The life expectancy of people with Down syndrome has increased greatly over the past several decades and many people with the condition now live into older age.Reference Yang, Rasmussen and Friedman 1 , Reference Englund, Jonsson, Zander, Gustafsson and Annerén 2 People with Down syndrome have a higher risk of developing Alzheimer's disease than the general population.Reference Wiseman, Al-Janabi, Hardy, Karmiloff-Smith, Nizetic and Tybulewicz 3 There is good evidence that cholinesterase inhibitors (donepezil, rivastigmine or galantamine) and memantine, a non-competitive N-methyl-d-aspartate receptor antagonist, can improve cognitive function and behaviour in people with Alzheimer's disease who do not have intellectual disabilities;Reference Birks 4 , Reference McShane, Areosa Sastre and Minakaran 5 however, people with Down syndrome have been excluded from most trials of antidementia drugs and the evidence that they are effective in this group remains inconclusive.Reference Mohan, Bennett and Carpenter 6 – Reference Hanney, Prasher, Williams, Jones, Aarsland and Corbett 9
The aim of this study was to determine the effect of cholinesterase inhibitors or memantine on survival and cognitive and adaptive function in a large clinical cohort of people with Down syndrome and Alzheimer's disease.
Method
Data source
Data were obtained from the Aging with Down Syndrome and Intellectual Disability (ADSID) database, a research database conceived following the regular meetings of a regional dementia in intellectual disability special interest group.Reference Sheehan, Sinai, Bass, Blatchford, Bohnen and Bonell 10 The ADSID database contains the clinical information of over 1000 adults with intellectual disability who have been assessed in specialist memory clinics for adults with intellectual disability. Data were collected by the authors from the clinical records held by psychiatry of intellectual disability community teams across London and the south of England. Demographic and clinical details were extracted from the patient notes, pseudonymised at source and added to the database, which is held securely at University College London.
Data include, degree and aetiology of intellectual disability, physical and psychiatric comorbidities (with a particular emphasis on those that are common in people with Down syndrome and those that are related to cognitive functioning such as thyroid disorder, past or current history of depression, epilepsy and sensory impairment) and psychotropic drug prescription. Dates of clinical assessments and the results of standardised cognitive tests are also recorded. Many of those included have been under regular clinic follow-up and have contribute data from several sequential assessments. Date and cause of death is recorded, where applicable. The database contains information from clinical assessments conducted between 2000 and 2013, when the research database was superseded.
Ethics
Ethical approval for use of the ADSID database in research studies was obtained from the Newcastle and North Tyneside 1 Research Ethics Committee (reference 10/H0906/30). Special permission was received from the National Information Governance Board for Health and Social Care Ethics and Confidentiality Committee to process patient identifiable information at source without consent (reference ECC 5-04(h)/2010). The Caldicott Guardian of organisations providing information authorised data collection and transfer of pseudonymised data.
Study participants
We included all people in the ADSID database with Down syndrome and a diagnosis of Alzheimer's disease made after January 2000. Data collection finished in 2013. A record of Down syndrome in the individual's clinical notes or results of genetic tests indicating Down syndrome was taken as evidence of the condition. The diagnosis of dementia was made by the person's own clinical team and the assessment process was determined by local protocols, as there is no agreed standard for dementia assessment in Down syndrome. In line with current best practice guidance, diagnostic assessments were expected to have been undertaken by an experienced clinician and to include medical history, neuropsychiatric assessment, and physical and mental state examination, and exclusion of other causes of decline. 11 If there was uncertainty about the diagnosis of Down syndrome or Alzheimer's disease the participant was excluded from the study. We collected a minimum data-set for each participant and excluded those for whom insufficient data were available.
The cohort was divided into groups based on prescription of medication for dementia, first into those who had been prescribed medication and those who had not, then by medication class and drug prescribed. A separate category was created for individuals who had been prescribed both cholinesterase inhibitors and memantine. The dates of starting, stopping and switching medication were recorded, where available. Where the date of starting medication was not known, the date of dementia diagnosis was considered the most likely date of prescription and used as a proxy.
Outcomes and measures
The Dementia Questionnaire for People with Learning Disabilities (DLD) was used to measure baseline and changes in cognitive and adaptive function.Reference Eurlings, Evenhuis and Kengen 12 The DLD is a routinely-used standardised informant-based questionnaire consisting of 50 items grouped into eight categories that the informant is asked to rate on a linear scale based on observations made over the preceding 6 months. Completing the DLD yields two subscores; a sum of cognitive scores (SCS, covering memory and orientation); a sum of social scores (SSS, covering aspects of behaviour and adaptive function). Higher scores indicate more severe impairment. The DLD is a sensitive tool in tracking changes over time in people with Down syndrome and cognitive decline, and is recommended as a tool to supplement clinical assessment in people with intellectual disability in the National Institute for Health and Care Excellence dementia pathway.Reference Evenhuis 13 – Reference McCarron, McCallion, Reilly and Mulryan 15
Baseline score was defined as the DLD at the point of diagnosis (or the last recorded DLD prior to diagnosis), first assessment and second assessment DLD scores were the first and second subsequent clinical assessments after a diagnosis of dementia, which occurred at variable times after diagnosis based on local clinical policies and individual need. Clinicians were also asked to rate patients as having early-, middle- or late-stage dementia based on the overall clinical picture and their clinical judgement.
Where available, the latest recorded DLD score prior to (or at) dementia diagnosis and the DLD scores at first and second follow-up appointments after the diagnosis were compared between those prescribed and not prescribed acetylcholinesterase inhibitors or memantine.
Statistical analysis
Numerical data were summarised using mean, standard deviation or median and range depending on data distribution. Categorical data were summarised using count and percentages. We carried out simple analyses using the independent t-test or Mann–Whitney U-test, and the chi-squared test to compare the groups defined by dementia medication status. We used multivariable linear regression to assess the difference in DLD score between the groups, adjusting for pre-treatment values.Reference Altman 16
The primary outcome measure was death. Survival time was defined as the time in years between the date of diagnosis of Alzheimer's disease and the date of death. Participants were censored if they were alive or dead with no known date of death. We used the Kaplan–Meier method to estimate the median survival times. Survival time was defined as the time between dementia diagnosis and last assessment or date of death. Participants who were alive were censored at the date of the last clinic assessment. Participants who died were censored at the date of death. We censored the small number of participants who had died but for whom date of death was missing, at the date of the last clinic assessment. We used log-rank tests to evaluate the survival distributions of different groups (antidementia medication status and medication class).Reference Lee, Lee and Wang 17
We examined the effect of the following pre-defined set of potential confounding variables on survival: age at dementia diagnosis; gender; degree of intellectual disability; antipsychotic use; past history of depression (recorded diagnosis or prescription of antidepressant medication); current or past history of thyroid disease (recorded diagnosis or prescription of thyroid-specific medication); history of epilepsy preceding dementia diagnosis; and vision or hearing impairment.
Factors associated with death were analysed with Cox's proportional hazard model.Reference Harrell 18 We used 20% as the threshold for statistical significance in the univariable analysis to identify the variables that indicate a reasonable degree of association with the outcome and that were then added to the multivariable model.Reference Mickey and Greenland 19 We also used the backward elimination selection procedure with a 20% significance level to verify the stability of the variable selection process.Reference Ambler, Seaman and Omar 20 The final multivariable model included site, age at dementia diagnosis, gender and degree of intellectual disability. We tested the validity of the proportional hazard assumption by plotting log-minus-log survival curves and carrying out Schoenfeld tests.Reference Harrell 18
Statistical analyses were performed using Stata, version 13. No formal sample size calculation was carried out. It is recommended that at least ten events per estimated parameter are required in order to avoid the problem of overfitting, which can arise if the model contains fewer events compared with the number of variables in the Cox regression model.Reference Harrell 18 Missing data were not imputed.
Results
Data were collected from 18 psychiatry of intellectual disability clinical teams across four broad geographical regions. A total of 310 individuals with Down syndrome and dementia were included (174 male, 56.1%). Approximately a third (35.2%) of the cohort had mild–moderate intellectual disability (IQ range 35–69), a third (33.2%) had severe–profound intellectual disability (IQ <35), and the degree of intellectual disability was not recorded for the remainder (31.6%).
Descriptive characteristics of study participants
In total, 145 (47%) of the study participants were prescribed antidementia medication and 165 (53%) were not prescribed such medication (Table 1). Those prescribed antidementia medication were younger at diagnosis (53.8 v. 56.6 years, P = 0.0003) and a greater proportion had mild–moderate intellectual disability (P<0.0001).
Table 1 Comparison of baseline demographic and clinical characteristics by antidementia medication status of the study participants (n = 310)
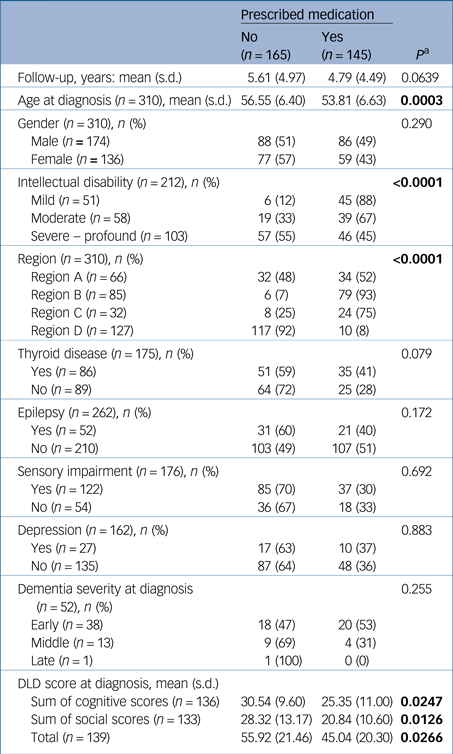
a. P-values obtained from: two-sample independent t-test, Mann–Whitney U-test, and chi-squared tests as appropriate for the scale and distribution of the variable. Results in bold are significant.
Total DLD score, SCS and the SSS at diagnosis, were significantly higher in the group not prescribed medication, indicating that this group had more severe symptoms of dementia at diagnosis. A greater proportion of those prescribed antidementia drugs were assessed as having early dementia by clinicians, and a greater proportion in the non-prescribed group had middle or late dementia, although the differences in these proportions were not statistically significant.
There was no significant difference in gender or rates of measured comorbidities between those prescribed and not prescribed antidementia medication.
Impact of acetylcholinesterase inhibitors or memantine on survival
The Kaplan–Meier survival curves following diagnosis of dementia for those prescribed and not prescribed antidementia drugs show a significant difference in survival time between those prescribed and not prescribed antidementia medication (Fig. 1(a)). Median survival time on any antidementia drug was 5.59 years (95% CI 4.67–6.67) whereas median survival time of those not prescribed any antidementia drug was 3.45 years (95% CI 2.91–4.13; log-rank test P<0.0001). Prescription of cholinesterase inhibitors, either alone or in combination with memantine, conferred a survival advantage; median survival time for those prescribed an acetylcholinesterase inhibitor was 6.15 years (95% CI 4.44–?.??). (The upper confidence limit in this case was not calculable from the data. The median survival time represents the time beyond which 50% of subjects are expected to survive. Confidence intervals for the median survival time are not based on standard errors but are obtained by inverting the respective 100 (1–alpha)% confidence interval for the survivor function. A missing upper confidence limit in this case indicates that this limit could not be determined. For these groups the estimated upper confidence limit of the survivor function never falls below 0.5.Reference Cleves 38 ) The median survival time of those people prescribed both memantine and an acetylcholinesterase inhibitor was 5.31 years (95% CI 3.65–6.10) (Fig. 1(b)). In contrast, median survival time on no medication was 3.45 years (95% CI 2.91–4.13, log-rank test P = 0.0001).

Fig. 1 Difference in survival time: (a) between those prescribed and not prescribed anti-dementia medication and (b) by anti-dementia drug type or no medication.
Factors that conferred a higher hazard of death during the observation period were, increased age at diagnosis, more severe intellectual disability and having pre-existing epilepsy (supplementary Table 1; available at http://doi.org/10.1192/bjp.2017.21). These were entered into a multivariable Cox regression (with region and gender as fixed factors) to investigate the effect of different variables on survival after a diagnosis of dementia (Table 2).
Table 2 Adjusted and unadjusted hazard ratio (HR) for death, derived from a Cox regression model

AChE, acetylcholinesterase inhibitor.
a. The model included: age at diagnosis, gender, region and level of intellectual disability.
b. Some participants were on two medications: donepezil and rivastigmine (n = 1), donepezil and galantamine (n = 1), and rivastigmine and galantamine (n = 7).
Those who were prescribed antidementia medication had a lower risk of death than those who were not prescribed antidementia medication, but this association was not statistically significant after accounting for confounding variables (adjusted hazard ratio 0.65, 95% CI 0.32–1.32, P = 0.235). There were no statistically significant differences between different classes of antidementia medication, although prescription of cholinesterase inhibitors (either alone or in combination with memantine) was consistently associated with a lower hazard ratio of death than prescription of memantine alone. There was no difference in hazard ratio of death between the different cholinesterase inhibitors. We conducted a sensitivity analysis where the regression model did not include level of intellectual disability as this variable included a high degree of missing data; in this analysis (including 254 people) the adjusted hazard ratio of death for those prescribed antidementia medication was 0.45 (95% CI 0.25–0.82, P = 0.009).
DLD score
The median time between the baseline and first assessment after diagnosis was 191 days (6.1 months, interquartile range (IQR)=139–303 days) and between first and second assessments was 183 days (5.9 months, IQR=166–233 days). The cognitive score (SCS) was significantly lower in the group that received medication at first follow-up assessment (indicating better cognitive functioning); this advantage was lost by the time of the second follow-up appointment. The sum of social scores (SSS, indicating functional abilities) showed no differences between the groups (Table 3).
Table 3 Estimate of the effects of anti-dementia medication on Dementia Questionnaire for People with Learning Disabilities (DLD) scores at first and second assessment, estimated from a multiple linear regression model
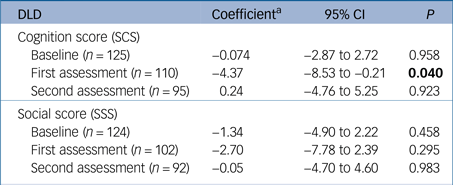
SCS, sum of cognitive scores; SSS, sum of social scores.
Result in bold is significant.
a. Regression coefficient adjusted for pre-treatment score. The coefficient represents the mean difference (on medication – not on medication).
Discussion
Summary of results
This observational study is the first to investigate survival of people with Down syndrome and Alzheimer's disease who were prescribed cholinesterase inhibitors and/or memantine. Survival analysis demonstrates an advantage in patients prescribed medication over patients who were not prescribed medication for Alzheimer's disease; however, when we accounted for the effect of confounders in a Cox regression model, the effect of antidementia medication prescription on survival was mitigated and became non-significant.
There was a trend in adjusted analyses for a survival advantage in those prescribed cholinesterase inhibitors compared with those prescribed memantine alone, who had a tendency to die sooner, although this finding needs to be interpreted with caution given the small number of people in the analysis. There were no significant differences between the different cholinesterase inhibitors (donepezil, galantamine and rivastigmine). Performing a sensitivity analysis that excluded degree of intellectual disability from the model enabled a larger sample size to explore the effect of missing data, although at the expense of removing a factor likely to influence survival, and in this analysis the positive effect of antidementia drugs on survival time was significant.
We considered the potential symptomatic benefit of cholinesterase inhibitors and memantine using the DLD, a validated carer-rated measure of function over several domains. The cognitive subscale of the DLD, covering short-term memory, long-term memory and orientation, showed a significantly difference at the first follow-up assessment (on average 6 months after the baseline) in favour of those prescribed medication. This difference was not mirrored by differences in the social (behaviour) subscale and had been lost by the second follow-up assessment (on average 12 months after baseline).
There were conspicuous differences in rates of prescribing between the four regions from which data were collected. Some of these differences may be accounted for by the different clinical characteristics of people seen by each team, although as each community team could be expected to see a broadly similar group of patients, the data also highlight likely variation in practice. This is perhaps not surprising given the lack of available evidence in this area on which clinicians can base decisions. There are currently no national- or regional-level data in the UK that compare rates of diagnosis of dementia or prescribing of antidementia drugs in people with intellectual disability, and the prevalence of prescribing is likely to have changed over the time period that our data were derived. The results suggest that care pathways should be standardised for this group to ensure equitable treatment, given some evidence of early benefit at the cognitive level.
Comparison with existing literature
The effect of antidementia drugs on survival in people without Down syndrome is uncertain; some studies report increased survival in people prescribed antidementia drugs,Reference Wu, Hu, Chow, Chou, Huang and Wang 21 , Reference Zhu, Livote, Scarmeas, Albert, Brandt and Blacker 22 yet other studies do not.Reference Suh, Ryu, Lee, Han, Ju and Kee 23 , Reference Rountree, Chan, Pavlik, Darby and Doody 24 A underlying mechanism by which antidementia medications might reduce mortality has not been established but it has been hypothesised that the effect is mediated by protective effects of the drugs on atherosclerotic cardiovascular risk.Reference Nordström, Religa, Wimo, Winblad and Eriksdotter 25 However, the relevance of such a mechanism to people with Down syndrome might be offset by the relatively low observed rates of atherosclerotic disease in this group.Reference Vis, Duffels, Winter, Weijerman, Cobben and Huisman 26
Our results accord with a number of existing small studies that have explored the symptomatic benefit of cholinesterase inhibitors for people with Down syndrome and Alzheimer's disease and shown potential for limited benefit of these drugs.Reference Lott, Osann, Doran and Nelson 27 – Reference Atri, Hendrix, Pejović, Hofbauer, Edwards and Molinuevo 31 The benefit of cholinesterase inhibitors has been reported to occur within the first 3–6 months of treatment,Reference Lott, Osann, Doran and Nelson 27 , Reference Kishnani, Sullivan, Walter, Spiridigliozzi, Doraiswamy and Krishnan 32 consistent with our own results. Our findings are also in keeping with recent evidence from clinical studies of cholinesterase inhibitors in adults with sporadic Alzheimer's disease, also showing a larger response during the first 6 months of treatment,Reference Perera, Khondoker, Broadbent, Breen and Stewart 33 and donepezil treatment decreased the annual rate of hippocampal atrophy in a recent randomised controlled trial in prodromal Alzheimer's disease.Reference Dubois, Chupin, Hampel, Lista, Cavedo and Croisile 34 Better cognitive functioning could permit people to attend to and better manage their physical health, thus improving survival.
Strengths and limitations of the study
This study adds to the limited evidence base on the effectiveness of antidementia medication in people with Down syndrome and Alzheimer's disease. It is the first study to provide outcome data comparing different classes of antidementia drugs, and on the use of galantamine in this population. Data were collected over several years from a range of real-world clinical services covering urban and rural locations. The people included have a range of concomitant health issues as might be expected in a population of ageing adults with Down syndrome and are likely to be representative of those who are known to specialist community services nationally.
The widespread acceptance and use of cholinesterase inhibitors and memantine in people with Down syndrome and Alzheimer's disease in contemporary clinical care 35 makes it difficult to justify a controlled drug trial where some participants do not receive these drugs and the observational design of our study is therefore appropriate in this context. Our results suggest significant cognitive benefit during the initial 6 months of treatment of cholinesterase inhibitors in individuals with Down syndrome who have been diagnosed with Alzheimer's disease, which requires further exploration.
There are certain limitations to this observational study. It is not possible to determine a causal relationship between prescription of cholinesterase inhibitors and survival. There were significant baseline differences between the groups prescribed and not prescribed antidementia medication. Those who were not prescribed medication were older, more likely to have severe–profound intellectual disability, and had more severe dementia symptoms at baseline. Although we adjusted for major measured confounders in the analysis, because of lack of randomisation the results might be subject to residual confounding and influenced by unknown or unmeasured variables that could account for some of the differences associated with medication treatment we have observed.
There are difficulties in recognising and diagnosing dementia in people with Down syndrome who have pre-existing cognitive impairment. It is possible that a small number of people may have been misdiagnosed; however, we have shown in a previous study that clinical diagnoses of dementia made in these specialist teams are valid and reliable.Reference Sheehan, Sinai, Bass, Blatchford, Bohnen and Bonell 10
Owing to this being a study using routine clinical data, there were missing data and variation in time points of assessment that limited our analyses and the strength of the conclusions that we are able to draw. We did not have data on the dose of medication or adherence with treatment. The low numbers of people prescribed memantine prevent us from drawing strong inferences based on the obtained results; however, our results are in keeping with a randomised controlled trial that has shown that this drug is not effective for Alzheimer's disease in Down syndrome.Reference Hanney, Prasher, Williams, Jones, Aarsland and Corbett 9
Further work is needed to investigate the effect of all antidementia drugs on a broader set of cognitive and functional outcomes, including longer-term outcomes such as time to admission to higher care settings.
Implications
While we await advances in the understanding of the pathophysiology of Alzheimer's disease relevant to people with Down syndrome that might permit interventions to delay the onset or even prevent it,Reference Wiseman, Al-Janabi, Hardy, Karmiloff-Smith, Nizetic and Tybulewicz 3 identifying optimum treatment for those living with the disease must be a priority. Notwithstanding the limitations of this study, the results of this work lend support to the prescription of cholinesterase inhibitors as first-line pharmacological intervention for people with Down syndrome and Alzheimer's disease and suggest that they might increase survival and reduce the rate of cognitive decline in the early stages after diagnosis. Although people with Down syndrome are likely to have comorbid health conditions as they age, cholinesterase inhibitors are relatively safe in this population,Reference Lott, Osann, Doran and Nelson 27 , Reference Kishnani, Spiridigliozzi, Heller, Sullivan, Doraiswamy and Krishnan 36 and anecdotal evidence suggests that adverse side-effects should not be a barrier to prescription if supplemented by regular monitoring.
Alzheimer's disease is a major concern for people with Down syndrome who are increasingly living into older age. Dementia has wide-ranging implications for the person affected, their family and paid carers, and the system of medical and social supports that are likely to be required as the disease progresses.Reference Janicki and Dalton 37 Early diagnosis and improved care pathways are likely to have a positive impact on the provision of other aspects of care. A clinical trial of treatment with cholinesterase inhibitors during the early symptomatic stages of cognitive decline in Down syndrome before a formal dementia diagnosis should be considered to test whether early response can be maximised, and to determine if cognitive benefit is related to functional improvement and is of cost–benefit.
Supplementary material
Supplementary material is available online at http://doi.org/10.1192/bjp.2017.21.
Funding
The Baily Thomas Charitable Fund.
Acknowledgements
The authors wish to thank the clinicians and services that submitted data to the ADSID database.







eLetters
No eLetters have been published for this article.