Bipolar disorder is a prevalent and disabling mental disorder.Reference Merikangas, Jin, He, Kessler, Lee and Sampson1 Bipolar disorder is associated with mild cognitive impairments,Reference Roux, Etain, Cannavo, Aubin, Aouizerate and Azorin2,Reference Mann-Wrobel, Carreno and Dickinson3 which persist during periods of euthymia, with a prevalence between 4 and 67%Reference Roux, Etain, Cannavo, Aubin, Aouizerate and Azorin2 defined at usual clinically defined thresholds. Patients with bipolar disorder also experience deficits in psychosocial functioning, which persist during periods of euthymia.Reference Rosa, Reinares, Michalak, Bonnin, Sole and Franco4 Residual depressive symptoms are crucial determinants of functioning,Reference Marangell, Dennehy, Miyahara, Wisniewski, Bauer and Rapaport5 and a growing body of evidence suggests the role of cognition as a determinant of functioning in bipolar disorder.Reference Depp, Mausbach, Harmell, Savla, Bowie and Harvey6 Over the past few decades, mental health research has endorsed the perspective of recovery and completed its focus on symptom remission with functional rehabilitation. Several treatment approaches have been proposed to promote functional recovery in euthymic bipolar disorder,Reference Sanchez-Moreno, Martinez-Aran and Vieta7 such as enhancing cognition. However, there is no evidence that enhancing cognition would improve functioning. One randomised controlled trial reported that cognitive remediation produced significant improvements in patients with interepisode bipolar disorder with psychotic features for several cognitive domains.Reference Lewandowski, Sperry, Cohen, Norris, Fitzmaurice and Ongur8 However, this study failed to prove a significant improvement in functioning despite functional and cognitive changes being associated. They did not test the direction of the relationship between these two variables. Other studies also report that cognition predicts later functioning over various durations of follow-up.Reference Bonnin, Martinez-Aran, Torrent, Pacchiarotti, Rosa and Franco9–Reference Tabares-Seisdedos, Balanza-Martinez, Sanchez-Moreno, Martinez-Aran, Salazar-Fraile and Selva-Vera11 Again, none of these studies tested the alternative hypothesis that functioning would predict later cognitive performances or compared the strength of the cross-lagged relationships. An additional approach consists of directly targeting functioning with a specific psychosocial rehabilitation program, such as functional remediation, which aims to develop cognitive strategies, psychoeducation about cognition, and problem-solving in the context of everyday life. This type of intervention showed efficacy in improving functional outcome in euthymic bipolar disorder relative to control conditions, but it did not improve cognitive performance better than in the control conditions.Reference Torrent, Bonnin, Martínez-Arán, Valle, Amann and González-Pinto12 A follow-up evaluation reported that the functional improvement persisted over time, along with verbal memory improvements, which were correlated with the total functioning score only in the functional remediation group.Reference Bonnin, Torrent, Arango, Amann, Sole and Gonzalez-Pinto13 Hence, a crucial clinical point is to know whether cognitive remediation should precede or follow functional remediation in bipolar disorder. According to a bottom-up model (neurocognitive processes precede psychosocial consequences), cognitive remediation should precede functional remediation, whereas a top-down model (impaired functioning leads to cognitive difficulties through non-specific factors, such as lack of motivation) advocates for the reverse sequence. Further research on the longitudinal relationship between cognition and functioning in bipolar disorder is warranted, as it may help clinicians adapt the treatment of their patients and provide new elements about the dynamics of this relationship. We aimed to study this relationship in a large cohort of euthymic patients with bipolar disorder, using structural equation modelling, with the main hypothesis that neurocognition would predict later functioning, whereas the reverse would not be true.
Method
Study design and characteristics of the recruiting network
This multicentre longitudinal study included patients recruited into the FondaMental Advanced Centers of Expertise for Bipolar Disorders (FACE-BD) cohort within a French national network of ten centres (Bordeaux, Colombes, Créteil, Grenoble, Marseille, Monaco, Montpellier, Nancy, Paris and Versailles). This network was set up by the Fondation FondaMental (www.fondation-fondamental.org), who created an infrastructure and provided resources to follow clinical cohorts and comparative-effectiveness research in patients with bipolar disorder.
Participants
The diagnosis of bipolar disorder was based on the Structured Clinical Interview for DSM-IV-TR (SCID) criteria.14 Out-patients with type 1, type 2 or bipolar disorder not otherwise specified, aged between 18 and 65 years, were eligible for this analysis. All patients included in the analyses were euthymic at the three times of testing (T0: inclusion, T12: 12 months, T24: 24 months), according to the DSM-IV-TR criteria, with scores on the Montgomery–Asberg Depression Rating Scale (MADRS) ≤ 10Reference Montgomery and Asberg15 and the Young Mania Rating Scale (YMRS) < 12.Reference Young, Biggs, Ziegler and Meyer16 This cut-off was chosen to conform to previous recommendations about euthymia threshold with the same tools.Reference Jamrozinski, Gruber, Kemmer, Falkai and Scherk17 Patients who met the following criteria at any time of testing were excluded: history of neurological disorder, dyslexia, dysorthographia, dyscalculia, dysphasia, dyspraxia, substance-related disorders in the previous month (except tobacco use) or electroconvulsive therapy in the past year.
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving human patients were approved by the local ethics committee (Comité de Protection des Personnes Ile de France IX) on 18 January 2010, under French laws for non-interventional studies (observational studies without any risk, constraint or supplementary or unusual procedure concerning diagnosis, treatment or monitoring). The board required that all patients be given an informational letter but waived the requirement for written informed consent. However, verbal consent was witnessed and formally recorded.
Assessment tools
The following sociodemographic variables were collected at T0: gender, age, education level, employment status, independent housing, marital status and judiciary protection.
Clinical assessments at T0, T12 and T24
The following clinical variables were recorded using the SCID: age at onset of bipolar disorder, number and type of previous mood episodes, subtype of bipolar disorder and history of psychotic symptoms. Predominant polarity was determined following previous recommendations.Reference Mazzarini, Pacchiarotti, Colom, Sani, Kotzalidis and Rosa18 The Clinical Global Impression – Severity scale assessed the severity of the disease.Reference Guy and Guy19 We used a yes/no questionnaire for recording patient treatment at the time of evaluation: lithium carbonate, anticonvulsants, antipsychotics, antidepressants or anxiolytics. Mania was measured by YMRS. Depression was measured by MADRS. Psychosocial functioning was measured by the Functioning Assessment Short Test (FAST), which encompasses six domains: autonomy, occupational functioning, cognition, financial issues, interpersonal relationships and leisure.Reference Rosa, Sánchez-Moreno, Martínez-Aran, Salamero, Torrent and Reinares20
Battery of cognitive tests at T0 and T24
Experienced neuropsychologists administered the tests in a fixed order that was the same for every centre. Testing lasted approximately 120 min, including 5–10-min breaks. The standardised test battery complied with the recommendations of the International Society for Bipolar Disorders.Reference Yatham, Torres, Malhi, Frangou, Glahn and Bearden21 This evaluation was not performed at T12 (neuropsychological assessments are planned to be performed every two years in the design of the cohort. Such spacing was decided to minimize practice effects). It included 11 tests, among which five were subtests from the Wechsler Adult Intelligence Scale (WAIS) version IIIReference Wechsler22 or version IV,Reference Wechsler, Coalson and Raiford23 as the French version of the WAIS-IV was used as it became available. The battery evaluated six domains:
(a) Verbal memory: California Verbal Learning TestReference Delis24 short and long delay free recall and total recognition;
(b) Working memory: WAIS digit span (total score) and spatial span (forward and backward scores) from the Wechsler Memory Scale version III;Reference Wechsler25
(c) Executive functions: colour/word condition of the Stroop test,Reference Golden26 semantic and phonemic verbal fluencyReference Lezak and Lezak27 and Trail-Making Test (TMT) part B;Reference Reitan28
(d) Processing speed: digit symbol coding (WAIS-III) or coding (WAIS-IV), WAIS symbol search and TMT part A;
(e) Attention: Conners’ Continuous Performance Test II (omissions and detectability);Reference Conners and Staff29
(f) Verbal and perceptual reasoning: WAIS vocabulary and matrices.
Raw scores were transformed to demographically corrected standardised z-scores based on normative data.Reference Golden26,Reference Conners and Staff29–Reference Roussel and Godefroy31 Higher scores reflected better performance. Participants with >37.5% of missing neuropsychological data were excluded.Reference Reichenberg, Harvey, Bowie, Mojtabai, Rabinowitz and Heaton32 Some data obtained using this battery have been published previously.Reference Roux, Raust, Cannavo, Aubin, Aouizerate and Azorin33 We computed a mean score for each cognitive domain.
Statistical analyses
First, we compared the patients who completed the 2-year follow-up and those who dropped out, using Welch's t-tests for continuous variables and χ2 tests for categorical variables at baseline. Then, we sought a main effect of the time of testing on cognition, functioning and MADRS scores using linear mixed models, with subject as a random effect. When significant effects were found, we ran post hoc Bonferroni pairwise comparisons. Effect sizes for t-tests were computed with Cohen's d (difference of the means, divided by pooled s.d.) using 0.2, 0.5 and 0.8 as lower bounds for small, medium and large effects, and effect sizes for χ2 tests were computed with Cramer's V.
Evaluation of the measurement invariance of latent variables
Cognition was defined as a latent variable with six indicators (cognitive domains) and functioning was defined as a latent variable with six indicators (domains evaluated by FAST). We tested their longitudinal invariance to ensure that the constructs remained equally reliable across time, thus permitting their inclusion in our models (details in Supplementary material available at https://doi.org/10.1192/bjp.2019.180).
Longitudinal structural models
We used structural equation modelling with a cross-lagged panel design,Reference Little34 using lavaan Reference Rosseel35 in R (version 3.5.1, on Windows 10). Missing data were managed with full-information maximum likelihood. We used a robust maximum likelihood estimator. The required sample size was estimated following the procedure described in Supplementary material.
We computed Pearson's correlations between the variables of interest. The models included cognition and functioning as latent variables and MADRS was included as a covariate to control for depressive symptoms. Cognition and functioning were allowed to be concurrently correlated and indicators in latent variables were allowed to correlate with themselves at other time points to account for autocorrelations due to repeated measures. We examined consensual fit indices with recommended cut-off criteria for good fitReference Hu and Bentler36: Comparative Fit Index (CFI)Reference Bentler37 and Tucker–Lewis Index (TLI)Reference Tucker and Lewis38 > 0.95, root-mean-square error of approximation (RMSEA) ≤ 0.05 (P of close-fit > 0.05, and 90% CI) and standardised root-mean-square residual (SRMR) < 0.08. We tested robustness by calculating the expected value of the cross-validation index (ECVI); a lower ECVI indicates greater robustness.Reference Browne and Cudeck39
We followed the procedure used by De Jonge et al.Reference Jonge, Dormann, Janssen, Dollard, Landeweerd and Nijhuis40 We compared successive models to test the existence, magnitude and significance of different potential directed relationships between cognition and functioning:
(a) Autoregressive model: only longitudinal autoregressive paths (CT0 → CT24, FT0 → FT12 → FT24), no longitudinal relationship between cognition and functioning;
(b) Expected model: autoregressive + paths CT0 → FT12 and CT0 → FT24, cognition affects functioning at further time points;
(c) Reverse model: autoregressive + paths FT0 → CT24 and FT12 → CT24, functioning affects cognition at further time points;
(d) Reciprocal model: expected + reverse model, cognition and functioning affect each other at different time points.
Models were compared using χ2 tests. First, we compared the expected, reverse and reciprocal models to the autoregressive model to retain the model(s) that fit the data significantly better than the autoregressive model. Then, we compared the reciprocal model with the previously retained unidirectional model(s). We discarded the reciprocal model if it did not fit the data better than the retained unidirectional model.
Results
Participants
We included 887 participants between January 2009 and October 2015. The selection procedure is presented in the Supplementary material. A total of 55.2% of participants were lost during the follow-up. The final sample included 272 patients (in accordance with the estimated required sample size). Sociodemographic, clinical and neuropsychological characteristics of the sample are reported in Table 1.
Table 1 Sociodemographic, clinical and neuropsychological characteristics of the sample at T0

Cognitive scores are reported with a * when the group performance is significantly below the theoretical mean (0).
MADRS, Montgomery–Asberg Depression Rating Scale; YMRS, Young Mania Rating Scale; FAST, Functional Assessment Short Test; CGI, Clinical Global Impression.
We found several very small to small differences between completers and non-completers. The non-completers were younger; less educated; more frequently under treatment; had worse functioning on cognition, finance and interpersonal subscores; were less frequently married and living independently; and performed worse in verbal and perceptual reasoning (see Supplementary Table 1).
Measurement invariance of latent variables: cognition and functioning
The confirmatory factor analysis run on cognition at T0 yielded good fit indices: CFI = 0.985, TLI = 0.972, RMSEA ≤ 0.05 (P-value = 0.52, 90% CI = 0–0.09), SRMR = 0.036. The confirmatory factor analysis run on functioning at T0 also yielded good fit indices: CFI = 0.999, TLI = 0.998, RMSEA ≤ 0.05 (P-value = 0.71, 90% CI = 0–0.09), SRMR = 0.021. All factor loadings were significant.
Cognition achieved scalar invariance (constrained structure, factor loadings and intercepts) with good fit: CFI = 0.97, TLI = 0.965, RMSEA ≤ 0.05 (P-value = 0.49, 90% CI = 0–0.08), SRMR = 0.05. Functioning also achieved scalar invariance, with good fit: CFI = 0.989, TLI = 0.985, RMSEA ≤ 0.05 (P-value = 0.90, 90% CI = 0–0.06), SRMR = 0.041.
Comparisons of observed variables between T0, T12 and T24
We found overall cognitive (except for attention) and functional improvements, with very small to small effect sizes. Most measures improved between baseline and T12 and then remained stable between T12 and T24 (see Supplementary Table 2). Latent cognition (βT24–T0 = 0.17, z = 3.02, P = 0.002) and latent functioning (βT24–T0 = −0.42, z = 4.29, P < 0.001) improved from T0 to T24. More precisely, functioning improved from T0 to T12 (βT12–T0 = −0.39, z = −3.53, P < 0.001) but not from T12 to T24 (βT12–T0 = −0.02, z = −0.3, P = 0.77).
Model comparisons
Zero-order correlations are presented in Supplementary Table 3. The proportion of missing data in the model was 11.9%. Model comparisons are reported in Table 2. The expected model fit the data significantly better than the autoregressive model (P < 0.001), whereas the reverse model did not (P = 0.265). The reciprocal model, which fit the data significantly better than the autoregressive model (P < 0.001), did not fit the data significantly better than the expected model (P = 0.661). The model which had the best fit indices was the expected model (see Table 3). Hence, we retained the expected model (autoregressive paths and directed paths from cognition at baseline to functioning at later time points (Fig. 1).
Table 2 Pairwise comparisons of the models
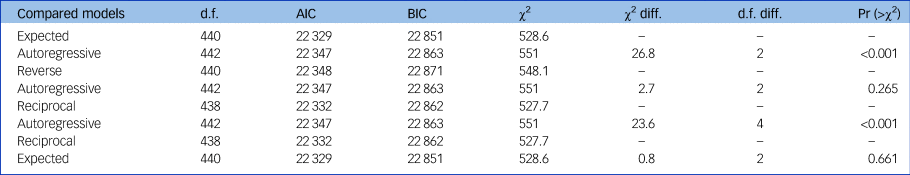
d.f., degrees of freedom; AIC, Akaike Information Criterion; BIC, Bayesian Information Criterion; diff., difference; Pr, probability.
Table 3 Fit indices of the models tested

CFI, Comparative Fit Index; TLI, Tucker-Lewis Index; RMSEA, Root Mean Square Error of Approximation; SRMR, Standardized Root Mean Squared Residual; ECVI, Expected Cross-Validation Index.

Fig. 1 Final structural equation model with standardised path coefficients.
For readability, the names of indicators are truncated. Rectangles indicate the observed variables, ovals the latent variables, single-headed arrows the regressions (freely estimated regression weight), double-headed arrows the correlations or covariances. For readability, the serial correlations between indicators in the latent variables were not reported in the Figure but were indeed estimated in the model. The squared multiple correlation R 2 value for the dependent variables is presented above them. Significance levels are as follows: *** P < 0.001, ** P < 0.01, * P < 0.05.
A, autonomy; Att, attention; C, cognition; EF, executive functions; F, finances; IP, interpersonal relationship; L, leisure; MADRS, Montgomery–Asberg Depression Rating Scale; PS, processing speed; T0, inclusion; T12, 12 months; T24, 24 months; VM, verbal memory; VPR, visual and perceptual reasoning; W, work; WM, working memory.
Description of the retained model
The model explained 58% of the variance in functioning. We report estimated parameters in Supplementary Table 4. The path from baseline cognition to functioning at T12 was significant, suggesting that baseline cognitive performance predicts functioning at T12 (ßstd. = 0.37, z = 3.68, P < 0.001) after controlling for depressive symptoms. However, functioning at T24 was not predicted by baseline cognition (ßstd. = 0.05, z = 0.35, P = 0.73). Cognition and functioning were concurrently associated (ßstd. = 0.48, z = 2.23, P = 0.03) at T24, but not T0.
Depressive symptoms and functioning were concurrently associated at each assessment: higher depression was associated with worse functioning (ßstd. between 0.39 and 0.54, P < 0.001). Cognition and functioning were relatively stable across time, with large significant autoregressive coefficients. We tested whether controlling for residual manic symptoms would change the results. Adding YMRS score as a covariate led to a significant drop in model fit (χ2 P = 0.045), and did not alter the relationships between cognition and functioning.
It is possible that the path from functioning at T12 to cognition at T24 was not significant because of the improvement of functioning between T0 and T12, which might have reached a plateau, obscuring a putative influence on cognition. We tested this hypothesis by running additional analyses with only two time points (T0 and T24), ignoring T12. The results were similar to those obtained with three time points: we found a significant relationship from cognition to functioning in the expected model (ßstd. = 0.2, z = 2.34, P = 0.02), which was again the best-fitting model. These results confirm the unidirectional nature of the relationship between cognition and functioning and suggest that the link between baseline cognition and functioning at T24 was fully mediated by functioning at T12.
Discussion
Main findings and comparison with other studies
Our study is the first to use longitudinal structural equation modelling to examine the relationships between cognition and psychosocial functioning in bipolar disorder. There was a significant moderate effect of baseline neurocognition on psychosocial functioning 12 months after inclusion, after controlling for residual depressive symptoms and previous functional state, as hypothesised. In contrast, psychosocial functioning had no significant effect later cognition. The model that best fit the data was that which included a relationship from cognition to functioning but not the reverse. This result confirms our hypothesis, according to which cognition is a determinant of functioning. This result suggests that causal effects move from primary impairments in biological processes to functional processes. The data reported here suggest that the predictive power of baseline cognition for later functioning weakened after 12 months.
Other longitudinal studies have found comparable relationships between cognition and functioning despite important methodological differences. Several studies found that cognition, especially verbal memory, may predict functional outcome after 1,Reference Tabares-Seisdedos, Balanza-Martinez, Sanchez-Moreno, Martinez-Aran, Salazar-Fraile and Selva-Vera11 4Reference Bonnin, Martinez-Aran, Torrent, Pacchiarotti, Rosa and Franco9 and 15 years.Reference Burdick, Goldberg and Harrow10 However, the sample size of these studies was limited (<50) and all patients were not euthymic at follow-up. In a transdiagnostic longitudinal study, Lee et al. Reference Lee, Hermens, Redoblado-Hodge, Naismith, Porter and Kaur41 found that baseline cognition predicted functioning a year later among patients with depression, bipolar disorder or psychosis; the strength of the relationship between baseline cognition and functioning 1 year later (ßstd. = 0.33) was comparable with our results (ßstd. = 0.37). Several studies on schizophrenia also suggested a causal relationship from cognition to functioning, but not from functioning to cognition, using cross-lagged panel models.Reference Horan, Green, DeGroot, Fiske, Hellemann and Kee42
Our study also showed that residual depressive symptoms were significantly associated with functioning at every evaluation, but not with cognition. This is in accordance with previous research establishing that depressive symptoms are important determinants of functioning, even in euthymic patients.Reference Marangell, Dennehy, Miyahara, Wisniewski, Bauer and Rapaport5 The absence of significant association between cognition and depression is at variance with previous studies.Reference Bonnín, González-Pinto, Solé, Reinares, González-Ortega and Alberich43 This might be explained by methodological discrepancies: use of different depression scales and different criteria for euthymia. We used MADRS, which mainly focuses on core depressive symptoms and functional impairment. Therefore, it might be less sensitive to assessing subsyndromal depressive symptoms affecting cognition, such as depressive cognitive attitudes, but be sensitive enough to show a link between residual depression and functioning. Other scales such as the Beck Depression Inventory – II were shown to be more sensitive to establishing a significant relationship between residual depression and cognition than MADRS.Reference Volkert, Kopf, Kazmaier, Glaser, Zierhut and Schiele44 Further studies should confirm our results with alternative depression scales.
We also found overall small cognitive and functional improvements over time. Practice effects cannot be ruled out, as the cognitive improvement in our study (mean Cohen's d = 0.31) was comparable with the practice effect size (d = 0.33) found in a study of neurocognitive change in individuals with schizophrenia and controls who took neurocognitive tests at 6-week intervals.Reference Goldberg, Goldman, Burdick, Malhotra, Lencz and Patel45 The recruitment of a control group would have allowed to control for the impact of practice effect on the small cognitive improvement we found. In addition, centres of expertise provide patients with personalised recommendations concerning disease management and treatment, which might have contributed to the improvement of their clinical and functional outcomes. The magnitude of cognitive improvement reported here was greater than that found from cognitive remediation intervention,Reference Lewandowski, Sperry, Cohen, Norris, Fitzmaurice and Ongur8 whereas the magnitude of functional improvement was smaller for cognitive, interpersonal, autonomy and occupational domains than that found from functional remediation interventions.Reference Torrent, Bonnin, Martínez-Arán, Valle, Amann and González-Pinto12 The sample we have recruited was not severely impaired, as 77.8% of participants were employed and the mean FAST score (12.7) was just above the threshold for impairment.Reference Tabares-Seisdedos, Balanza-Martinez, Sanchez-Moreno, Martinez-Aran, Salazar-Fraile and Selva-Vera11 Moreover, cognitive performance was only mildly impaired. Further studies of more severely impaired patients are warranted to confirm our findings.
Finally, our results support the temporal stability of the six-factor structure of FAST, demonstrating the reliability of this measure.
Limitations
Our study had several limitations. The cross-lagged panel design was incomplete: our study would have benefited from an intermediary neuropsychological assessment at T12, which would have provided more information about the dynamics of change, as our analysis of reciprocal relationships between cognition and functioning was not based on the same temporal segment. However, an additional neuropsychological evaluation would have magnified the practice effect. Importantly, additional analyses revealed that the results remained consistent when the analysis was run on the same temporal segment (between baseline and T24, omitting T12). It is not possible to directly infer causality from cross-lagged panel modelling. Although our results support a unidirectional relationship between cognition and functioning, we cannot rule out the putative effect of another unstudied variable. The relationship should be further investigated using alternative designs, such as randomised clinical trials that actively manipulate cognition and functioning through cognitive and functional remediation. Cross-lagged panel models do not allow for disentangling the within-individual process and between-individual differences; one strategy to account for this would be to use derived models, such as random intercept cross-lagged panel models.Reference Hamaker, Kuiper and Grasman46 Moreover, there was a global trend of improvement in cognition and functioning during the follow-up. The results should thus be replicated in samples with stable or declining cognition and functioning, or with dual change score models, which allow for dividing change into a constant change (overall rate of change across all time points) and a proportional change (depending on the adjacent measurement occasions).Reference Mund and Nestler47 These two alternative methods require at least three evaluations, whereas cognition was only measured twice in our study.
Furthermore, our results may only generalise to euthymic adult out-patients with bipolar disorder, as a consequence of our inclusion criteria. Functional assessment could have benefited from additional sources of information and more objective measures, e.g. from relatives or structured evaluation of objective functional performance in situ. FAST indeed measures the clinician's subjective appraisal about patient functioning based on what the patient reports during a structured interview. Furthermore, medication was not included in the models, although it has been reported to have an impact on cognition in bipolar disorder.Reference Roux, Etain, Cannavo, Aubin, Aouizerate and Azorin2 We only included in our models the concurrent level of depressive symptoms measured by MADRS, and did not include potential mood episodes that could have occurred between evaluations.
A final drawback was the loss of more than half of the patients to follow-up. No survey was proposed to the non-completers; it was thus impossible to investigate the reasons for such attrition. However, the differences between completers and non-completers were very small to small, suggesting a minor attrition bias.
Clinical implications
These findings highlight that improvement in functioning depends on a set of influential factors that start with cognition. Our results also suggest that interventions seeking to improve functioning should be based on a neuropsychological assessment. Our study supports the potential value of cognitive improvement for patients with bipolar disorder to alleviate long-term functional disability. Aside from psychoeducation, the two most promising psychosocial interventions in bipolar disorder are cognitiveReference Lewandowski, Sperry, Cohen, Norris, Fitzmaurice and Ongur8 and functional remediation.Reference Torrent, Bonnin, Martínez-Arán, Valle, Amann and González-Pinto12 However, little is known about the optimal temporal sequence for these interventions. Our results may be compatible with a service model of staged interventions that aims to improve cognitive performances before or simultaneously with functional remediation, because functional improvement is expected from cognitive improvement, whereas no cognitive improvement is expected from functional remediation. We call for cross-over randomised controlled trials to evaluate the extent to which the cognitive followed by functional remediation sequence is the best option to improve psychosocial and cognitive functioning over time.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.180.
Funding
This work was supported by the Centre Hospitalier de Versailles, Fondation FondaMental, Créteil, France; and the Investissements d'Avenir Programs managed by the Agence Nationale de la Recherche (references ANR-11-IDEX-0004-02 and ANR-10-COHO-10-01).
Acknowledgements
All co-authors were invited to proofread and amend the manuscript. We thank the Centre Hospitalier de Versailles and William Hempel (Alex Edelman & Associates) for editorial assistance.
The FACE-BD Collaborators are listed as follows: FACE-BD Clinical Coordinating Centre (Fondation FondaMental): B. Etain, E. Olié and M. Leboyer; FACE-BD Data Coordinating Centre (Fondation FondaMental): V. Barteau, O. Godin, H. Laouamri and K. Souryis. FACE-BD Clinical Sites and Principal Collaborators in France are listed as follows: AP-HP, DHU PePSY, Pôle de Psychiatrie et ’Addictologie des Hôpitaux Universitaires H Mondor, Créteil: S. Hotier, A. Pelletier and J.P Sanchez; AP-HP, GH Saint-Louis–Lariboisière–Fernand Widal, Pôle Neurosciences, Paris: F. Bellivier, M. Carminati, B. Etain, E. Marlinge and J. Meheust; Hôpital C. Perrens, Centre Expert Trouble Bipolaire, Service de Psychiatrie Adulte, Pôle 3-4-7, Bordeaux: B. Antoniol, A. Desage, S. Gard, A. Jutant, K. Mbailara, I. Minois and L. Zanouy; Département d'Urgence et Post Urgence Psychiatrique, CHRU Montpellier, Montpellier: S. Bonnet, F. Coppola, P. Courtet, D. Ducasse, M. Gachet, L. Matos, F. Molière, B. Noisette, E. Olié and G. Tarquini; Département de Psychiatrie, Hôpital Sainte Marguerite, Marseille: J. M. Azorin, R. Belzeaux, N. Corréard, J. L. Consoloni, F. Groppi, L. Lescalier. J. Montant and N. Viglianese; Service de Psychiatrie et Psychologie Clinique, CHU de Nancy, Hôpitaux de Brabois, Vandoeuvre Les Nancy: R. Cohen, J.P. Kahn, P. Kieffer and O. Wajsbrot-Elgrabli; Clinique Universitaire de Psychiatrie, CHU de Grenoble et des Alpes, Grenoble: T. Bougerol, B. Fredembach, S. Garçon, P. Grignon, A. Perrin and M. Polosan; Centre Hospitalier de Versailles, Service Universitaire de Psychiatrie d'adultes, Le Chesnay: A.M. Galliot, I. Grévin, A.S. Cannavo, N. Kayser, M. Lardinois C. Passerieux and P. Roux; Service de Psychiatrie, Centre Hospitalier Princesse Grace, Monaco: V. Aubin, I. Cussac, M.A Dupont, J. Loftus and I. Medecin.







eLetters
No eLetters have been published for this article.