Formerly, when asthma was considered a psychosomatic disorder, it was believed that asthma and psychotic illness were mutually exclusive and hence they could not coexist. Reference Green1 Although it is now recognised that people with psychosis have numerous comorbid medical conditions, such as type 2 diabetes Reference Suvisaari, Perala, Saarni, Harkanen, Pirkola and Joukamaa2 and osteoporosis, Reference Partti, Heliövaara, Impivaara, Perälä, Saarni and Lönnqvist3 there is little information on the prevalence of respiratory diseases in individuals with psychosis. However, there are several studies that have reported excess mortality from respiratory diseases in people with schizophrenia. Reference Saha, Chant and McGrath4–Reference Crump, Winkleby, Sundquist and Sundquist6 Although most of the studies have not specified the individual respiratory causes of death, one replicated finding seems to be elevated mortality from pneumonia. Reference Brown, Kim, Mitchell and Inskip5,Reference Crump, Winkleby, Sundquist and Sundquist6 According to the few existing studies, serious mental illness might also be associated with an increased risk of chronic obstructive pulmonary disease (COPD) and asthma. Reference Crump, Winkleby, Sundquist and Sundquist6–Reference Hsu, Chien, Lin, Chou and Chou11 Most of the studies have relied on register-based diagnoses of both respiratory and psychiatric disorders. There are only a few reports that have measured lung function with spirometry in people with psychotic disorders. Reference Filik, Sipos, Kehoe, Burns, Cooper and Stevens12–Reference Vancampfort, Probst, Stubbs, Soundy, De Herdt and De Hert14 Smoking is prevalent among individuals with schizophrenia Reference Dalack, Healy and Meador-Woodruff15,Reference Kalman, Morissette and George16 and is considered the most important cause of COPD. 17 Other conceivable risk factors for respiratory disease in individuals with psychosis include obesity and type 2 diabetes, both of which have been associated with restrictive lung disease. Reference Wannamethee, Shaper and Whincup18,Reference Van den Borst, Gosker, Zeegers and Schols19
We utilised data from a general population survey representative of the Finnish adult population, Reference Aromaa and Koskinen20 a part of which was a substudy of psychotic disorders, which were diagnosed utilising the Research Version of the Structured Clinical Interview for DSM-IV-TR (SCID-I) Reference First, Spitzer, Gibbon and Williams21 and medical records. The aims of the study were: (a) to compare lung function as measured by spirometry between people with psychosis and the general population, (b) to investigate whether psychotic disorders are associated with lower spirometry values after controlling for common risk factors for reduced lung function, (c) to examine the prevalence of asthma, COPD, chronic bronchitis and pneumonia in individuals with psychotic disorders, (d) to investigate whether psychotic disorders are associated with elevated serum cotinine levels.
Method
Study design and sample
The study is based on the Health 2000 Survey, a nationwide health examination study conducted in Finland between September 2000 and June 2001. Reference Aromaa and Koskinen20,Reference Heistaro22 The two-stage cluster sample comprised 8028 participants aged 30 or over (3637 men (45.3%) and 4391 women (54.7%)) and was stratified to represent the adult population of Finland. Participants aged 80 or over were oversampled (2:1) with relation to their proportion in the population. The survey consisted of a home interview and a comprehensive health examination carried out by specially trained personnel in the local health centre, or a condensed interview and health examination of non-respondents at home. Supplementary information was collected with questionnaires. In addition, register information on the whole sample was available. Participation in different measurements and phases of the survey is presented in detail in online Table DS1. Study participants gave written informed consent, and the Ethics Committee of the Hospital District of Helsinki and Uusimaa approved the survey.
Diagnostic assessment of psychotic disorders
Psychotic disorders were diagnosed in a substudy of the Health 2000 survey; the diagnostic process has been previously described in detail. Reference Perälä, Suvisaari, Saarni, Kuoppasalmi, Isometsä and Pirkola23 We screened the participants of the Health 2000 sample for a possible psychotic disorder, and interviewed screen-positive and a random sample of screen-negative participants with the SCID-I. Reference First, Spitzer, Gibbon and Williams21 The participants were screened from the total sample if they received a diagnosis of a possible or definite psychotic disorder from the physician in the health examination, if they reported having been diagnosed with a psychotic disorder, or if they had symptoms suggestive of a psychotic or bipolar I disorder in the Composite International Diagnostic Interview (CIDI), Reference Wittchen, Lachner, Wunderlich and Pfister24 conducted as part of the health examination. In addition, participants were screened from several healthcare registers on the basis of: hospital treatment with a diagnosis of psychotic disorder, disability pension for psychotic disorder, reimbursed antipsychotic medication or prescribed mood-stabilising medication without a neurological diagnosis.
In total, 63.4% of the participants who were screened positive based on the preliminary data participated in the SCID-I. The participants who did not participate in the SCID-I were diagnosed by means of hospital and out-patient medical records for all lifetime treatments in psychiatric and primary healthcare units. Patient records were also acquired for the participants who participated in the SCID-I. Final best-estimate DSM-IV-TR-based 25 diagnoses were made using all available information by three clinicians (J.S., J.P., S.I.S.), between whom kappa values ranged from 0.74 to 0.97 for different psychotic disorders. Reference Perälä, Suvisaari, Saarni, Kuoppasalmi, Isometsä and Pirkola23
In this article, lifetime-ever primary psychotic disorders are grouped into schizophrenia, other non-affective psychoses (schizophreniform disorder, schizoaffective disorder, delusional disorder, brief psychotic disorder, psychotic disorder not otherwise specified) and affective psychoses (major depressive disorder with psychotic features and bipolar I disorder).
Spirometry measurements
Respiratory function was measured with a Vitalograph 2150 spirometer (Vitalograph Ltd, Buckingham, UK) as part of the health examination. The measurements were performed by specially trained technicians following international guidelines and instructions. The procedures used have been outlined in detail elsewhere. Reference Heistaro22,Reference Vasankari, Impivaara, Heliövaara, Heistaro, Liippo and Puukka26 The main outcome variables were forced vital capacity (FVC) (i.e. the maximal volume of air that can be forcefully expelled from the lungs after maximal inhalation, in litres) and forced expiratory volume in 1 s (FEV1) (i.e. the volume of air that can be forcefully expelled from the lungs in the first second after maximal inhalation, in litres). From a minimum of two technically acceptable and consistent efforts, the highest readings of FEV1 and FVC were recorded and used in the analyses. Finnish reference values Reference Viljanen27 were used to compute the individual FEV1 and FVC values as a percentage of those predicted for corresponding age, gender and height in healthy, non-smoking adults.
Based on spirometry results, pulmonary obstruction was defined as a FEV1/FVC <70%; and restriction as a FVC <80% of the predicted value and a FEV1/FVC >70% to exclude obstruction. A bronchodilation test was also performed as part of the spirometry measurements if obstruction was detected, but only spirometry results without bronchodilatation are reported in this study.
Diagnosis of respiratory disorders and the respiratory examination
Asthma was diagnosed if the participant reported having been diagnosed with asthma by a physician and receiving medication for asthma; or if the participant had been admitted to a hospital with asthma (ICD-9 code 493 or ICD-10 codes J45, J46) 28,29 between the years 1987 and 2008 according to National Hospital Discharge Register (HILMO). COPD was diagnosed if the participant had been admitted to a hospital with COPD (ICD-9 codes 491.2, 496 or ICD-10 code J44); or had a spirometry result indicative of COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria: FEV1/FVC <70% and FEV1 <80% of the predicted value (GOLD stages II–IV). 17 COPD was not diagnosed if the participant had self-reported asthma or a bronchodilatation response indicative of asthma on spirometry (an increase of 12% or more in FEV1 following bronchodilation). From the HILMO we also identified if the participant had been admitted to a hospital for pneumonia (ICD-9 codes 480–486 or ICD-10 codes J12–J18) between the years 1987 and 2008.
Information about having had an influenza vaccination, as well as symptoms of chronic bronchitis was enquired about in the symptom interview, conducted as part of the health examination. In Finland influenza vaccination is routinely offered free of charge to people at risk of complications of influenza and to professionals in the healthcare sector. Chronic bronchitis was defined according to the recommendations for population studies as cough and sputum production occurring nearly daily for at least 3 months of the year and for at least 2 consecutive years. Reference Heistaro22
Respiratory examination was part of the health examination performed by a physician and included lung auscultation along with the inspection of deformities in the thorax and abnormalities in breathing: difficulties in expiration or inspiration, the use of accessory respiratory muscles or breathing through the mouth.
Smoking and serum cotinine measurements
Self-reported daily smoking was defined according to the World Health Organization's recommendation: regular smoking, during which the participant had smoked at least 100 cigarettes, had smoked for at least 1 year and had smoked during the day of the interview or the day before. Previous smoking was defined as having quit regular smoking at least 1 month ago. Never smoking comprised never having been a regular smoker and occasional smoking was defined as not being a regular smoker at the time of the study. Heavy smokers were classified as daily smokers who smoked more than one pack (20) of cigarettes, cigars or pipe tobacco a day.
Cotinine measurements were employed as an objective method for determining nicotine intake. Reference Keskitalo, Broms, Heliovaara, Ripatti, Surakka and Perola30 Cotinine is the principal plasma metabolite of nicotine and has a half-life of approximately 16 h; thus, in addition to reflecting recent nicotine intake, it gives information on cumulative intake of ~7 days, as well as nicotine intake from passive smoking. Reference Keskitalo, Broms, Heliovaara, Ripatti, Surakka and Perola30,Reference Vartiainen, Seppälä, Lilsunde and Puska31 Blood samples were obtained either in the health examination proper or in the condensed health examination. Cotinine (ng/mL) was determined from serum with liquid-phase radioimmunoassay methodology (Nicotine Metabolite DOUBLE ANTIBODY kit, Diagnostic Products Corporation, Los Angeles, CA, USA). The inter-assay coefficient of variation was 12.3%.
Medications
Information on medication was collected in the home interview, and was registered based on self-reported current medication use. Participants had been asked to bring their prescriptions or medications with them to the interview to assist in reporting current medication.
Other variables
Height as well as waist and hip circumference were recorded as part of the health examination according to recommendations for anthropometric measurements in population studies. Reference Heistaro22 Waist-to-hip ratio was used as a measure of abdominal fat, which is considered particularly important in restricting lung function. Reference Wannamethee, Shaper and Whincup18
Self-reported leisure time physical activity was recorded according to the four categories of the Gothenburg scale Reference Heistaro22 but for the analyses we used a dichotomised scale: sedentary (category I) and active (categories II–IV). Participants were categorised as being physically active if they reported (a) walking, cycling or undertaking similar exercise for at least 4 h per week, (b) fitness training for at least 3 h per week or (c) competitive sports regularly several times a week.
Type 2 diabetes was diagnosed using the WHO 1999 criteria by combining information from laboratory measurements, health examination and register data. Metabolic syndrome was diagnosed according to the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP-III) criteria 32 using information from laboratory measurements and the health examination. Both diagnostic processes have been described in detail in earlier studies. Reference Suvisaari, Perala, Saarni, Harkanen, Pirkola and Joukamaa2,Reference Suvisaari, Saarni, Perälä, Suvisaari, Härkänen and Lönnqvist33
Statistical analysis
Analyses were completed using the SUDAAN software system (release 10.0). The two-stage cluster sampling design was accounted for in the analyses, and post-stratification weights estimated by Statistics Finland were used to adjust for non-response as well as the oversampling of participants aged 80 years and over. Reference Aromaa and Koskinen20 Because of these weighting adjustments, robust standard error estimates, instead of the conventional standard deviation, were calculated to indicate variability around the mean values of continuous variables. Moreover, instead of numbers we prefer to report percentages taking into account the sampling design.
Differences between participants with a lifetime diagnosis of a psychotic disorder (schizophrenia, other non-affective psychoses or affective psychosis) and participants without psychosis were tested using the t-test for continuous variables; for categorical variables, either the chi-squared test or Fisher's exact test was used, when appropriate. Analyses requiring the use of Fisher's exact test were performed using the SAS software system (version 9.1). Odds ratios (OR and 95% CI), adjusted for age and gender, were calculated using logistic regression. Statistical significance was consistently evaluated using the 0.05-level, two-tailed tests.
We employed regression analyses to investigate the independent roles of psychotic disorders as determinants of reduced lung function in the total study population. Linear regression analyses were conducted for the FEV1 and FVC per cent predicted (% predicted) values as the dependent variables and logistic analyses for restriction and obstruction, respectively. The independent variables were preselected based either on previous literature or theoretical meaningfulness, and entered simultaneously into the models together with the psychosis diagnoses. We used the Satterthwaite-adjusted F-statistic to test the statistical significance of the regression coefficients.
Results
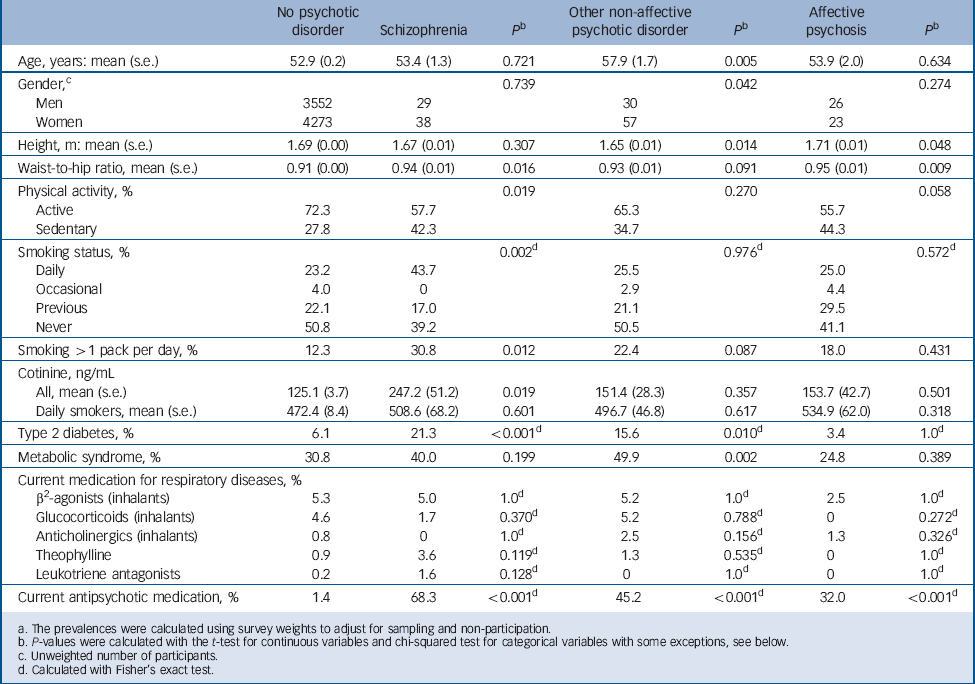
The characteristics for the sample are summarised in Table 1. Both the relatively high mean age and the excess of women in the sample are explained by the fact that the sample is representative of the Finnish population aged 30 years and over with no upper age limit. Self-reported daily smoking and heavy smoking were significantly more common in participants with schizophrenia compared with participants without psychosis. Likewise, participants with schizophrenia had significantly higher serum cotinine (S-Cot) levels compared with participants without psychosis, even after adjusting for age and gender (β = 127.6 ng/mL, 95% CI 31.4–223.9 ng/mL, P = 0.009) in a linear regression model.
Table 1 Demographic characteristics, variables related to respiratory health and current antipsychotic medication for participants with and without psychotic disorder a
| No psychotic disorder |
Schizophrenia | P b | Other non-affective psychotic disorder |
P b | Affective psychosis |
P b | |
|---|---|---|---|---|---|---|---|
| Age, years: mean (s.e.) | 52.9 (0.2) | 53.4 (1.3) | 0.721 | 57.9 (1.7) | 0.005 | 53.9 (2.0) | 0.634 |
| Gender, c | 0.739 | 0.042 | 0.274 | ||||
| Men | 3552 | 29 | 30 | 26 | |||
| Women | 4273 | 38 | 57 | 23 | |||
| Height, m: mean (s.e.) | 1.69 (0.00) | 1.67 (0.01) | 0.307 | 1.65 (0.01) | 0.014 | 1.71 (0.01) | 0.048 |
| Waist-to-hip ratio, mean (s.e.) | 0.91 (0.00) | 0.94 (0.01) | 0.016 | 0.93 (0.01) | 0.091 | 0.95 (0.01) | 0.009 |
| Physical activity, % | 0.019 | 0.270 | 0.058 | ||||
| Active | 72.3 | 57.7 | 65.3 | 55.7 | |||
| Sedentary | 27.8 | 42.3 | 34.7 | 44.3 | |||
| Smoking status, % | 0.002 d | 0.976 d | 0.572 d | ||||
| Daily | 23.2 | 43.7 | 25.5 | 25.0 | |||
| Occasional | 4.0 | 0 | 2.9 | 4.4 | |||
| Previous | 22.1 | 17.0 | 21.1 | 29.5 | |||
| Never | 50.8 | 39.2 | 50.5 | 41.1 | |||
| Smoking >1 pack per day, % | 12.3 | 30.8 | 0.012 | 22.4 | 0.087 | 18.0 | 0.431 |
| Cotinine, ng/mL | |||||||
| All, mean (s.e.) | 125.1 (3.7) | 247.2 (51.2) | 0.019 | 151.4 (28.3) | 0.357 | 153.7 (42.7) | 0.501 |
| Daily smokers, mean (s.e.) | 472.4 (8.4) | 508.6 (68.2) | 0.601 | 496.7 (46.8) | 0.617 | 534.9 (62.0) | 0.318 |
| Type 2 diabetes, % | 6.1 | 21.3 | <0.001 d | 15.6 | 0.010 d | 3.4 | 1.0 d |
| Metabolic syndrome, % | 30.8 | 40.0 | 0.199 | 49.9 | 0.002 | 24.8 | 0.389 |
| Current medication for respiratory diseases, % | |||||||
| β2-agonists (inhalants) | 5.3 | 5.0 | 1.0 d | 5.2 | 1.0 d | 2.5 | 1.0 d |
| Glucocorticoids (inhalants) | 4.6 | 1.7 | 0.370 d | 5.2 | 0.788 d | 0 | 0.272 d |
| Anticholinergics (inhalants) | 0.8 | 0 | 1.0 d | 2.5 | 0.156 d | 1.3 | 0.326 d |
| Theophylline | 0.9 | 3.6 | 0.119 d | 1.3 | 0.535 d | 0 | 1.0 d |
| Leukotriene antagonists | 0.2 | 1.6 | 0.128 d | 0 | 1.0 d | 0 | 1.0 d |
| Current antipsychotic medication, % | 1.4 | 68.3 | <0.001 d | 45.2 | <0.001 d | 32.0 | <0.001 d |
a. The prevalences were calculated using survey weights to adjust for sampling and non-participation.
b. P-values were calculated with the t-test for continuous variables and chi-squared test for categorical variables with some exceptions, see below.
c. Unweighted number of participants.
d. Calculated with Fisher's exact test.
There were no differences in the use of COPD medications between participants with schizophrenia and the general population. As previously reported, Reference Suvisaari, Perala, Saarni, Harkanen, Pirkola and Joukamaa2 type 2 diabetes was more prevalent in participants with schizophrenia and other non-affective psychoses. Metabolic syndrome was significantly more common in individuals with other non-affective psychoses compared with the general population.
Spirometry results
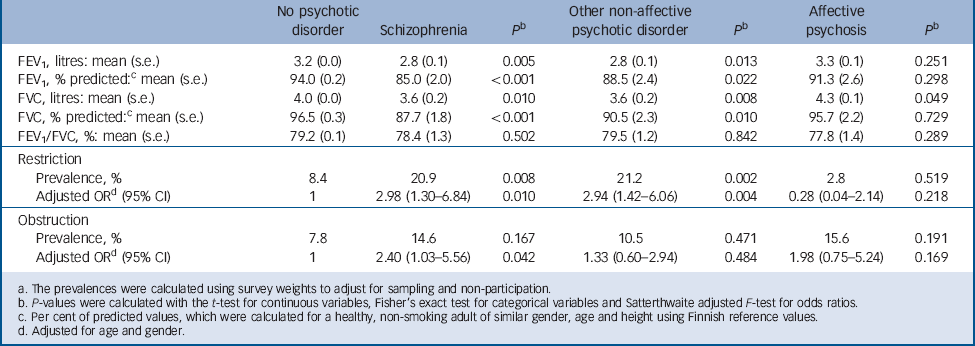
The spirometry results are presented in Table 2. Particularly participants with schizophrenia but also participants with other non-affective psychoses, had statistically significantly lower FEV1 and FVC values but a normal FEV1/FVC ratio compared with participants without psychosis, which suggests a restrictive ventilatory pattern. Accordingly, pulmonary restriction was significantly more prevalent both in participants with schizophrenia and those with other non-affective psychoses. Moreover, schizophrenia was associated with significantly increased odds of pulmonary obstruction after adjusting for age and gender. In total, 35.5% of participants with schizophrenia and 31.7% of participants with other non-affective psychoses had either restrictive or obstructive pulmonary impairment compared with 16.3% in the general population (P = 0.017 and P = 0.032, respectively).
Table 2 Spirometry results for participants with and without psychotic diosrder a
| No psychotic disorder |
Schizophrenia | P b | Other non-affective psychotic disorder |
P b | Affective psychosis |
P b | |
|---|---|---|---|---|---|---|---|
| FEV1, litres: mean (s.e.) | 3.2 (0.0) | 2.8 (0.1) | 0.005 | 2.8 (0.1) | 0.013 | 3.3 (0.1) | 0.251 |
| FEV1, % predicted: c mean (s.e.) | 94.0 (0.2) | 85.0 (2.0) | <0.001 | 88.5 (2.4) | 0.022 | 91.3 (2.6) | 0.298 |
| FVC, litres: mean (s.e.) | 4.0 (0.0) | 3.6 (0.2) | 0.010 | 3.6 (0.2) | 0.008 | 4.3 (0.1) | 0.049 |
| FVC, % predicted: c mean (s.e.) | 96.5 (0.3) | 87.7 (1.8) | <0.001 | 90.5 (2.3) | 0.010 | 95.7 (2.2) | 0.729 |
| FEV1/FVC, %: mean (s.e.) | 79.2 (0.1) | 78.4 (1.3) | 0.502 | 79.5 (1.2) | 0.842 | 77.8 (1.4) | 0.289 |
| Restriction | |||||||
| Prevalence, % | 8.4 | 20.9 | 0.008 | 21.2 | 0.002 | 2.8 | 0.519 |
| Adjusted OR d (95% CI) | 1 | 2.98 (1.30–6.84) | 0.010 | 2.94 (1.42–6.06) | 0.004 | 0.28 (0.04–2.14) | 0.218 |
| Obstruction | |||||||
| Prevalence, % | 7.8 | 14.6 | 0.167 | 10.5 | 0.471 | 15.6 | 0.191 |
| Adjusted OR d (95% CI) | 1 | 2.40 (1.03–5.56) | 0.042 | 1.33 (0.60–2.94) | 0.484 | 1.98 (0.75–5.24) | 0.169 |
a. The prevalences were calculated using survey weights to adjust for sampling and non-participation.
b. P-values were calculated with the t-test for continuous variables, Fisher's exact test for categorical variables and Satterthwaite adjusted F-test for odds ratios.
c. Per cent of predicted values, which were calculated for a healthy, non-smoking adult of similar gender, age and height using Finnish reference values.
d. Adjusted for age and gender.
Respiratory diseases and symptoms
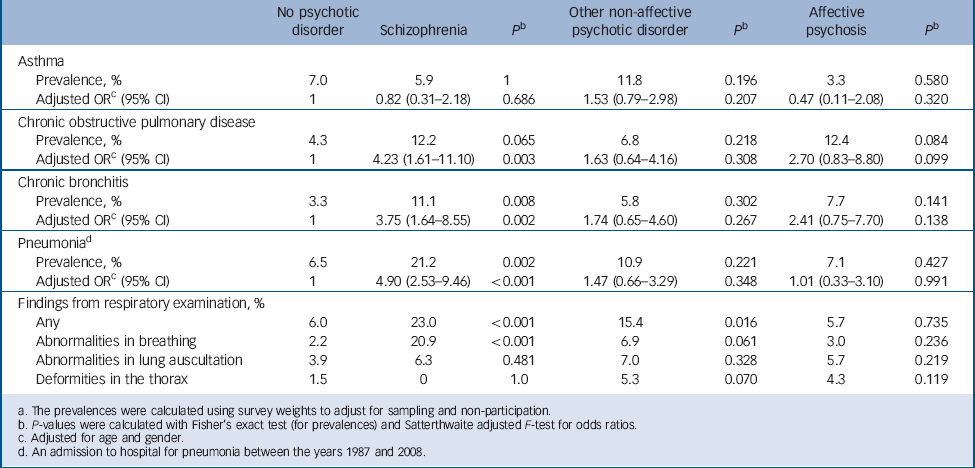
The prevalence and adjusted odds ratios of respiratory diseases and symptoms are shown in Table 3. Schizophrenia was associated with significantly increased odds of both COPD and chronic bronchitis after adjusting for age and gender. Participants with schizophrenia also had significantly more admissions to hospital for pneumonia between the years 1987 and 2008 even after controlling for age and gender. We investigated the possibility of the lack of influenza vaccinations being a confounder: participants with schizophrenia had an odds ratio of 1.20 (95% CI 0.53–2.71, P = 0.668) of having had an influenza vaccination during the past 12 months compared with the general population.
Table 3 Prevalences and adjusted odds ratios for respiratory diseases and symptoms for participants with and without psychotic diosrder a
| No psychotic disorder |
Schizophrenia | P b | Other non-affective psychotic disorder |
P b | Affective psychosis |
P b | |
|---|---|---|---|---|---|---|---|
| Asthma | |||||||
| Prevalence, % | 7.0 | 5.9 | 1 | 11.8 | 0.196 | 3.3 | 0.580 |
| Adjusted OR c (95% CI) | 1 | 0.82 (0.31–2.18) | 0.686 | 1.53 (0.79–2.98) | 0.207 | 0.47 (0.11–2.08) | 0.320 |
| Chronic obstructive pulmonary disease | |||||||
| Prevalence, % | 4.3 | 12.2 | 0.065 | 6.8 | 0.218 | 12.4 | 0.084 |
| Adjusted OR c (95% CI) | 1 | 4.23 (1.61–11.10) | 0.003 | 1.63 (0.64–4.16) | 0.308 | 2.70 (0.83–8.80) | 0.099 |
| Chronic bronchitis | |||||||
| Prevalence, % | 3.3 | 11.1 | 0.008 | 5.8 | 0.302 | 7.7 | 0.141 |
| Adjusted OR c (95% CI) | 1 | 3.75 (1.64–8.55) | 0.002 | 1.74 (0.65–4.60) | 0.267 | 2.41 (0.75–7.70) | 0.138 |
| Pneumonia d | |||||||
| Prevalence, % | 6.5 | 21.2 | 0.002 | 10.9 | 0.221 | 7.1 | 0.427 |
| Adjusted OR c (95% CI) | 1 | 4.90 (2.53–9.46) | <0.001 | 1.47 (0.66–3.29) | 0.348 | 1.01 (0.33–3.10) | 0.991 |
| Findings from respiratory examination, % | |||||||
| Any | 6.0 | 23.0 | <0.001 | 15.4 | 0.016 | 5.7 | 0.735 |
| Abnormalities in breathing | 2.2 | 20.9 | <0.001 | 6.9 | 0.061 | 3.0 | 0.236 |
| Abnormalities in lung auscultation | 3.9 | 6.3 | 0.481 | 7.0 | 0.328 | 5.7 | 0.219 |
| Deformities in the thorax | 1.5 | 0 | 1.0 | 5.3 | 0.070 | 4.3 | 0.119 |
a. The prevalences were calculated using survey weights to adjust for sampling and non-participation.
b. P-values were calculated with Fisher's exact test (for prevalences) and Satterthwaite adjusted F-test for odds ratios.
c. Adjusted for age and gender.
d. An admission to hospital for pneumonia between the years 1987 and 2008.
Abnormalities in breathing were observed significantly more often in participants with schizophrenia in the physician's clinical examination compared with participants without psychosis. In detailed analysis, the result was accounted for by the habit of mouth breathing, which was significantly more prevalent in participants with schizophrenia (20.9%) compared with the general population (1.3%, P<0.001).
Regression analysis for spirometry results
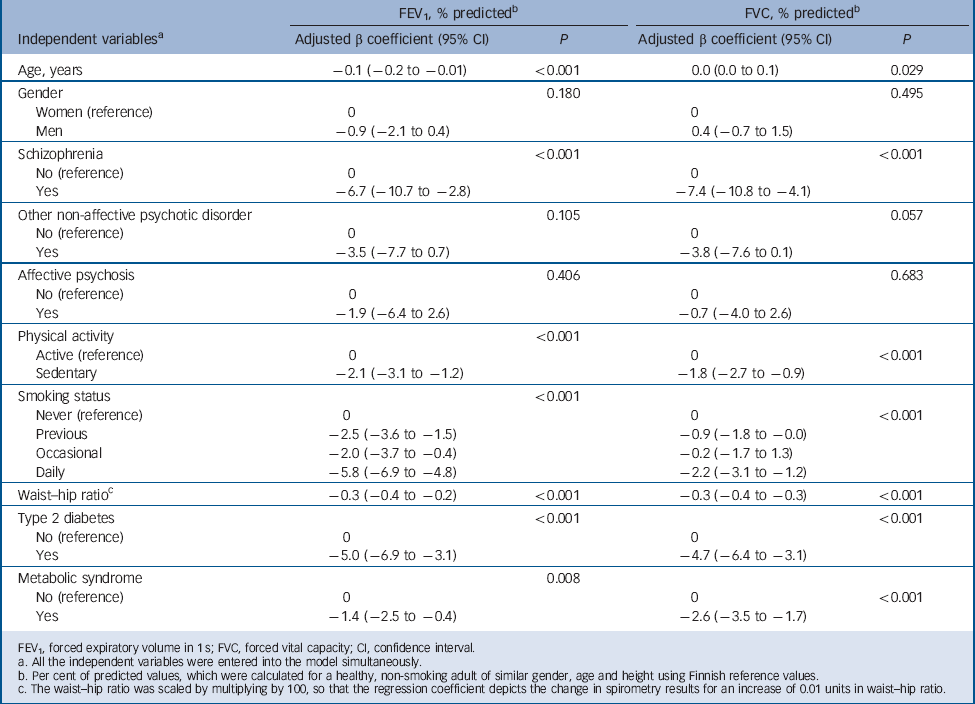
Tables 4 and 5 present the multiple linear and logistic regression models, respectively. Of the three psychosis diagnoses, only schizophrenia remained independently associated with significantly lower FEV1 and FVC % predicted values in the linear regression models; however, the independent association of other non-affective psychoses with FVC % predicted values approached borderline significance (P = 0.057). Only other non-affective psychoses remained independently associated with restrictive lung disease; no other significant associations were detected in the logistic regression models between psychosis diagnoses and restriction/obstruction after accounting for all the potential confounders. Restriction was statistically significantly associated with waist-to-hip ratio, type 2 diabetes, metabolic syndrome, physical activity, age and gender; and obstruction with smoking status, age and gender in the logistic regression models.
Table 4 Results of multiple linear regression analyses with the spirometry values of the total general population sample as dependent variables and psychotic disorders as independent variables along with potential confounding factors
| FEV1, % predicted b | FVC, % predicted b | |||
|---|---|---|---|---|
| Independent variables a | Adjusted β coefficient (95% CI) | P | Adjusted β coefficient (95% CI) | P |
| Age, years | −0.1 (−0.2 to −0.01) | <0.001 | 0.0 (0.0 to 0.1) | 0.029 |
| Gender | 0.180 | 0.495 | ||
| Women (reference) | 0 | 0 | ||
| Men | −0.9 (−2.1 to 0.4) | 0.4 (−0.7 to 1.5) | ||
| Schizophrenia | <0.001 | <0.001 | ||
| No (reference) | 0 | 0 | ||
| Yes | −6.7 (−10.7 to −2.8) | −7.4 (−10.8 to −4.1) | ||
| Other non-affective psychotic disorder | 0.105 | 0.057 | ||
| No (reference) | 0 | 0 | ||
| Yes | −3.5 (−7.7 to 0.7) | −3.8 (−7.6 to 0.1) | ||
| Affective psychosis | 0.406 | 0.683 | ||
| No (reference) | 0 | 0 | ||
| Yes | −1.9 (−6.4 to 2.6) | −0.7 (−4.0 to 2.6) | ||
| Physical activity | <0.001 | |||
| Active (reference) | 0 | 0 | <0.001 | |
| Sedentary | −2.1 (−3.1 to −1.2) | −1.8 (−2.7 to −0.9) | ||
| Smoking status | <0.001 | |||
| Never (reference) | 0 | 0 | <0.001 | |
| Previous | −2.5 (−3.6 to −1.5) | −0.9 (−1.8 to −0.0) | ||
| Occasional | −2.0 (−3.7 to −0.4) | −0.2 (−1.7 to 1.3) | ||
| Daily | −5.8 (−6.9 to −4.8) | −2.2 (−3.1 to −1.2) | ||
| Waist-hip ratio c | −0.3 (−0.4 to −0.2) | <0.001 | −0.3 (−0.4 to −0.3) | <0.001 |
| Type 2 diabetes | <0.001 | <0.001 | ||
| No (reference) | 0 | 0 | ||
| Yes | −5.0 (−6.9 to −3.1) | −4.7 (−6.4 to −3.1) | ||
| Metabolic syndrome | 0.008 | |||
| No (reference) | 0 | 0 | <0.001 | |
| Yes | −1.4 (−2.5 to −0.4) | −2.6 (−3.5 to −1.7) | ||
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; CI, confidence interval.
a. All the independent variables were entered into the model simultaneously.
b. Per cent of predicted values, which were calculated for a healthy, non-smoking adult of similar gender, age and height using Finnish reference values.
c. The waist-hip ratio was scaled by multiplying by 100, so that the regression coefficient depicts the change in spirometry results for an increase of 0.01 units in waist-hip ratio.
Table 5 Results of logistic regression analyses for the total general population sample with restriction and obstruction as dependent variables and psychotic disorders as independent variables along with potential confounding factors
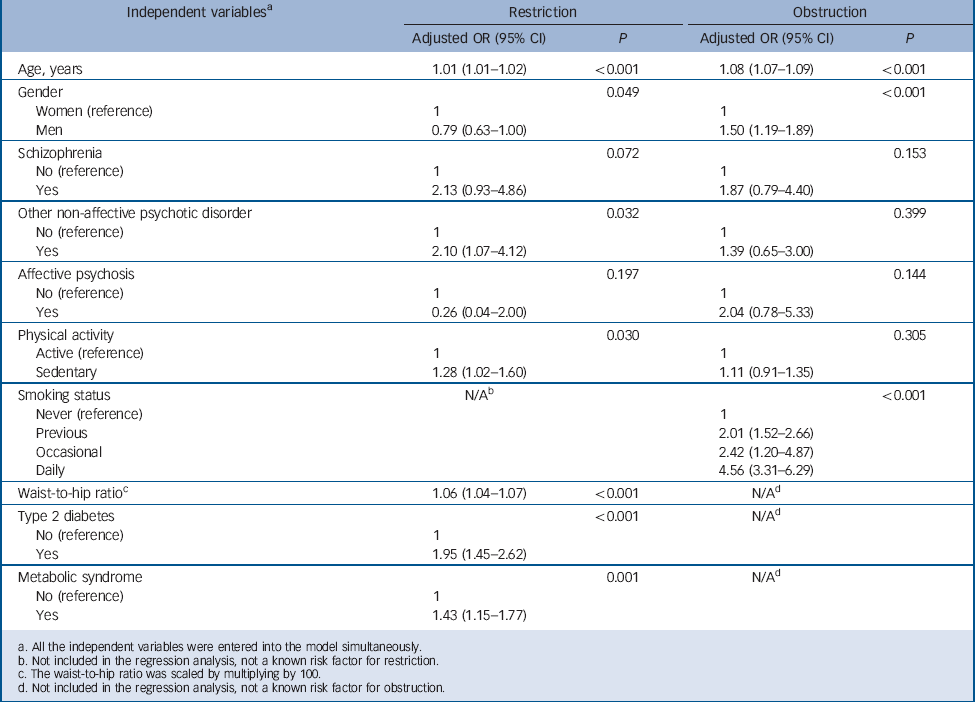
| Independent variables a | Restriction | Obstruction | ||
|---|---|---|---|---|
| Adjusted OR (95% CI) | P | Adjusted OR (95% CI) | P | |
| Age, years | 1.01 (1.01–1.02) | <0.001 | 1.08 (1.07–1.09) | <0.001 |
| Gender | 0.049 | <0.001 | ||
| Women (reference) | 1 | 1 | ||
| Men | 0.79 (0.63–1.00) | 1.50 (1.19–1.89) | ||
| Schizophrenia | 0.072 | 0.153 | ||
| No (reference) | 1 | 1 | ||
| Yes | 2.13 (0.93–4.86) | 1.87 (0.79–4.40) | ||
| Other non-affective psychotic disorder | 0.032 | 0.399 | ||
| No (reference) | 1 | 1 | ||
| Yes | 2.10 (1.07–4.12) | 1.39 (0.65–3.00) | ||
| Affective psychosis | 0.197 | 0.144 | ||
| No (reference) | 1 | 1 | ||
| Yes | 0.26 (0.04–2.00) | 2.04 (0.78–5.33) | ||
| Physical activity | 0.030 | 0.305 | ||
| Active (reference) | 1 | 1 | ||
| Sedentary | 1.28 (1.02–1.60) | 1.11 (0.91–1.35) | ||
| Smoking status | N/A b | <0.001 | ||
| Never (reference) | 1 | |||
| Previous | 2.01 (1.52–2.66) | |||
| Occasional | 2.42 (1.20–4.87) | |||
| Daily | 4.56 (3.31–6.29) | |||
| Waist-to-hip ratio c | 1.06 (1.04–1.07) | <0.001 | N/A d | |
| Type 2 diabetes | <0.001 | N/A d | ||
| No (reference) | 1 | |||
| Yes | 1.95 (1.45–2.62) | |||
| Metabolic syndrome | 0.001 | N/A d | ||
| No (reference) | 1 | |||
| Yes | 1.43 (1.15–1.77) | |||
a. All the independent variables were entered into the model simultaneously.
b. Not included in the regression analysis, not a known risk factor for restriction.
c. The waist-to-hip ratio was scaled by multiplying by 100.
d. Not included in the regression analysis, not a known risk factor for obstruction.
Attrition
The participation rates for spirometry were: 75.6% for participants without psychotic disorder, 64.2% for participants with schizophrenia, 65.5% for participants with other non-affective psychoses, and 67.3% for participants with affective psychosis (online Table DS1). We analysed the possible effect of attrition on the results (see online supplement DS1 for symptom-specific details with methods and detailed results). Briefly, people with schizophrenia for whom spirometry was not performed or was not successful had more severe symptoms and a less favourable outcome for the disorder compared with the participants. Similarly, non-participants with affective psychosis had more severe symptoms in a few symptom variables than the participants. Among people with ONAP, no differences were observed in any symptom or outcome measure between participants and non-participants
Discussion
Main findings
In this population-based study, schizophrenia and other non-affective psychoses were associated with impaired lung function as measured by spirometry. In total, approximately a third of participants with schizophrenia and other non-affective psychoses had either restrictive or obstructive lung disease compared with a figure of 16.3% in the general population. Schizophrenia remained associated with low spirometry values even after adjusting for age, gender, smoking, abdominal obesity, type 2 diabetes, metabolic syndrome and physical activity. Moreover, the odds of pneumonia, COPD and chronic bronchitis were significantly increased in participants with schizophrenia in comparison with the general population. We also found significantly higher cotinine levels in participants with schizophrenia compared with participants without psychosis.
Spirometry results
To our knowledge, there are only three previous studies that have measured lung function with spirometry in people with psychotic disorders. Reference Filik, Sipos, Kehoe, Burns, Cooper and Stevens12–Reference Vancampfort, Probst, Stubbs, Soundy, De Herdt and De Hert14 The two most recent studies by Vancampfort et al were based on the same sample of 80 predominantly male patients with schizophrenia: the first study found an association between impaired walking ability and reduced lung function; and the second study an association between metabolic syndrome and restrictive lung disease in patients with schizophrenia. Reference Vancampfort, Probst, Stubbs, Soundy, De Herdt and De Hert13,Reference Vancampfort, Probst, Stubbs, Soundy, De Herdt and De Hert14 In the first study the authors also reported reduced spirometry values in patients with schizophrenia compared with healthy controls, but whether restrictive lung disease is more common in schizophrenia compared with the general population was not investigated. Reference Vancampfort, Probst, Stubbs, Soundy, De Herdt and De Hert13 Moreover, in another study of mostly male patients with schizophrenia-spectrum psychoses, Filik et al found both low FEV1 and FVC values, but the contrast to the general population participants was slightly greater for FVC than for FEV1 values: 90.6% of the patients had a less than predicted FVC compared with a figure of 39% in the general population. Reference Filik, Sipos, Kehoe, Burns, Cooper and Stevens12 The finding is consistent with our results of increased prevalence of restrictive lung disease in individuals with schizophrenia and other non-affective psychoses.
Restrictive lung disease is a multifactorial clinical condition featuring a reduction in lung volumes, which has been associated with increased mortality risk in the general population. Reference Scarlata, Pedone, Fimognari, Bellia, Forastiere and Incalzi34,Reference Guerra, Sherrill, Venker, Ceccato, Halonen and Martinez35 In our study, restriction was most strongly associated with abdominal obesity, metabolic syndrome and type 2 diabetes. Abdominal and thoracic fat deposition may have simple mechanical restrictive effects on lung volumes. Reference Wannamethee, Shaper and Whincup18 Further, it is assumed that the association between metabolic syndrome and restrictive lung disease is mediated by abdominal obesity. Reference Vancampfort, Probst, Stubbs, Soundy, De Herdt and De Hert14,Reference Paek, Jung, Hwang, Lee, Lee and Lee36 However, other mechanisms, such as insulin resistance and inflammation, have also been suggested. Reference Vancampfort, Probst, Stubbs, Soundy, De Herdt and De Hert14,Reference Paek, Jung, Hwang, Lee, Lee and Lee36 Diabetes has been independently associated with impaired lung function and restrictive lung disease in particular. Reference Van den Borst, Gosker, Zeegers and Schols19 It remains unclear whether there is a causal relationship between diabetes and impaired pulmonary function, but, for example, diabetes-related microvascular damage and stiffening of the thorax and lung parenchyma have been proposed as possible pathophysiological mechanisms. Reference Van den Borst, Gosker, Zeegers and Schols19 It is worth noting that a restrictive spirometric pattern is not known to be associated with smoking. Reference Guerra, Sherrill, Venker, Ceccato, Halonen and Martinez35
Our results suggest that abdominal obesity, type 2 diabetes, metabolic syndrome and sedentary lifestyle contribute to restrictive lung disease in people with schizophrenia and other non-affective psychoses. However, even after adjusting for these factors, the effect of other non-affective psychoses remained significant, and the effect of schizophrenia nearly significant, suggesting that other factors are also influencing the risk of restrictive lung disease in individuals with these disorders. In conclusion, factors underlying spirometric restriction often remain unclear Reference Guerra, Sherrill, Venker, Ceccato, Halonen and Martinez35 and our cross-sectional study design does not allow any conclusion with regard to causality.
In addition to restriction, schizophrenia was associated with significantly increased odds of obstruction in spirometry. Obstructive lung disease is characterised by a reduction in the FEV1/FVC ratio and its most common causes are asthma and COPD. Accordingly, participants with schizophrenia had increased odds of COPD, which is an anticipated finding considering that smoking is the dominant cause of COPD 17 and smoking was more prevalent among participants with schizophrenia compared with those without psychosis. Schizophrenia was not an independent predictor of obstruction in the logistic regression model after adjustment for smoking. The use of COPD medications (inhaled anticholinergics, beta2-agonists and glucocorticoids) did not differ from the general population among participants with schizophrenia, despite the increased odds of COPD, suggesting that COPD often remains undiagnosed or untreated in people with schizophrenia.
Respiratory diseases
There are only a few studies that have investigated the prevalence of respiratory diseases in psychotic disorders. Copeland et al studied whether schizophrenia was associated with a higher risk of COPD and pneumonia during the last year of life among Veterans Health Administration patients who had died while in hospital treatment. Reference Copeland, Mortensen, Zeber, Pugh, Restrepo and Dalack9 After adjusting for confounding variables, they found increased odds of COPD (OR = 1.9, 95% CI 1.7–2.3) and of pneumonia (OR = 1.5, 95% CI 1.3–1.7) in their predominantly male study population. Reference Copeland, Mortensen, Zeber, Pugh, Restrepo and Dalack9 In another register-based study, the OR for COPD was 1.9 (95% CI 1.5–2.3) and 1.8 (95% CI 1.3–2.4) for asthma in patients with schizophrenia or schizoaffective disorder. Reference Carney, Jones and Woolson8 Moreover, Crump et al found more than a two-fold greater risk of COPD in patients with schizophrenia followed for 7 years in a national cohort study. Reference Crump, Winkleby, Sundquist and Sundquist6 In two separate studies based on a large population-based sample derived from the Taiwan National Health Research Insurance database, Chen et al found slightly increased odds (OR = 1.3, 95% CI 1.2–1.4) of asthma for patients with schizophrenia under the age of 45; Reference Chen, Lee and Lin10 and Hsu et al reported an odds ratio of 1.7 (95% CI 1.4–1.9) for COPD for adult patients with schizophrenia. Reference Hsu, Chien, Lin, Chou and Chou11 The latter study, however, erroneously included asthma in the diagnosis of COPD. Potentially unreliable diagnostics for respiratory diseases is a shared limitation of the above quoted register-based studies, particularly considering that the studies did not include lung function measurements.
Contrary to the previous studies, we did not find significantly increased odds of asthma in any of the psychosis groups. Nevertheless, we found that people with schizophrenia had over three times the odds of having chronic bronchitis compared with those without psychosis after adjusting for age and gender. This finding is similar to that of Himelhoch et al, Reference Himelhoch, Lehman, Kreyenbuhl, Daumit, Brown and Dixon7 which, however, also included people with bipolar disorder and major depressive disorder. There are few studies on the association of bipolar disease with pulmonary conditions. We found a high prevalence of COPD and obstruction among individuals with affective psychosis, but the finding was not statistically significant – possibly because of a lack of power.
Pneumonia and schizophrenia
We found significantly increased age- and gender-adjusted odds of admission to hospital with a diagnosis of pneumonia between the years 1987 and 2008 for individuals with schizophrenia. Our finding is in accordance with two recent studies that have reported an increased risk of pneumonia in patients with schizophrenia. Reference Crump, Winkleby, Sundquist and Sundquist6,Reference Seminog and Goldacre37 Moreover, a few recent studies have found excess mortality from pneumonia in people with schizophrenia. Reference Brown, Kim, Mitchell and Inskip5,Reference Crump, Winkleby, Sundquist and Sundquist6 Pneumonia and pulmonary tuberculosis accounted for most of the excess deaths as far back as when asylums were in operation, and were then considered as hazards of institutional care. Reference Healy, Le Noury, Harris, Butt, Linden and Whitaker38 However, self-reported previously diagnosed tuberculosis was non-existent in the Health 2000 spirometry population, Reference Vasankari, Impivaara, Heliövaara, Heistaro, Liippo and Puukka26 whereas studies conducted in other populations have shown an increased risk for tuberculosis among patients with schizophrenia compared with the general population. Reference DE Hert, Correll, Bobes, Cetkovich-Bakmas, Cohen and Asai39
Possible risk factors for pneumonia in individuals with schizophrenia might include comorbid chronic cardiovascular and lung disease (especially COPD), smoking, and alcohol misuse, which in earlier literature have been recognised as risk factors for pneumonia requiring hospital treatment in the general population. Reference Koivula, Sten and Makela40 Vulnerability to severe infections may be aetiologically related to schizophrenia. Reference Benros, Nielsen, Nordentoft, Eaton, Dalton and Mortensen41 Lack of proper preventative measures might also be a contributory factor; however, we observed that individuals with schizophrenia had received an influenza vaccination over the past 12 months at least as often as individuals without psychosis. It may be hypothesised that people with schizophrenia are less likely to seek and receive timely and appropriate care for their symptoms, which may lead to clinically more severe pneumonia requiring admission to hospital. In a previous study, Chen et al discovered a higher prevalence (1.3–1.8 increased odds ratios) of adverse clinical outcomes among people with schizophrenia who were admitted to hospital with pneumonia, but interestingly no significant increase in in-hospital mortality of pneumonia. Reference Chen, Lin and Lin42
Furthermore, the use of antipsychotic drugs has been associated with an increased risk of pneumonia. Reference Trifiro, Gambassi, Sen, Caputi, Bagnardi and Brea43,Reference Kuo, Yang, Liao, Chen, Lee and Shau44 Possible hypothesised underlying mechanisms include the anticholinergic and antihistaminergic side-effects of antipsychotics, which are thought to increase the risk for aspiration pneumonia by causing a dryness of the mouth, pharyngeal and oesophageal dysfunction and sedation. Reference Trifiro, Gambassi, Sen, Caputi, Bagnardi and Brea43,Reference Kuo, Yang, Liao, Chen, Lee and Shau44 Regrettably, because we only had cross-sectional information on antipsychotic medication, we were unable to analyse the association between antipsychotic drug use and risk of pneumonia.
Respiratory examination findings
Mouth breathing was significantly more common among individuals with schizophrenia compared with the general population. Mouth breathing is associated with lower blood oxygen concentration, which in turn is associated with high blood pressure and cardiac failure. Reference Jefferson45 Moreover, mouth breathing may provoke apnoeic spells during sleep. Reference Jefferson45 Considering the above, more clinical attention should be directed to the habit of mouth breathing and its adverse effects among people with schizophrenia.
Smoking and schizophrenia
To our knowledge, our study is the first to compare cotinine levels between people with psychosis and the general population. The mean serum cotinine level of participants with schizophrenia was almost two-fold in comparison with the general population; this is an objective indicator of the higher prevalence of smoking among people with schizophrenia compared with the general population. Consistently, self-reported daily smoking was also more prevalent in those with schizophrenia than in the general population – but less prevalent compared with earlier clinical samples. Reference Dalack, Healy and Meador-Woodruff15,Reference Kalman, Morissette and George16 However, our results are in accord with the results of previous population-based studies. Reference Lasser, Boyd, Woolhandler, Himmelstein, McCormick and Bor46 The prevalence of smoking is also dependent on the definition of smoking, which varies across studies. In our study we employed the World Health Organization's definition for daily smoking, which is rather restrictive compared with the definitions used in many other studies. The higher average age and female predominance of our schizophrenia study sample compared with most earlier studies might also have affected the prevalence of smoking.
Strengths and weaknesses
The nationally representative population-based study sample and the thorough diagnostic assessment of psychotic disorders can be considered as major strengths of the study. Another strength of the study is the objective measurement of lung function using spirometry. However, estimates of total lung capacity, which are required to confirm a diagnosis of restrictive lung disease, were not available. Nonetheless, a find of a reduction in FVC without obstruction using spirometry has traditionally been used as a proxy for this condition. Reference Scarlata, Pedone, Fimognari, Bellia, Forastiere and Incalzi34
The fact that spirometry is an effort-dependent technique, should be considered especially when studying individuals with psychosis. For example, cognitive impairment may cause inability to follow spirometry instructions and negative symptoms may impair performance. In fact, the technician reported unsatisfactory spirometry technique and difficulty in following instructions for some of the participants with psychosis. However, the spirometry results were discarded if they did not meet the internationally recommended quality criteria.
Accordingly, in the analysis of attrition, people with schizophrenia – and to a lesser extent also those with affective psychosis – who did not participate in the spirometry or failed to produce successful spirometry results seemed to have more severe symptoms compared with the spirometry participants. Thus, it may also be hypothesised that the actual level of lung function is even lower in these patient groups than our results suggest.
In the regression analysis of spirometry results we were able to account for important potential confounders, such as obesity and diabetes. However, despite the large general population sample, the number of people with psychosis for whom acceptable spirometry results were obtained was too small for meaningful regression analysis of the predictors of low spirometry values solely within this subgroup. We only had information on pneumonia requiring hospital treatment and no data are available for the more common milder forms of pneumonia treated in ambulatory settings. The study sample was confined to the Finnish population and thus the findings may not be directly generalised to other populations. Finally, because of the cross-sectional design of the study, causal inferences cannot be made.
Implications
In conclusion, schizophrenia is associated with poor lung function, as well as with increased odds of COPD and pneumonia – all of which are associated with increased mortality risk Reference Himelhoch, Lehman, Kreyenbuhl, Daumit, Brown and Dixon7,17,Reference Scarlata, Pedone, Fimognari, Bellia, Forastiere and Incalzi34,Reference Guerra, Sherrill, Venker, Ceccato, Halonen and Martinez35,Reference Koivula, Sten and Makela40 and are plausible major contributors to the burden of excess respiratory mortality observed among people with schizophrenia. Thus, awareness of the importance of timely screening for these conditions should be promoted among clinicians and heightened attention should be paid to modifiable risk factors, especially smoking and abdominal obesity as a component of metabolic syndrome. Moreover, physical activity should be promoted, since it might delay decline in lung function. Reference Pelkonen, Notkola, Lakka, Tukiainen, Kivinen and Nissinen47 It has been suggested that people with schizophrenia should routinely receive pneumococcal vaccination as a preventative measure. Reference Seminog and Goldacre37 Routine influenza vaccination should be considered, as well, if our findings are replicated in future studies.
Funding
The study was supported by grants from the Stanley Medical Research Institute (J.S.), the Academy of Finland (J.L., J.S.), the Yrjö Jahnsson Foundation (J.S.), the Paulo Foundation (K.P.), the Foundation of the Finnish Anti-Tuberculosis Association (K.P.) and the Finnish Cultural Foundation, South Savo Regional fund (K.P.).








eLetters
No eLetters have been published for this article.