Premenstrual dysphoric disorder (PMDD) is typified by mood symptoms, including irritability, depressed mood, anxiety and emotional lability, that consistently recur in the premenstrual phase of the menstrual cycle and seriously impact quality of life. Reference Backstrom, Brown, Dennerstein, Endicott and Epperson1,Reference Halbreich, Borenstein, Pearlstein and Kahn2 The pooled prevalence in mixed samples has been estimated at 3.2% in a recently published meta-analysis, Reference Reilly, Patel, Unachukwu, Knox, Wilson and Craig3 but around 12% of women require medical management for premenstrual symptoms. Reference Halbreich, Borenstein, Pearlstein and Kahn2 Although mood symptoms decline or disappear after the start of each menstruation, the disease burden is severe Reference Dennerstein, Lehert, Backstrom and Heinemann4 and, in some cases, PMDD is associated with suicidality. Reference Wikman, Sacher, Bixo, Hirschberg, Kopp Kallner and Epperson5 The strict DSM-5 criteria for PMDD state that five or more different mental and somatic symptoms, including at least one of the core symptoms depressed mood, anxiety, irritability and mood swings, need to be present in the premenstrual period and become minimal or absent during the postmenstrual week. 6 Furthermore, the symptoms should incur significant distress and/or have a significant impact on relationships, social functioning and work or student performance. There is wide variety in how the symptoms manifest in terms of type of dominating symptom and duration during the luteal phase, but the intra-individual pattern is usually stable over time. Reference Kaiser, Janda, Kleinstauber and Weise7 Of the core symptoms, irritability and anxiety are more common than depression and mood swings, Reference Pearlstein, Yonkers, Fayyad and Gillespie8 and irritability is, not surprisingly, strongly associated with impairment of relationships. Reference Kaiser, Janda, Kleinstauber and Weise7,Reference Dennerstein, Lehert, Backstrom and Heinemann9
A PMDD diagnosis should be based on at least 2 months of prospective daily ratings of symptoms on a validated instrument and not be an exacerbation of an underlying condition (e.g. major depression or an anxiety disorder). 6 Although PMDD is not considered an entirely psychiatric condition (despite the shared symptomatology and inclusion in DSM-5), comorbidity with psychiatric disorders is common Reference Kim, Gyulai, Freeman, Morrison, Baldassano and Dube10 and earlier trauma seems to be a significant risk factor for PMDD later in life. Reference Pilver, Levy, Libby and Desai11,Reference Yang, Thornorethardottir, Hauksdottir, Aspelund, Jakobsdottir and Halldorsdottir12
Menstrual cycle-linked symptom pattern in PMDD
A normal menstrual cycle is characterised by pronounced fluctuations in circulating levels of ovarian steroids. The pathophysiology of PMDD is unclear, but a temporal association with circulating progesterone and its metabolite allopregnanolone during the premenstrual phase has been established Reference Backstrom, Bixo, Johansson, Nyberg, Ossewaarde and Ragagnin13 (Fig. 1). However, none of these steroids are useful as a biomarker for PMDD, because peripheral luteal phase levels in PMDD are not different from those in asymptomatic individuals. Reference Sundstrom-Poromaa, Comasco and Sumner14 Instead, the prevailing hypothesis today is that individuals with PMDD are vulnerable to hormonal fluctuations. Reference Backstrom, Bixo, Johansson, Nyberg, Ossewaarde and Ragagnin13 A prerequisite for PMDD symptoms is that a corpus luteum develops during menstrual cycles. This is evidenced by studies showing that PMDD symptoms are abolished in spontaneously anovulatory cycles, as well as during induced anovulation by gonadotropin-releasing hormone (GnRH) analogues. Reference Hammarback and Backstrom15,Reference Schmidt, Nieman, Danaceau, Adams and Rubinow16 However, in anovulatory women previously diagnosed with PMDD, recurrence of typical symptoms can be triggered by a combination of continuous oestradiol and native progesterone, or by a synthetic progestin in a cyclical regimen mimicking a physiological menstrual cycle pattern. Reference Segebladh, Borgstrom, Nyberg, Bixo and Sundstrom-Poromaa17,Reference Bjorn, Bixo, Nojd, Nyberg and Backstrom18 This further strengthens the hypothesis of an underlying individual susceptibility.
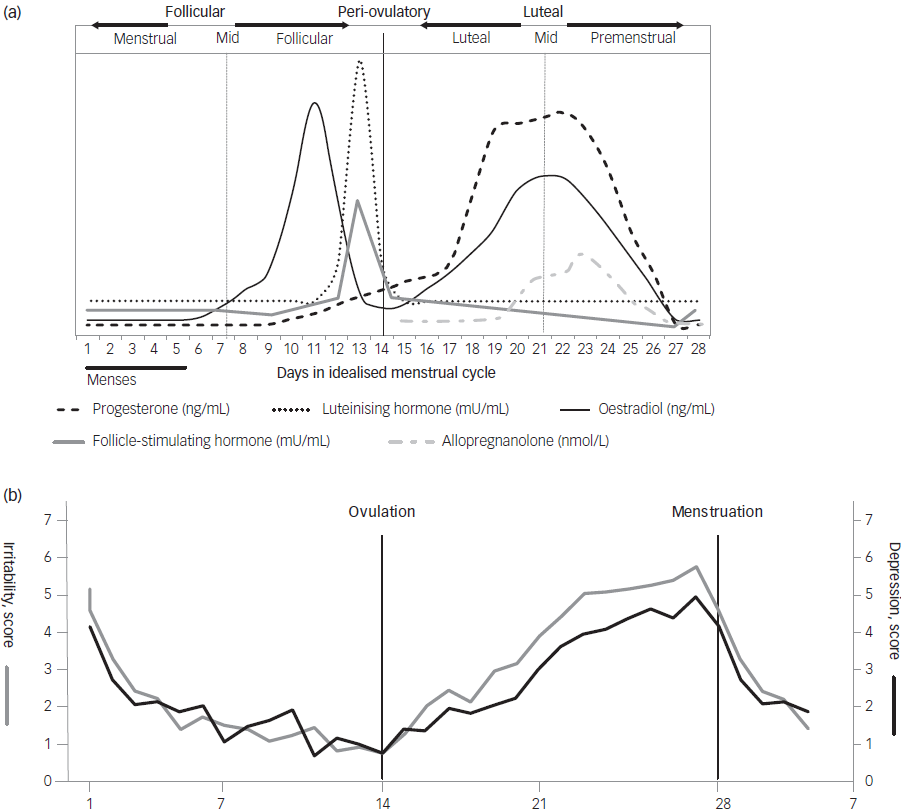
Fig. 1 (a) Hormonal changes during a normal, idealised menstrual cycle. The figure gives an overview of the fluctuations without displaying exact peripheral levels. Adapted from Draper et al129 and Bäckström et al.13 (b) Mean prospective scorings of the core symptoms irritability and depression in 38 menstrual cycles in 19 women with premenstrual dysphoric disorder (an idealised menstrual cycle corresponding to the allopregnanolone fluctuations displayed in the top panel). Adapted from Bäckström et al.13
Why then, are some women extremely sensitive to hormonal fluctuations during the luteal phase? In women with PMDD, serum levels of progesterone are closely linked to adverse mood (with a slight delay in peak symptoms). Progesterone has several target receptors in the brain. Genomic progesterone receptors, PR-A and PR-B are involved in reproductive function but are also expressed in areas associated with cognition and emotional processing. Reference Sundstrom-Poromaa, Comasco and Sumner14 Furthermore, a number of different membrane progesterone receptors and membrane binding sites (PGRMC) are known from animal research. Reference Bencker, Gschwandtner, Nayman, Griksiene, Nguyen and Nater19 However, studies trialling drugs that directly antagonise progesterone receptors, e.g. mifepristone, have failed to show positive effects on mood, at least when given after ovulation has occurred. Reference Chan, Mortola, Wood and Yen20 Instead, decades of research have highlighted the role of progesterone-derived neurosteroids as important modulators of mood and behaviour. The concept of neuroactive steroids is limited to steroids with specific targets in the central nervous system, while neurosteroids are defined as steroids that can be synthetised within the brain, de novo, from cholesterol or other precursors. The most well-known neurosteroids are allosteric modulators of the γ-amino-butyric acid (GABA)A receptor or the NMDA (N-methyl-D-aspartate) receptor. One of the most potent is allopregnanolone, which acts as a positive allosteric modulator of the GABAA receptor. PMDD symptoms arise in the context of allopregnanolone fluctuations (see Fig. 1). It is well documented that some individuals react paradoxically to sedatives such as benzodiazepines, barbiturates, alcohol and allopregnanolone, which are all active on the GABAA receptor, and thus experience anxiogenic rather than anxiolytic effects. In these cases the effect is bimodal: in high concentrations/dosages it is anxiolytic, sedative/anaesthetic and anti-epileptic, Reference Wang, Backstrom, Sundstrom, Wahlstrom, Olsson and Zhu21–Reference Backstrom, Gee, Lan, Sorensen and Wahlstrom23 but in low concentrations/dosages it is anxiogenic and promotes aggression. Reference Beauchamp, Ormerod, Jhamandas, Boegman and Beninger24–Reference Wenzel, Bartel, Eggebrecht, Philipp and Erbel31 The occurrence of these paradoxical reactions to GABAA agonists in the general population is 3% for severe irritability and aggression and up to 20% for moderate mood deterioration in specific patient groups, Reference Backstrom, Haage, Lofgren, Johansson, Stromberg and Nyberg32–Reference Matsumoto, Kaneko, Fujiki, Kusakabe, Nakayama and Tanaka34 prevalence figures similar to those for PMDD. Reference Reilly, Patel, Unachukwu, Knox, Wilson and Craig3,Reference Tschudin, Bertea and Zemp35 The bimodal, paradoxical response to allopregnanolone has been shown in both animal and human studies. Reference Beauchamp, Ormerod, Jhamandas, Boegman and Beninger24,Reference Fish, Faccidomo, DeBold and Miczek25,Reference Andreen, Sundstrom-Poromaa, Bixo, Nyberg and Backstrom36 In a study using oestrogen/progesterone cyclical treatment in postmenopausal women, negative mood symptoms were induced related to low luteal phase allopregnanolone concentrations, but decreased when higher concentrations were reached. Reference Andreen, Sundstrom-Poromaa, Bixo, Nyberg and Backstrom36 A functional magnetic resonance imaging (fMRI) study showed that luteal phase levels of progesterone/allopregnanolone induced paradoxically higher amygdala activation in response to an emotion-processing task. Reference van Wingen, van Broekhoven, Verkes, Petersson, Backstrom and Buitelaar37 High concentrations of allopregnanolone, on the other hand, are associated with reduced amygdala responses, reflecting the effects on the brain of benzodiazepines given in anxiolytic doses. Reference Paulus, Feinstein, Castillo, Simmons and Stein38
Positive allosteric modulators of the GABAA receptor
Allopregnanolone (3α-OH-5α-pregnan-20-one) is an endogenous progesterone metabolite and a potent positive allosteric modulator (PAM) of the GABAA receptor, enhancing GABAergic activity. Reference Majewska, Harrison, Schwartz, Barker and Paul39 The GABA system is the main inhibitory transmitter system in the brain, and the GABAA receptor is a membrane-bound chloride channel consisting of five subunits. At least 19 different subunits have been identified to date, making possible numerous combinations. Reference Olsen and Sieghart40 GABAA receptors are ubiquitously distributed within the brain and are located both in synapses (mediating phasic inhibition) and extrasynaptically (mediating tonic inhibition). Reference Farrant and Nusser41 GABAA receptor subunit composition varies in a site-specific manner throughout the brain, Reference Pirker, Schwarzer, Wieselthaler, Sieghart and Sperk42 and the precise composition of the receptor determines its pharmacodynamic response to GABAA-active drugs. Reference Olsen and Sieghart40,Reference Belelli and Lambert43,Reference Uusi-Oukari and Korpi44 Allopregnanolone is produced from progesterone by a two-step enzymatic conversion involving the enzymes 5 α-reductase type I and 3 α-hydroxysteroid dehydrogenase (Fig. 2). These two enzymes are expressed in the brain and colocalised in, for example, specific neurons in the cerebral cortex, hippocampus and amygdala.Reference Locci and Pinna45 In a human post-mortem study, levels of allopregnanolone in peripheral blood were reflected in the brain but also accumulated in specific areas, e.g. amygdala.Reference Bixo, Andersson, Winblad and Purdy46 Peripheral levels of allopregnanolone mirror those of progesterone during the menstrual cycle and in pregnancy,Reference Backstrom, Bixo, Johansson, Nyberg, Ossewaarde and Ragagnin13 but allopregnanolone is also secreted from the adrenal cortex as a reaction to stress.Reference Bengtsson, Backstrom, Brinton, Irwin, Johansson and Sjostedt47
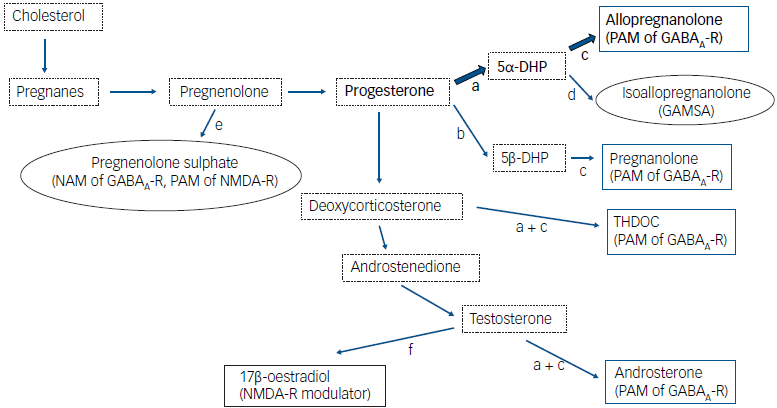
Fig. 2 Schematic biosynthesis pathways of neuroactive steroids. Bold arrows indicate the key pathways relevant for the pathophysiology of premenstrual dysphoric disorder. Blue boxes, positive GABA modulators; oval boxes, negative GABA modulators; dotted boxes, intermediary steroids. The most important enzymatic steps are depicted as a, 5α-reductase; b, 5β-reductase; c, 3α-hydroxysteroid dehydrogenase; d, 3β-hydroxysteroid dehydrogenase; e, sulphatase; and f, aromatase. GABA, γ-amino-butyric acid; PAM, positive allosteric modulator; NAM, negative allosteric modulator; GAMSA, GABAA receptor-modulating steroid antagonist; DHP, dihydroprogesterone; THDOC, tetrahydrodeoxycorticosterone; NMDA, N-methyl-D-aspartate.
Allopregnanolone acts as a PAM of the GABAA receptor in the lower nanomolar concentration range, but higher concentrations can activate the receptor directly without the presence of GABA. Reference Reddy and Estes48 Extrasynaptic receptors containing a delta subunit are known to be acutely sensitive to allopregnanolone, and some researchers have argued that delta-containing GABAA receptors are allopregnanolone’s main target at physiological concentration ranges. Reference Belelli, Casula, Ling and Lambert49 Like other PAMs of the GABAA receptor and pharmaceutical compounds such as benzodiazepines and barbiturates, allopregnanolone has sedative, anaesthetic and anti-epileptic effects in both animals and humans at high concentrations. Reference Timby, Balgard, Nyberg, Spigset, Andersson and Porankiewicz-Asplund50–Reference Norberg, Backstrom and Wahlstrom53 In addition, it has been shown to mediate anxiolytic effects in animal experiments. Reference Akwa, Purdy, Koob and Britton54 One of several possible physiological roles of endogenous allopregnanolone is as a neuroprotective agent, and it contributes to the normal development of the foetal brain during pregnancy. Reference Yawno, Yan, Hirst and Walker55,Reference Brunton, Russell and Hirst56 During the past 20 years, allopregnanolone research has expanded rapidly in several areas, mostly related to mood disorders. Reference Guo, Mao, Zhang, Xu, Zhao and Wang57 As a potential pharmaceutical, allopregnanolone is especially appealing since it is an endogenous steroid with a positive safety profile. Reference Epperson, Rubinow, Meltzer-Brody, Deligiannidis, Riesenberg and Krystal58 Allopregnanolone is approved by the American Food and Drug Administration for treatment of postpartum depression under the names Brexanolone (intravenous administration) and Zuranolone (oral administration). Reference Epperson, Rubinow, Meltzer-Brody, Deligiannidis, Riesenberg and Krystal58,Reference Barnes, Vogl and Nelson59 Other PAMs of the GABAA receptor include pregnanolone (an isomer of allopregnanolone), androsterone (3 α-OH-5 α-androstane-17-one, a metabolite of testosterone), and 5α-tetrahydrodeoxycorticosterone (a metabolite of corticosterone) Reference Majewska, Harrison, Schwartz, Barker and Paul39,Reference Zhu, Wang, Backstrom and Wahlstrom60,Reference Rahman, Lindblad, Johansson, Backstrom and Wang61 (see Fig. 2). Today, allopregnanolone is by far the most dominant and interesting PAM in the expanding research field of neurosteroids. Reference Guo, Mao, Zhang, Xu, Zhao and Wang57
GABAA receptor-modulating steroid antagonists and negative allosteric modulators of the GABAA receptor
Isoallopregnanolone (3β-OH-5α-pregnan-20-one) is a GABAA receptor-modulating steroid antagonist (GAMSA) that inhibits the effect of allopregnanolone. Its antagonising effect has been shown both in vitro and in animal experiments. Reference Lundgren, Stromberg, Backstrom and Wang62–Reference Wang, He, Eisenman, Fields, Zeng and Mathews64 As a metabolite of progesterone and an isomer to allopregnanolone, serum levels of endogenous isoallopregnanolone fluctuate similarly during different reproductive states. Reference Hill, Cibula, Havlikova, Kancheva, Fait and Kancheva65–Reference Havlikova, Hill, Kancheva, Vrbikova, Pouzar and Cerny67 During pregnancy, levels of isoallopregnanolone increase in parallel with allopregnanolone at a constant ratio of approximately 1:4 in peripheral blood, and during the menstrual cycle the ratio is between 1:4 and 1:2. Reference Kancheva, Hill, Cibula, Vcelakova, Kancheva and Vrbikova68 Isoallopregnanolone can be produced from allopregnanolone by either a two-step enzymatic reaction (with dihydroprogesterone produced as an intermediate) or direct epimerisation Reference Belyaeva, Chetyrkin, Clark, Kostereva, SantaCruz and Chronwall69,Reference Huang and Luu-The70 (see Fig. 2). The enzymes responsible for these conversions are present within the brain, but are most abundant in the liver. The physiological role of isoallopregnanolone is at present unknown; in early studies of neuroactive steroids, the compound was even used as an inert control. Reference Gee, Chang, Brinton and McEwen71 Isoallopregnanolone has no effects of its own on the GABAA receptor in vitro. Reference Lambert, Belelli, Hill-Venning and Peters72 The effect of GAMSA on GABAA receptor function seems to be specific to neuroactive steroids such as allopregnanolone, because it does not influence the action of other GABAA receptor agonists such as benzodiazepines or barbiturates, or GABA itself. Reference Lundgren, Stromberg, Backstrom and Wang62,Reference Stromberg, Haage, Taube, Backstrom and Lundgren73
Pregnenolone sulphate is a negative allosteric modulator (NAM) of the GABAA receptor, but it is also a GAMSA because it inhibits the effects of allopregnanolone on GABAergic transmission and a PAM of the NMDA receptor (Fig. 2). The effect of GAMSA has been shown in vitro in rat neurons using patch-clamp technique Reference Haage, Backstrom and Johansson74 and, more recently, more direct inhibitory actions of pregnenolone sulphate on the GABAA receptor have been shown. Reference Seljeset, Liebowitz, Bright and Smart75
Experimental effects of PAMs, GAMSA and NAMs in humans*
Measurement of saccadic eye velocity (SEV) is used to quantify GABAA receptor sensitivity Reference de Visser, van der Post, de Waal, Cornet, Cohen and van Gerven76 and, in studies of healthy women in the follicular phase, intravenous allopregnanolone dose-dependently increased subjective sedation and decreased maximal SEV. Reference Timby, Balgard, Nyberg, Spigset, Andersson and Porankiewicz-Asplund50 However, when isoallopregnanolone was added, the effect on SEV was inhibited. Reference Bengtsson, Nyberg, Hedstrom, Zingmark, Jonsson and Backstrom77
Studies using fMRI have shown that exogenous administration of PAMs influences the patterns of activity in brain networks that are highly relevant to the pathophysiology of mood disorders. Reference Menon78 For example, in healthy males, pregnanolone administration resulted in a sevenfold rise in allopregnanolone that was related to reduced amygdala and insula responses to emotional stimuli, as compared with placebo. Reference Sripada, Marx, King, Rampton, Ho and Liberzon79 In addition, pregnanolone decreased connectivity between the amygdala and important nodes of the default mode network (precuneus, dorsomedial prefrontal cortex and hippocampus), which related to a reduction in self-reported negative affect. Reference Sripada, Welsh, Marx and Liberzon80 A study of healthy female participants showed that administration of progesterone resulting in luteal phase levels of allopregnanolone led to increased amygdala responses to emotional stimuli, Reference van Wingen, van Broekhoven, Verkes, Petersson, Backstrom and Buitelaar37 while higher allopregnanolone levels in the same individuals related to reduced responses. Reference van Wingen, van Broekhoven, Verkes, Petersson, Backstrom and Buitelaar81 These equivocal findings may be due to the studies’ small sample sizes, but also to either the specific dosage of the substance administered or the sex of the participants. The response most likely also relates to the complex dose-dependent effect of allopregnanolone, where higher doses are known to induce anxiolysis and moderate doses are linked to paradoxical mood effects in susceptible persons. Reference Backstrom, Haage, Lofgren, Johansson, Stromberg and Nyberg32 No neuroimaging studies have examined the effect of a GAMSA or a NAM of the GABAA receptor on brain function. However, in naturally cycling women without premenstrual symptoms, increased peripheral levels of isoallopregnanolone relative to allopregnanolone have been related to lower amygdala activity in response to emotional stimuli during the luteal phase of the menstrual cycle. Reference Stiernman, Dubol, Comasco, Sundstrom-Poromaa, Boraxbekk and Johansson82
Effects of neurosteroids in PMDD
In women with PMDD, premenstrual mood improves when serum levels of allopregnanolone are decreased, either by inhibition of ovarian progesterone/allopregnanolone production or hampering of progesterone metabolism to allopregnanolone using a 5α reductase inhibitor. Reference Martinez, Rubinow, Nieman, Koziol, Morrow and Schiller83,Reference Nyberg, Backstrom, Zingmark, Purdy and Poromaa84 Like other GABAA receptor agonists – for example, benzodiazepines and barbiturates – allopregnanolone may induce tolerance and this has been demonstrated in animal studies. Reference Turkmen, Backstrom, Wahlstrom, Andreen and Johansson85 In the SEV model, women with PMDD were shown to have an altered sensitivity to intravenous injections of allopregnanolone in comparison with healthy controls. PMDD women were more sensitive during the luteal phase as opposed to during the follicular phase, whereas the opposite was seen in healthy controls. The conclusion from these results was that women with PMDD might not be able to develop a physiological tolerance to allopregnanolone during the luteal phase. Reference Timby, Backstrom, Nyberg, Stenlund, Wihlback and Bixo86
In a study of postmenopausal women sequentially treated with oestradiol and progesterone, prior PMDD was a risk factor for development of negative mood depending on the level of metabolism to allopregnanolone. Reference Andreen, Sundstrom-Poromaa, Bixo, Nyberg and Backstrom36,Reference Andreen, Nyberg, Turkmen, van Wingen, Fernandez and Backstrom87 However, not only is allopregnanolone of importance but also oestradiol concentrations can drive negative mood reactions. When anovulatory women with PMDD were given a combination of oestradiol and progesterone, a higher dose of the former caused a more pronounced negative mood. Reference Segebladh, Borgstrom, Nyberg, Bixo and Sundstrom-Poromaa17
Direct allopregnanolone administration has never been evaluated in women with PMDD using neuroimaging methods. However, emotion processing-related fMRI studies suggest effects of PMDD in key regions of the limbic system, including the amygdala, anterior cingulate cortex and insula, as well as the prefrontal cortex across the menstrual cycle during which allopregnanolone levels are fluctuating (see Fig. 3 and Dubol et al). Reference Dubol, Epperson, Lanzenberger, Sundstrom-Poromaa and Comasco88 Reductions in grey matter volume have also been reported in the luteal phase in PMDD. Reference Dubol, Epperson, Lanzenberger, Sundstrom-Poromaa and Comasco88 However, recent studies have shown that widespread reductions of cortical thickness in PMDD seem to be independent of menstrual cycle phase. Reference Dubol, Stiernman, Sundstrom-Poromaa, Bixo and Comasco89 Structural brain differences may thus reflect an underlying vulnerability to developing PMDD symptoms and are relatively stable in the context of fluctuating steroid levels. However, functional measures of brain activity have previously been associated with circulating steroids: for instance, activity of the amygdala during the luteal phase has been linked to progesterone and progesterone-derived neurosteroid levels in women with PMDD. Reference Stiernman, Dubol, Comasco, Sundstrom-Poromaa, Boraxbekk and Johansson82,Reference Gingnell, Morell, Bannbers, Wikstrom and Sundstrom Poromaa90 Aberrant activation of the amygdala is commonly observed in mood and anxiety disorders. Reference Menon78 Interestingly, increased amygdala activity during the luteal phase has been related to lower transcriptional expression of the delta subunit of the GABAA receptor in peripheral immune cells in women with PMDD. Reference Stiernman91 Delta-containing GABAA receptors are exclusively extrasynaptic and mediate tonic inhibition, and are thus considered to be an important determinant of baseline neuronal excitability. Reference Zheleznova, Sedelnikova and Weiss92 Furthermore, the delta subunit is thought to endow the GABAA receptor with a heightened sensitivity to allopregnanolone, and brain expression of this receptor is linked to reproductive status in rodents. Reference Belelli, Casula, Ling and Lambert49,Reference Maguire, Stell, Rafizadeh and Mody93–Reference Maguire, Ferando, Simonsen and Mody96 Deficits in delta-GABAA receptor expression are linked to affective disturbances in animal models. Reference Maguire, Stell, Rafizadeh and Mody93,Reference Maguire and Mody95 Whether delta-containing GABAA receptors are differentially expressed at the regional brain level in women with PMDD remains to be determined. Nonetheless, aberrant regulation of delta-containing GABAA receptors represents an interesting hypothesis explaining the differential behavioural and pharmacodynamic responses observed in these women in response to fluctuating allopregnanolone levels.
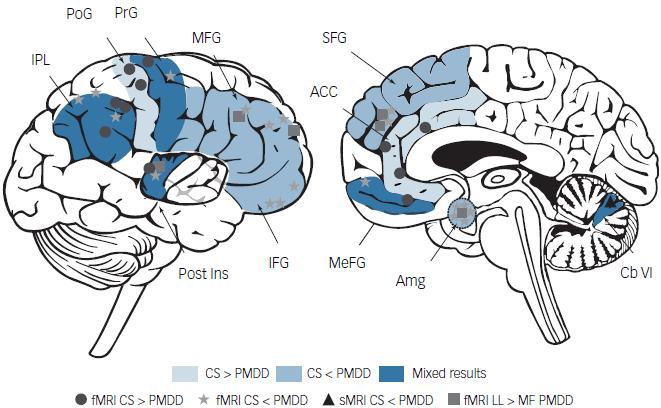
Fig. 3 Functional and structural brain differences in women with premenstrual dysphoric disorder (PMDD). Regions in light blue correspond to higher activity reported in control subjects compared with women with PMDD. Regions shown in medium blue correspond to higher activity or grey matter volume reported in women with PMDD compared with control subjects. Regions shown in dark blue correspond to mixed patterns of functional differences reported between women with PMDD and control subjects. ACC, anterior cingulate cortex; Amg, amygdala; Cb VI, cerebellum VI lobule; CS, control subjects; fMRI, functional MRI; IFG, inferior frontal gyrus; IPL, inferior parietal lobule; LL, late luteal phase; MeFG, medial frontal gyrus; MF, mid-follicular phase; MFG, middle frontal gyrus; PMDD, premenstrual dysphoric disorder; PoG, postcentral gyrus; Post Ins, posterior insula; PrG, precentral gyrus; SFG, superior frontal gyrus; sMRI, structural MRI. Reprinted with permission from Dubol et al.88
Like allopregnanolone, its isomer pregnanolone is a PAM of the GABAA receptor but its effect is less studied in PMDD. However, a recent study has revealed that pregnanolone levels in the luteal phase are affected by treatment with a selective serotonin reuptake inhibitor (SSRI), a common treatment for PMDD. Reference Miller, Standeven, Morrow, Payne, Epperson and Hantsoo97 In women with PMDD, SSRI treatment has been shown to normalise sensibility to pregnanolone in an experimental SEV model. Reference Sundstrom and Backstrom98 SSRIs appear to have steroidogenic effects, even at low doses, and to increase the synthesis of allopregnanolone. Reference Pinna99
Pregnenolone sulphate is a NAM of the GABAA receptor but also a PAM of the NMDA receptor. Studies in PMDD women are scarce, but there are some indications that pregnenolone sulphate is also of importance regarding symptom severity in this group. Reference Wang, Seippel, Purdy and Backstrom100
The role of oestradiol in PMDD pathophysiology
The ovaries are the main source of circulating oestradiol in women of fertile age, but variations in regional intracerebral distribution are also due to brain-derived 17β-oestradiol from neurons and astrocytes. Reference Brann, Lu, Wang, Sareddy, Pratap and Zhang101 17β-oestradiol has a high affinity to the genomic receptors ERα and ERγ, which are abundant in the human brain. In addition to reproductive processes, both receptor types appear to play a role in neuronal functioning and neurodegenerative diseases. Reference Sato, Takayama, Saito and Inoue102 In addition to genomic receptors, the membrane-bound ERα has been shown to promote the pre-ovulatory surge in luteinising hormone. Reference Faure, Corona, Roomans, Lenfant, Foidart and Cornil103 However, 17β-oestradiol has also been shown to have a rapid and direct neuromodulatory effect that appears to be mediated by NMDA receptors. Reference Brann, Lu, Wang, Sareddy, Pratap and Zhang101,Reference Kow and Pfaff104 Contrary to the effect of PAMs, oestradiol is thus pro-excitatory, at least in areas such as the hippocampus. Reference Zadran, Qin, Bi, Zadran, Kim and Foy105 In addition to reproduction and sexual behaviour, oestradiol appears to promote synaptic plasticity, cognitive function and neuroprotection. Reference Brann, Lu, Wang, Sareddy, Pratap and Zhang101 In women with premenstrual dysphoria, increased oestradiol levels have been shown to aggravate the detrimental effects of progesterone on mood when administered during the luteal phase, or in combined menopausal hormone therapy with synthetic progestins. Reference Seippel and Backstrom106–Reference Bjorn, Sundstrom-Poromaa, Bixo, Nyberg, Backstrom and Backstrom108 However, fluctuations of oestradiol might be more harmful than the actual circulating levels. Reference Schmidt, Martinez, Nieman, Koziol, Thompson and Schenkel109 Symptoms of mental disorders in women usually fluctuate over the menstrual cycle and, in an exploratory study a relationship between perimenstrual oestradiol levels and suicide ideations, could be confirmed. Reference Schmalenberger, Mulligan, Barone, Nagpal, Divine and Maki110 Moreover, treatment with oestradiol, to prevent the rapid decline of circulating levels perimenstrually, reduced suicidal ideations and improved cognitive function. Reference Schmalenberger, Mulligan, Barone, Nagpal, Divine and Maki110 Furthermore, an increased risk of developing PMDD has been found in women with oestrogen receptor alpha mutations. Reference Huo, Straub, Roca, Schmidt, Shi and Vakkalanka111 However, very little is known about the mechanisms underlying oestradiol effects on the brain. Brain imaging studies have revealed oestradiol-mediated effects on brain morphology during the menstrual cycle in healthy women. Reference Zsido, Williams, Barth, Serio, Kurth and Mildner112 In particular, the plasticity of brain regions important for cognitive functions, such as the CA1 area of the hippocampus, appeared to be affected by fluctuations in ovarian steroids. Reference Zsido, Williams, Barth, Serio, Kurth and Mildner112 Very few neuroimaging studies have investigated associations between oestradiol levels and brain functional measures in PMDD populations. One small fMRI study (N = 28) showed a relationship between medial prefrontal cortex activation and oestradiol levels during the luteal phase in women with PMDD during emotion processing. Reference Gingnell, Bannbers, Wikstrom, Fredrikson and Sundstrom-Poromaa113 However, subsequent studies have not replicated this finding. Reference Stiernman, Dubol, Comasco, Sundstrom-Poromaa, Boraxbekk and Johansson82,Reference Stiernman91
Treatment for PMDD
First-line treatment for PMDD today is an SSRI. 114 Although efficacy is equal or slightly better for continuous as compared with intermittent treatment, the latter regimen reduces problems with side-effects and mitigates withdrawal symptoms. Reference Reilly, Wallman, Clark, Knox, Craig and Taylor115,Reference Jespersen, Lauritsen, Frokjaer and Schroll116 Overall, the effect on mood symptoms is generally good although side-effects are common and compliance usually low. Reference Marjoribanks, Brown, O’Brien and Wyatt117,Reference Sundstrom-Poromaa, Bixo, Bjorn and Nordh118 Current recommendations also include complimentary non-pharmacological treatments and, for the less burdensome premenstrual syndrome (PMS), cognitive behavioural therapy is first-line treatment according to the American College of Obstetricians and Gynecologists. 114
Especially for women requesting contraception, combined oral contraceptives (COCs) could be a good alternative, and combinations of a low dose of ethinyl oestradiol and drospirenone have proved efficient in ameliorating PMDD symptoms. 114,Reference Lopez, Kaptein and Helmerhorst119 A more recent meta-analysis concluded that all types of COC are equally efficient when treating PMDD and PMS, but the effect on premenstrual depression was weak overall. Reference de Wit, de Vries, de Boer, Scheper, Fokkema and Janssen120
In addition, the effectiveness of non-contraceptive oestradiol preparations for treatment of PMS symptoms has been investigated in a meta-analysis. Reference Naheed, Kuiper and Uthman121 The purpose of this study was to inhibit ovulation and exert a moderate effect on mood symptoms, but no effect on quality of life was found. Overall, the studies were of low quality and adverse effects, due to the progestogen component that was added to protect the endometrium from hyperplasia, were common. Reference Naheed, Kuiper and Uthman121 Today, oestradiol is not recommended for treatment of premenstrual mood symptoms. 114
Since ovulation is a prerequisite for PMDD, treatment with a GnRH analogue is usually effective and an option when no other treatment works. 114,Reference Wyatt, Dimmock, Ismail and Jones122 The problem is that add-back must be given to protect the woman from osteoporosis and other negative effects of oestrogen deprivation, with the risk of triggering negative mood. Reference Segebladh, Borgstrom, Nyberg, Bixo and Sundstrom-Poromaa17 Interestingly, in a small observational study of 12 women with PMDD who responded to GnRH treatment, PMDD symptoms recurred initially when oestradiol and progestogen were added but, when steroid levels were stable for the remaining 2−3 months of treatment, PMDD symptoms declined. Reference Schmidt, Martinez, Nieman, Koziol, Thompson and Schenkel109
Ulipristal is a selective progesterone receptor modulator that has shown promising effects on PMDD symptoms in a placebo-controlled, randomised trial. Reference Comasco, Kopp Kallner, Bixo, Hirschberg, Nyback and de Grauw123 Due to reports of liver damage, ulipristal has been taken off the market in many countries but nevertheless the study constituted a proof of concept.
Isoallopregnanolone has been tested in two randomised, placebo-controlled trials as a treatment modality for PMDD. Reference Bixo, Ekberg, Poromaa, Hirschberg, Jonasson and Andreen124,Reference Backstrom, Ekberg, Hirschberg, Bixo, Epperson and Briggs125 Active drug and placebo were administered repeatedly as subcutaneous injections during the luteal phase, and treatment was double-blinded. A significant reduction of negative mood was detected on a validated PMDD scale during 9 days in the luteal phase, and no severe side-effects were reported. Reference Bixo, Ekberg, Poromaa, Hirschberg, Jonasson and Andreen124,Reference Backstrom, Ekberg, Hirschberg, Bixo, Epperson and Briggs125 Moreover, the effect size was similar to that obtained in other treatment studies with SSRIs or COCs in women with PMDD and using the same outcome measures. Reference Cohen, Miner, Brown, Freeman, Halbreich and Sundell126–Reference Yonkers, Brown, Pearlstein, Foegh, Sampson-Landers and Rapkin128
Future directions
In conclusion, women with PMDD react differently when exposed to neurosteroids, such as the progesterone metabolite allopregnanolone, a positive allosteric modulator of the GABAA receptor. This has been shown in fMRI studies, as well as clinically in anovulatory women given a cyclical treatment with bioidentical oestradiol and progesterone that mimic the menstrual cycle. A potential target for future drug development is the GABAA receptor itself, and/or drugs that modulate the effect of allosteric modulators – for example, allopregnanolone. The future drug development suggested above is costly and will take many years (from animal experiments to human experimentation and clinical drug trials). Nevertheless, improvement of PMDD treatment is possible based on current knowledge. Suppressed ovulation is successful, providing that levels of oestrogen and progestogen can be balanced and kept stable. Clinical trials with larger populations and longer follow-up could give useful insight into how to tailor these treatments. Selective modulators of steroid receptors and combinations of GnRH antagonists and add-back hormones are available for other indications, and could be tested in clinical trials for PMDD. The latter could be more feasible for patients than injectable GnRH analogues with separate add-back hormones, because this combined compound is administered orally as one tablet per day. Also, initial flare-up effects, which are common with GnRH analogues, are avoided.
Data availability statement
This narrative review is mainly based on previous research from which data are not available to the authors. Data from studies by the authors are available on request.
Author contributions
M.B., L.S. and T.B. contributed to the conception, drafting and final approval of this work. The authors are accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
T.B. has shares in Umecrine Cognition. M.B. and L.S. have no conflicts of interest.







eLetters
No eLetters have been published for this article.