sessions. At least one postnatal session was received by 85% of the mothers, with 78% receiving two sessions. Consultation was made to doctors for about 3% of the women following their first assessment by the PMCP. None of the participants were prescribed an antidepressant medication.
About 95% of participants in the LIT arm had two prenatal treatment contacts with the PMCP, 60% had three contacts and only 10% had four contacts. One postnatal treatment contact was made by 30% of the women.
There were three maternal deaths, all in the HIT group, none of which was from suicide. Eight miscarriages were recorded: five in the LIT arm and three in the HIT arm. There were 36 stillbirths, 25 (6%) in the HIT group and 11 (5%) in the LIT group. None of the adverse events were judged by the independent Trial Steering Committee to be related to the study procedures.
Costs and cost-effectiveness
Service costs per participant per month reduced appreciably and to a similar degree from their baseline values in both groups (Supplementary Table 3), falling from Naira 981 to 788 (at 6 months) and 379 (at 12 months) in the HIT group, and from Naira 809 to 485 (at 6 months) and 142 (at 12 months) in the LIT group. Total estimated costs over the full 1 year period following baseline were approximately double in the HIT group (Naira 7028/US$46.85 per participant) compared with the LIT group (Naira 3600/US$24.00 per participant). Converted to US dollars (US$1 was worth Naira 150 in 2015), these costs appear relatively low – reflecting very modest use of services in general – but may nevertheless impose a financial burden on families contributing towards the cost of services in the local context via direct payments (out-of-pocket payments).
Cost-effectiveness was assessed at 6 and 12 month follow-ups. Changes in service costs were linked to changes on the EPDS, with results showing that reductions in service cost over time were slightly greater in the LIT group, whereas outcomes were only marginally in favour of HIT. The extra cost per one-point improvement on the EPDS with HIT compared with LIT was Naira −653 (95% CI −5108 to 3975) at 6 months and Naira −128 (95% CI −1360 to 1186) at 12 months. Cost-effectiveness was also assessed with respect to disability; for this outcome the cost per one-unit improvement on WHODAS was Naira 186 (95% CI −1055 to 1408) at 6 months and Naira 58 (95% CI −521 to 568) at 12 months. In summary, HIT represents a cost-effective alternative to LIT, but since LIT was associated with similar changes in health, functioning and cost, there is no significant difference for the more intensive strategy.
Discussion
We found no evidence of any significant differences between HIT and LIT in the proportion of women who recovered from depression at 6 months after birth, nor did we detect any cost-effectiveness advantage for HIT. However, our data suggest that HIT may be more effective than LIT for the subgroup of women with more severe depressive symptoms at baseline. We also found some evidence that participants in the HIT group had fewer depressive symptoms as measured by mean EPDS score at the 12 month outcome as well as over the course of the 12 month follow-up after birth, indicating a greater level of symptom remission in the HIT group, but this effect may not be clinically significant. Other than significantly lower disability at 6 months, there was no evidence of any differences between the groups in the levels of attitude to motherhood, experience of stigma, and in the quality and quantity of stimulation they provided to their infants at home at the 6 and 12 month outcomes. Unlike as noted by others,Reference Rahman, Malik, Sikander, Roberts and Creed22 infant outcomes at 6 months including growth, reported illnesses, exposure to scheduled immunisations, and cognitive and motor development at 12 months were also similar between the groups. However, mothers receiving the high-intensity intervention were more likely to have provided exclusive breastfeeding to their infants than those receiving the low-intensity intervention at 6 months, a benefit that may be explained by the parenting skills training component of the HIT.
The study did not find substantial evidence to indicate that the HIT is superior to the LIT except among women with high baseline depression. The outcomes from the two interventions were also comparable to what has been reported in the literature either with the use of medication or psychological approaches.Reference Rahman, Fisher, Bower, Luchters, Tran and Yasamy12, Reference Rahman, Malik, Sikander, Roberts and Creed22, Reference Rojas, Fritsch, Solis, Jadresic, Castillo and González23 Extending observations made in high-income countries that show the effectiveness of psychological and psychosocial interventions for common perinatal mental disorders,Reference Dennis, Ross and Grigoriadis10 there are now several studies providing similar evidence for such forms of intervention in LMICs.Reference Clarke, King and Prost11 A recent meta-analysis of 13 studies conducted in LMICs showed that interventions delivered by mostly non-specialist health workers, within task-shifting or task-sharing approaches, provide significant benefit to mothers with perinatal depression as well as their infants.Reference Rahman, Fisher, Bower, Luchters, Tran and Yasamy12
The major findings of our study confirm the feasibility of this task-sharing model of care in the setting of the study. Although they differed in intensity and structure, both interventions met some of the essential features of psychological interventions delivered by non-specialists that have been found to be effective in LMICs for perinatal depression: active psychotherapeutic components delivered within an understanding of the contextual social problems of the women and with sensitivity to cultural and language factors.Reference Adeponle, Groleau, Kola, Kirmayer and Gureje25 When those features are present, even simple, low-intensity interventions are commonly effective for perinatal depression.Reference Chowdhary, Sikander, Atif, Singh, Ahmad and Fuhr26
The rate of depression in this sample is lower than is often reported.Reference Gelaye, Rondon, Araya and Williams1 Even though a score of ten or higher on EPDS has a specificity of 91.5% for DSM-IV major depression among perinatal women in Nigeria,Reference Adewuya, Ola, Dada and Fasoto27 we have used a higher cut-off score to focus on depression of at least moderate severity. A higher proportion of women screened positive in the HIT compared with the LIT arm. This was due primarily to two clinics which served communities with large populations of migrant farm workers. However, both arms were similar in terms of age, gestational age, education, pregnancy outcomes and baseline EPDS scores.Reference Tan, Gureje, Montgomery, Hepburn, Oladeji and Araya28
The fact that PMCPs were willing and able to use the mhGAP-IG as a clinical support tool in this trial suggests that the tool may have potentials for scaling up care for perinatal depression. However, the design of the study involved screening and assessment of women for the presence of perinatal depression by the study research team. There thus remains the need to demonstrate whether, with training and with the use of the mhGAP-IG, these frontline providers can reliably detect the condition themselves. The strengths of the EXPONATE trial include the use of validated tools with proven cultural appropriateness and acceptability in the settingReference Gureje, Odejide, Olatawura, Ikuesan, Acha, Bamidele, Ustün and Sartorius29, Reference Gureje, Lasebikan, Kola and Makanjuola30 and its focus on depression of at least moderate severity and therefore of undoubted clinical significance. A major limitation is the use of an enhanced care as usual approach, rather than typical care as usual, where most women with perinatal depression are either not detected or offered evidence-based treatment.Reference Gureje, Odejide, Olatawura, Ikuesan, Acha, Bamidele, Ustün and Sartorius29 The reasons for a lack of clear difference between the two trial arms could include: spontaneous remission of women with the less severe forms of the condition, a non-real world implementation of the mhGAP-IG in which detection of depression was by research assistants rather than by the providers, the use of non-specialists to supervise providers in the HIT arm or the fact that the performance of the PMCPs was not uniformly good. Also, although efforts were made to blind the outcome assessors, the possibility of unmasking and hence some information bias cannot be totally excluded. Another limitation is that, although the CIDI has been extensively used and validated by us,Reference Gureje, Lasebikan, Kola and Makanjuola30, Reference Gureje, Olowosegun, Adebayo and Stein31 and the trained interviewers have wide experience with its use, no formal test of reliability for its items were conducted for this study.
Our findings show that even though a high-intensity psychosocial intervention has some added benefits, a low-intensity, evidence-based intervention (consisting of the basic treatment specifications for depression described in the mhGAP-IG) may be sufficient to bring relief to the majority of women with perinatal depression in primary maternal care service. A low-intensity intervention may be more feasible as well as more affordable to deliver within routine service in busy primary maternal care clinics in low-resource settings.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.4.
Funding
EXPONATE was funded by Grand Challenges Canada (0082-04). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data and had final responsibility for the decision to submit for publication.







eLetters
No eLetters have been published for this article.