There are currently eight antipsychotic long-acting injections (LAIs) in widespread use around the world. Six of these are LAI preparations of first-generation antipsychotics (FGAs), including flupentixol, fluphenazine, haloperidol, perphenazine, pipotiazine and zuclopenthixol, and the remaining two are LAI formulations of the second-generation antipsychotics (SGAs) risperidone and olanzapine. The dosing characteristics of these LAIs are summarised in Table 1.
Table 1 Pharmacokinetic and pharmacodynamic characteristics of antipsychotic long-acting injections
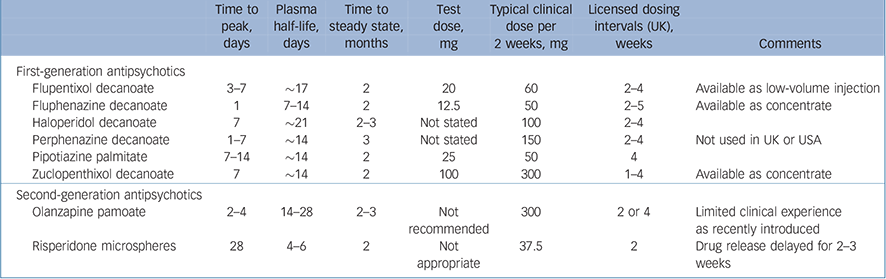
| Time to peak, days | Plasma half-life, days | Time to steady state, months | Test dose, mg | Typical clinical dose per 2 weeks, mg | Licensed dosing intervals (UK), weeks | Comments | |
|---|---|---|---|---|---|---|---|
| First-generation antipsychotics | |||||||
| Flupentixol decanoate | 3–7 | ∼17 | 2 | 20 | 60 | 2–4 | Available as low-volume injection |
| Fluphenazine decanoate | 1 | 7–14 | 2 | 12.5 | 50 | 2–5 | Available as concentrate |
| Haloperidol decanoate | 7 | ∼21 | 2–3 | Not stated | 100 | 2–4 | |
| Perphenazine decanoate | 1–7 | ∼14 | 3 | Not stated | 150 | 2–4 | Not used in UK or USA |
| Pipotiazine palmitate | 7–14 | ∼14 | 2 | 25 | 50 | 4 | |
| Zuclopenthixol decanoate | 7 | ∼14 | 2 | 100 | 300 | 1–4 | Available as concentrate |
| Second-generation antipsychotics | |||||||
| Olanzapine pamoate | 2–4 | 14–28 | 2–3 | Not recommended | 300 | 2 or 4 | Limited clinical experience as recently introduced |
| Risperidone microspheres | 28 | 4–6 | 2 | Not appropriate | 37.5 | 2 | Drug release delayed for 2–3 weeks |
Pharmacokinetic characteristics
Almost all antipsychotics have short plasma half-lives of 1–2 days at most. This means that antipsychotics have to be administered frequently in order to attain and maintain therapeutic effects. Although the elimination half-lives of these drugs cannot be altered to any important degree, chemical manipulation of the parent compound and changes in pharmaceutical formulation can significantly alter drug release or absorption characteristics. When absorption and/or release are extensively prolonged, effective plasma half-life can be extended (half-life is dependent on release rather than elimination).
Some FGAs possess a terminal alcohol (–OH) group which allows them to be combined with carboxylic acids by a process of esterification. Long-chain esters show high oil solubility and low water solubility. Long-acting injectable formulations of FGAs contain long-chain drug esters (e.g. decanoate, palmitate) dissolved in a vegetable oil. When injected intramuscularly, the oil forms a depot of drug: the drug ester slowly diffuses into the blood stream and is then rapidly hydrolysed to release the parent drug. Reference Barnes and Curson1 Most SGAs lack terminal –OH groups suitable for esterification. Modification of release characteristics is brought about by other means: encapsulating the drug into a biodegradable polymer (risperidone) or injecting a suspension (i.e. water-insoluble particulate matter in water) of drug compound (olanzapine pamoate).
First-generation LAIs
Flupentixol
Flupentixol is a thioxanthine antipsychotic. Flupentixol LAI is formulated as flupentixol decanoate in a low-viscosity vegetable oil (fractionated coconut oil). Flupentixol decanoate provides peak plasma levels 3–7 days after intramuscular injection and shows an apparent half-life of 17 days. Reference Jann, Ereshefsky and Saklad2,Reference Stauning, Kirk and Joorgensen3 Steady-state plasma levels can be expected to be achieved after 2 months or so of regular dosing. In practice, plasma levels may show marked variability independent of dose changes. Reference Tuninger and Levander4
Fluphenazine
Fluphenazine is a piperazine phenothiazine compound. Fluphenazine decanoate is available as an LAI in sesame oil. Plasma levels peak within 24 h of intramuscular injection, Reference Wiles and Gelder5 and the half-life is approximately 7–14 days. Reference Barnes and Curson1,Reference Curry, Whelpton, De Schepper, Vranckx and Schiff6 Plasma levels obtained vary up to 40-fold in patients receiving the same dose. Reference Wiles and Gelder5 Smoking significantly reduces plasma fluphenazine levels. Reference Ereshefsky, Jann, Saklad, Davis, Richards and Burch7
Haloperidol
Haloperidol is a butyrophenone and is available as haloperidol decanoate in sesame oil. Peak plasma levels are seen up to 7 days after intramuscular injection and plasma half-life is around 3 weeks. Reference Barnes and Curson1,Reference Jann, Ereshefsky and Saklad2 Steady-state plasma levels can be expected to be reached after 2–3 months of regular dosing. Reference Kissling, Moller, Walter, Wittmann, Krueger and Trenk8 As with fluphenazine, clearance of haloperidol is significantly increased by smoking. Reference Jann, Saklad, Ereshefsky, Richards, Harrington and Davis9 Nonetheless, variation in plasma levels is smaller in patients receiving haloperidol decanoate than in those receiving oral haloperidol. Reference Nayak, Doose and Nair10
Perphenazine
Perphenazine is a piperazine phenothiazine available as perphenazine decanoate in sesame oil, used mainly in northern Europe and Scandinavia. After intramuscular injection, peak plasma levels are obtained in 1–7 days and the half-life is approximately 2 weeks. Reference Knudsen, Hansen and Larsen11 Steady-state levels are obtained after 3 months. Reference Larsen and Hansen12 Variations in plasma levels during regular dosing are small, Reference Knudsen, Hansen and Larsen11 and plasma levels are directly correlated with dose. Reference Kistrup, Gerlach, Aaes-Jorgensen and Larsen13
Pipotiazine
Pipotiazine is a piperidine phenothiazine antipsychotic. The LAI formulation contains pipotiazine palmitate in coconut oil. This provides peak plasma levels after 1–2 weeks although no drug is released for at least 3 days. Reference Girard, Granier, Schmitt, Cotonat, Escande and Blanc14 Other sources suggest peak levels are seen within 24 h. Reference Barnes and Curson1,Reference Altamura, Sassella, Santini, Montresor, Fumagalli and Mundo15 Plasma half-life is around 2 weeks and the time to steady state is 2 months. Reference Barnes and Curson1,Reference Girard, Granier, Schmitt, Cotonat, Escande and Blanc14,Reference Altamura, Sassella, Santini, Montresor, Fumagalli and Mundo15
Zuclopenthixol
Like flupentixol, zuclopenthixol is a thioxanthine compound. Zuclopenthixol LAI is formulated as the decanoate ester dissolved in thin vegetable oil (fractionated coconut oil). Peak plasma levels of zuclopenthixol are achieved a week after injection. Reference Viala, Hou, Ba, Durand, Dufour and D'Agostino16 Plasma half-life has been estimated at 7.4 days and 19 days. Reference Poulsen, Olesen and Larsen17,Reference Jorgensen and Overo18 Zuclopenthixol LAI shows moderate inter- and intra-individual differences in plasma levels, Reference Viala, Ba, Durand, Gouezo, Hou and Jorgensen19 and marked differences between peak and trough plasma levels when given every 2 weeks (peak levels more than 3 times higher than trough). Reference Poulsen, Olesen and Larsen17 Steady-state plasma levels are achieved after around 2 months of regular dosing. Reference Viala, Ba, Durand, Gouezo, Hou and Jorgensen19
Second-generation LAIs
Olanzapine
Olanzapine LAI is a salt of pamoic acid and olanzapine (olanzapine pamoate) suspended in water. This formulation provides peak plasma levels some 2–4 days after intramuscular administration and a plasma half-life of 2–4 weeks. Reference Kurtz, Bergstrom, McDonnell and Mitchell20 Plasma levels obtained are directly proportional to dose given and time to steady state is around 2–3 months. Reference Kurtz, Bergstrom, McDonnell and Mitchell20 In 2-weekly dosing trough levels are around 50% of peak level; in monthly dosing trough levels are 75% lower than peak. Reference Kurtz, Bergstrom, McDonnell and Mitchell20 Olanzapine pamoate dissociates readily in aqueous environments (e.g. plasma) so its pharmacokinetic properties depend on the method of administration (see below).
Risperidone
As with olanzapine LAI, the formulation of risperidone LAI is materially different from the ester-in-oil preparations of first-generation antipsychotics so far described. Its LAI formulation contains risperidone encapsulated into polymeric microspheres which requires cold storage to retain its intended release characteristics. These microspheres are made up of biodegradable copolymer which is slowly hydrolysed in vivo to release risperidone. Reference Ramstack, Grandolfi, Mannaert, D'Hoore and Lasser21 After intramuscular injection a small amount of risperidone is released from the surface of the microspheres, Reference Ramstack, Grandolfi, Mannaert, D'Hoore and Lasser21 but then further release is delayed for 2–3 weeks, during which time erosion of the microspheres takes place. In 2-weekly dosing peak plasma levels are seen at around 4 weeks after the first injection and the plasma half-life is of the order of 4–6 days. Reference Gefvert, Eriksson, Persson, Helldin, Bjorner and Mannaert22 Steady-state plasma levels are obtained after 8 weeks, at which time peak-to-trough levels vary threefold. Reference Gefvert, Eriksson, Persson, Helldin, Bjorner and Mannaert22
Dose and administration
As with oral antipsychotics, dosing requirements for LAIs in individuals vary enormously. Examination of population data (as below) can provide some guidance on optimum dosing, but individual variation should always be borne in mind and dose adjustments should be according to patient response and tolerability.
Flupentixol decanoate is given initially as a test dose of 20 mg. After 7 days a further dose of 20–40 mg is given. Dosing is then at intervals of 2–4 weeks, at doses ranging from 50 mg every 4 weeks to 400 mg weekly. Dose–response relationships are less than clear. One study showed 40 mg every 2 weeks to be as effective in relapse prevention as 40 mg every 4 weeks. Reference Agrup-Andersson, Bengtsson, Erlandsson, Gottfries and Witzell-Ostlund23 Other trials lend some support to the use of 40 mg every 2 weeks as the optimal dose of this compound. Reference McCreadie, Flanagan, McKnight and Jorgensen24,Reference Johnson, Ludlow, Street and Taylor25 Both studies were double-blind. In one study, 40 mg every 2 weeks was shown to be as effective in improving ‘ward behaviour’ and better tolerated than 200 mg every 2 weeks in female patients over 13 weeks' treatment. Reference McCreadie, Flanagan, McKnight and Jorgensen24 In the other study a 50% dose reduction to 6 mg per week led to substantially increased risk of relapse compared with participants not undergoing dose reduction receiving on average 9 mg per week. Reference Johnson, Ludlow, Street and Taylor25 In contrast, another study in patients stabilised on higher doses showed that reducing the dose of flupentixol below 200 mg every 2 weeks significantly increased the risk of relapse. Reference Cookson26 This study was also a double-blind randomised trial but included only 18 participants.
Fluphenazine decanoate is initiated with a test dose of 12.5 mg. Maintenance doses are in the range 12.5–100 mg given every 2–5 weeks – the maximum dose is thus 100 mg every 2 weeks. Much lower doses appear to be effective – 25 mg every 2 weeks is no more effective than 25 mg every 6 weeks. Reference Carpenter, Buchanan, Kirkpatrick, Lann, Breier and Summerfelt27 There is some support for even lower doses, Reference Geller28 but controlled trials suggest that doses substantially less than 25 mg every 2 weeks greatly increase the risk of relapse. Reference Marder, Van Putten, Mintz, Lebell, McKenzie and May29,Reference Capstick30
For haloperidol decanoate no formal test dose is specified. Treatment is usually begun with a dose of 50 mg and maintenance doses are usually in the range 50–300 mg every 4 weeks (in the USA up to 20 times the previous oral haloperidol dose can be used). Doses of around 100 mg every 4 weeks are probably optimal; Reference Kane, Davis, Schooler, Marder, Casey and Brauzer31,Reference Taylor32 relapse prevention is not improved by higher doses. A dose of 25 mg every 4 weeks is probably ineffective, Reference Kane, Davis, Schooler, Marder, Casey and Brauzer31 whereas doses of around 50 mg every 4 weeks may be effective in some patients. Reference Kane, Davis, Schooler, Marder, Casey and Brauzer31,Reference Eklund and Forsman33
Perphenazine plasma levels of 1–5 nmol/l have been associated with good therapeutic response, Reference Omerov, Wistedt, Bolvig-Hansen and Larsen34 although a higher therapeutic range has been suggested. Reference Mazure, Nelson, Jatlow, Kincare and Bowers35 In a study that aimed to discover the minimum effective dose of perphenazine decanoate, it was found that this was around 100 mg every 2 weeks (range 21.6–270.5 per 2 weeks). Reference Kistrup, Gerlach, Aaes-Jorgensen and Larsen13 Plasma levels ranged from around 2 nmol/l to 18 nmol/l depending on dose. In a further study of responding patients receiving 108.5 mg perphenazine decanoate every 2 weeks, plasma levels averaged 5 nmol/l and ranged from around 2 nmol/l to 6 nmol/l. Reference Knudsen, Hansen and Larsen11 Other studies have shown that doses of around 100 mg every 2 weeks are effective and provide plasma levels in the range 3–11 nmol/l. Reference Knudsen, Hansen, Hojholdt and Larsen36,Reference Knudsen, Hansen, Hojholdt and Larsen37
Pipotiazine palmitate is usually first given as a test dose of 25 mg. Maintenance doses are usually within the range 50–200 mg every month (up to 250 mg in Canada). The dose–response relationship is poorly defined. An early study suggested that the optimal dose of pipotiazine palmitate was between 100 mg and 600 mg every 4 weeks. Reference Gallant, Mielke, Bishop, Oelsner and Guerrero-Figueroa38 Later trials found doses of 50–200 mg every 4 weeks to be adequately effective. Reference Schmidt39,Reference Burch and Ayd40
Zuclopenthixol decanoate is initiated with a test dose of 100 mg and maintenance doses vary from 200 mg every 4 weeks to 600 mg a week. In an open dose-reduction study of 23 patients, the minimum effective dose was shown to be, on average, 200 mg every 2 weeks (range 60–400 mg). Reference Solgaard, Kistrup, Aaes-Jorgensen and Gerlach41 In practice zuclopenthixol is often given at weekly or 2-weekly intervals. However, doses of 100–600 mg (mean 284 mg) given every 4 weeks have been shown to be as effective in preventing relapse as haloperidol decanoate (38–200 mg per month). Reference Wistedt, Koskinen, Thelander, Nerdrum, Pedersen and Molbjerg42 Fortnightly or monthly administration thus seems reasonable in practice in the out-patient setting for maintenance treatment.
Olanzapine pamoate is licensed to be given in doses ranging from 150 mg every 2 weeks to 300 mg every 2 weeks. Supplementation with oral olanzapine is not usually required owing to prompt early release of olanzapine from the formulation. In a placebo-controlled trial, doses of 210 mg per 2 weeks, 300 mg per 2 weeks and 405 mg per 4 weeks were equally effective and well tolerated. Reference Lauriello, Lambert, Andersen, Lin, Taylor and McDonnell43
Risperidone LAI is usually begun at a dose of 25 mg or 37.5 mg according to the dose of antipsychotic previously received. Ideally, patients should first be treated with oral risperidone to assess dose requirements and tolerability. Supplementary oral antipsychotic treatment is required for 4–6 weeks after the first risperidone LAI. Dosing is licensed only at 2-weekly intervals, although there is emerging evidence that monthly dosing might be effective. Reference Gharabawi, Gearhart, Lasser, Mahmoud, Zhu and Mannaert44 Doses of 25 mg, 37.5 mg, 50 mg and 75 mg every 2 weeks have been shown to be broadly equal in effectiveness in clinical trials. Reference Moller45–Reference Keks, Ingham, Khan and Karcher47 The maximum licensed dose is 50 mg every 2 weeks (a dose of 75 mg per 2 weeks causes significantly more extrapyramidal symptoms). Reference Kane, Eerdekens, Lindenmayer, Keith, Lesem and Karcher46 In practice, the lowest licensed dose of 25 mg every 2 weeks may be relatively ineffective. Reference Taylor, Young and Patel48,Reference Bai, Ting, Chen, Chang, Wu and Hung49
Pharmacodynamics
Both first-generation and second-generation antipsychotics administered by any route are presumed to exert their antipsychotic action by blocking central dopamine receptors of the D2 receptor subtype. All LAIs are potent in vitro antagonists of D2 receptors. In vivo, potency in humans is usually determined by measuring striatal D2 occupancy using methods such as positron emission tomography (PET) and single photon emission computerised tomography (SPECT). Therapeutic response seems to be associated with striatal D2 occupancies of 65% or greater, whereas hyperprolactinaemia and extrapyramidal side-effects occur at occupancies of 72% and 78% respectively, Reference Kapur, Zipursky, Jones, Remington and Houle50 although these observations are based on a single study including a limited number of individuals. Antipsychotic action may not be derived from activity in the striatum, but striatal occupancies correlate closely with response to antipsychotic treatment. Reference Agid, Mamo, Ginovart, Vitcu, Wilson and Zipursky51
Few studies have examined striatal D2 occupancy afforded by the use of LAIs. The earliest PET study found occupancies of 50–80% for patients receiving haloperidol decanoate 50–200 mg a month, pipotiazine palmitate 100 mg a month and fluphenazine decanoate 200 mg a month. Reference Baron, Martinot, Cambon, Boulenger, Poirier and Caillard52 Occupancy levels appeared to increase in the 20 days following injection. A later study of four patients receiving 30–50 mg haloperidol decanoate a month showed that peak D2 occupancies were in the range 66–82% a week after injection. Reference Nyberg, Farde and Halldin53 In an extension of this study (n=8, including two patients who participated in the previous study), D2 occupancies peaked at 60–82% a week after injection (doses 30–50 mg every 4 weeks). Reference Nyberg, Farde, Halldin, Dahl and Bertilsson54 Four weeks later occupancies were in the range 20–74%. The patients remained well despite these low occupancies.
A relatively recent study of patients responding to perphenazine decanoate found that at plasma levels of 1.8–9 nmol/l striatal D2 occupancy ranged from 66% to 82%. Reference Talvik, Nordstrom, Larsen, Jucaite, Cervenka and Halldin55 Doses used were in the range 38–108 mg per 2 weeks.
One study has examined D2 occupancy produced by olanzapine pamoate. Reference Mamo, Kapur, Keshavan, Laruelle, Taylor and Kothare56 Participants were switched from oral olanzapine to the LAI (300 mg every 4 weeks) and followed up for 6 months. Dopamine D2 receptor occupancy averaged 69% for oral olanzapine (5–20 mg per day) but then fell to around 55% in the first month before rising to just over 60% (all trough samples). There was no change in patients' clinical condition over the study period.
Two studies have determined D2 occupancy associated with the use of risperidone LAI. In the first, 28 patients received 2-weekly injections of 25 mg, 50 mg or 75 mg risperidone and D2 occupancy was estimated 2 weeks after the fifth injection (i.e. at steady-state trough plasma levels): the D2 occupancies were 25–48% for 25 mg, 59–83% for 50 mg and 62–72% for 75 mg. Reference Gefvert, Eriksson, Persson, Helldin, Bjorner and Mannaert22 In the second study D2 occupancies were determined for nine participants up to 3 days after injection and less than 5 days before the next injection. Reference Remington, Mamo, Labelle, Reiss, Shammi and Mannaert57 All participants had at least five injections before PET determinations were carried out. Occupancies were 53–75% for two patients receiving 25 mg, 59–85% for five patients receiving 50 mg and 71–85% for two patients receiving 75 mg.
Data on D2 occupancies associated with the use of LAIs are somewhat perplexing. They appear to show apparently subtherapeutic D2 occupancies associated with some doses, at least at some time points, without attendant relapse. This may mean that persistently high striatal D2 receptor occupancy is not required for full therapeutic effect, or that other D2 activity outside the striatum or other receptor activities are relevant. On the other hand, the low occupancies seen with low doses of haloperidol and risperidone do fit with clinical data which suggest that these doses are suboptimal. Reference Kane, Davis, Schooler, Marder, Casey and Brauzer31,Reference Eklund and Forsman33,Reference Taylor, Young and Patel48,Reference Bai, Ting, Chen, Chang, Wu and Hung49
Adverse effects
Long-acting injection formulations of FGAs might be expected to give rise to acute extrapyramidal symptoms, tardive dyskinesia and symptoms related to hyperprolactinaemia. Reference Haddad, Taylor and Niaz58 The frequency of movement disorders and tardive dyskinesia with FGA–LAIs is similar to that seen with oral FGAs. Reference Adams, Fenton, Quraishi and David59 Consequently the clinical utility of the LAIs is often seen to be severely limited by the likelihood of these important adverse effects (Appendix 1).
Flupentixol decanoate typically causes extrapyramidal symptoms, sedation and dry mouth. Reference Eberhard and Hellbom60 In one study of patients receiving 40 mg every 3 weeks, 97% showed signs of extrapyramidal symptoms and 82% received anti-Parkinsonian medication. Reference Knights, Okasha, Salih and Hirsch61 Fluphenazine decanoate (25 mg every 3 weeks) in the same trial caused extrapyramidal symptoms in 97% of patients and 93% required anti-Parkinsonian medication. Rates of other important side-effects such as incident depression were virtually identical for flupentixol and fluphenazine (53% v. 55%). Reference Knights, Okasha, Salih and Hirsch61 Fluphenazine decanoate is also associated with blurred vision and excess salivation. Reference Johnson62 Weight gain of more than 1 kg is common. Reference Chouinard, Annable and Campbell63 Changes in prolactin levels seem to be dose-related. Reference Bechelli, Iecco, Acioli and Pontes64
Haloperidol decanoate is also associated with a dose-dependent rise in plasma prolactin and in one study caused extrapyramidal symptoms severe enough to warrant anti-Parkinsonian drugs in around 90% of patients (dose averaging around 250 mg every 2–4 weeks). Reference Chouinard, Annable and Campbell63 Although haloperidol decanoate is often assumed to give the highest risk of extrapyramidal symptoms, this has not been demonstrated in comparative studies. Reference Kissling, Moller, Walter, Wittmann, Krueger and Trenk8,Reference Wistedt, Koskinen, Thelander, Nerdrum, Pedersen and Molbjerg42,Reference Wistedt, Persson and Hellbom65 Weight gain may be less common than with fluphenazine decanoate. Reference Wistedt66
Perphenazine decanoate, like other first-generation drugs, is associated with acute extrapyramidal symptoms. Reported prevalences of such symptoms or use of anticholinergic drugs range from 29% Reference Knudsen, Hansen, Hojholdt and Larsen36 to 55%. Reference Kistrup, Gerlach, Aaes-Jorgensen and Larsen13 Other studies suggest high rates of extrapyramidal symptoms and hyperprolactinaemia with perphenazine enanthate, Reference Lindholm, Gullberg, Ohman and Sedvall67,Reference Ahlfors, Dencker, Gravem and Remvig68 but this ester of perphenazine is known to result in much higher plasma levels of perphenazine than perphenazine decanoate. Reference Knudsen, Hansen, Auken, Waehrens, Hojholdt and Larsen69 The total number of patients examined in well-conducted trials with either ester appears to be only 313. Reference David, Quraishi and Rathbone70 Injection site abscesses have been reported for perphenazine enanthate. Reference Starmark, Forsman and Wahlstrom71 Weight gain seems not to occur with perphenazine decanoate. Reference Knudsen, Hansen, Hojholdt and Larsen36
Pipotiazine palmitate (mean dose 65 mg per month) has been shown to cause similar rates of extrapyramidal symptoms as haloperidol decanoate (mean dose 100 mg per month): 36% and 29% respectively. Reference Bechelli, Iecco, Acioli and Pontes64 In the same study weight gain of more than 5 kg was more common in those receiving pipotiazine (39% v. 16%). As a piperidine phenothiazine, pipotiazine might be expected to show a low incidence of extrapyramidal symptoms. There is limited evidence that pipotiazine has a relatively low potential for causing these symptoms compared with other LAIs. Reference Burch and Ayd40
Zuclopenthixol typically causes extrapyramidal symptoms and mild autonomic effects such as postural dizziness and blurred vision. Reference Wistedt, Koskinen, Thelander, Nerdrum, Pedersen and Molbjerg42 In one study of doses ranging from 50 mg to 300 mg every 2 weeks, 61% of participants experienced extrapyramidal symptoms and 78% required anti-Parkinsonian medication. Reference Solgaard, Kistrup, Aaes-Jorgensen and Gerlach41
A meta-analysis of comparative trials of FGA–LAI doses revealed no differences in the rates of extrapyramidal symptoms and tardive dyskinesia compared with oral medication and few important differences between individual LAIs. Reference Adams, Fenton, Quraishi and David59 Other sources suggest an increased incidence of extrapyramidal symptoms and tardive dyskinesia with LAIs compared with oral antipsychotics. Reference Barnes and Curson1 Both reviews reported an increased risk of movement disorders with fluphenazine decanoate compared with other FGA–LAIs. Reference Haddad, Taylor and Niaz58 All FGA–LAIs are associated with acute and chronic local reactions at injection sites, particularly when used in high doses or injection volumes. Reference Hay72
Adverse effects seen with olanzapine pamoate are similar to those seen with oral olanzapine. Common side-effects include increased plasma triglycerides, weight gain, sedation and increased appetite. Reference Lauriello, Lambert, Andersen, Lin, Taylor and McDonnell43 Extrapyramidal symptoms have not been reported more frequently than with oral olanzapine and the incidence of tardive dyskinesia is not known.
There has been no report of any important difference in adverse events between oral olanzapine and the LAI formulation, with the exception of a safety risk that emerged in clinical trials: post-injection syndrome. Reference McDonnell, Sorsaburu, Brunner, Detke, Anderson and Bergstrom73 This came to light when an unanticipated degree of sedation was observed in a small number of patients following an injection. This adverse event has occurred in 24 patients (up to end September 2007), an incidence of 1.2% of patients treated with olanzapine pamoate or 0.07% of injections given. Post-injection syndrome consists of sedation, confusion, dizziness, altered speech/dysarthria, somnolence and/or unconsciousness. These effects usually occur within an hour of injection, but the median time ranged from 20 min to 3 h after injection. To date all patients have fully recovered from this adverse event, usually within 3–72 h, without permanent sequelae and the majority (67%) have continued to receive the LAI formulation. Blood samples of olanzapine plasma concentrations were taken during the events and were found to be substantially elevated (Fig. 1).
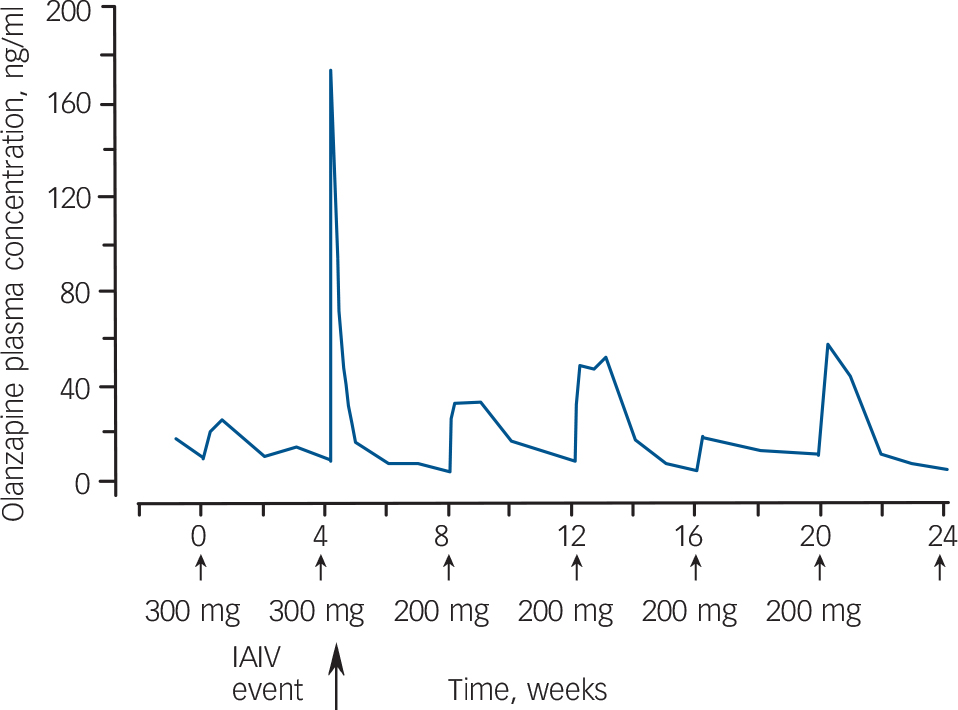
Fig. 1 Olanzapine plasma concentration profiles for a period during which six injections were administered to a patient who experienced an inadvertent intravascular (IAIV) event after the second injection. Reference Lilly75
Solubility experiments have revealed that when olanzapine pamoate LAI is injected into the muscle as intended, the dissolution of the salt is gradual and results in a slow release of drug into the blood stream. However, if the salt comes into contact with a considerable amount of blood or plasma, as occurs if the needle punctures a vessel or enters a rich capillary bed during administration, the salt dissolves and therefore dissociates more quickly. Other factors thought to affect the dissolution rate of the pamoate salt are the volume and rate of blood flow and the degree of vascular injury. Reference Lilly74
The patient should be advised about the potential risk of post-injection syndrome and needs to be observed for 3 h in a healthcare facility each time that the injection is administered. Reference Lilly74 Prior to giving the injection the clinician should determine that the patient will not have to travel alone to his or her next destination. During the post-injection observation period it should be confirmed that the patient is alert and oriented and that there is no sign or symptom of overdose. If post-injection syndrome is suspected, close medical supervision and monitoring should continue until examination indicates that signs and symptoms have resolved. For the remainder of the day after injection, patients should be advised to be vigilant for signs and symptoms of overdose secondary to post-injection adverse reactions, should be able to obtain assistance if needed and should not drive or operate machinery.
Risperidone LAI is associated with relatively low rates of movement disorder. Reference Moller45 In one study extrapyramidal symptoms were seen in 10% of participants receiving 25 mg every 2 weeks and in 24% of those receiving 50 mg. Reference Kane, Eerdekens, Lindenmayer, Keith, Lesem and Karcher46 Other studies confirm this low rate of movement disorder. Reference Keks, Ingham, Khan and Karcher47,Reference Schmauss, Sacchetti, Kahn and Medori76 Weight gain averages around 3 kg in the longer term (up to 1 year). Reference Moller45 Risperidone LAI is associated with an incidence of increased plasma prolactin similar to that seen with oral risperidone but the magnitude of this increase is lower, Reference Moller45 perhaps because risperidone LAI is associated with lower plasma levels of the active drug moiety. Reference Bai, Ting, Chen, Chang, Wu and Hung49 Injection site pain and local reactions have been carefully evaluated – pain is not usually severe and injection site reactions are rare. Reference Moller45 Outside clinical trials, injection site pain leading to treatment withdrawal is uncommon. Reference Taylor, Young and Patel48,Reference Niaz and Haddad77 Tardive dyskinesia has been shown to emerge in 1.19% of treated individuals over 1 year. Reference Gharabawi, Bossie, Zhu, Mao and Lasser78 A similarly low incidence is also seen in more susceptible populations such as elderly patients. Reference Kissling, Glue, Medori and Simpson79 (Comparative data for FGA–LAIs are scant. One study observed an increase in the prevalence of oral tardive dyskinesia from 8% to 22% over a 3-year period during which fluphenazine LAI or flupentixol LAI were used.) Reference Gibson80
Initiation and switching to LAIs
The use of LAIs is confined to those who have already received antipsychotic medication: these injections should never be given to antipsychotic-naive patients. Treatment with LAIs is therefore initiated in those already taking antipsychotics or those who, for whatever reason, have recently stopped taking them. Initiation of LAIs is complicated by a number of factors (Appendix 2). First-generation antipsychotic LAIs are usually first given as a test dose, or at least at a dose at the lower end of the licensed range. These test doses are aimed at establishing patient tolerability of the drug and vehicle administered. However, test doses given in this way only really establish or otherwise the tolerability of a single, small dose of the LAI. Their use has little bearing on likely tolerability in multiple, higher-dose treatment because plasma levels at steady state are considerably higher than after a single dose. For example, in one study in which patients were given fluphenazine decanoate 50 mg every week, plasma levels quadrupled between the end of the first week and the end of the sixth week. Reference Ereshefsky, Saklad, Jann, Davis, Richards and Seidel81 This substantial rise in plasma levels despite there being no change in dose is likely to be seen with all LAIs because of their shared long plasma half-lives, and makes sensible dose titration almost impossible. An added difficulty here is that population dose–response relationships are poorly defined for most LAIs (olanzapine, risperidone and haloperidol are the exceptions). In practice, therefore, dose optimisation is a matter largely of conjecture, and patient tolerability is often used as a proxy for dose limitation. Furthermore, the use of oral antipsychotics as ‘cover’ during the early stages of LAI treatment often leads to prolonged polypharmacy (there is evidence that prescribers tend not to change regimens once patients improve, presumably because to do so would be to risk relapse). Reference Taylor, Mir, Mace and Whiskey82
Conclusion
All antipsychotic LAIs enable infrequent administration and assurance of adherence (or at least ready detection of non-adherence). However, appropriate prescribing of LAI preparations is clearly complicated by their shared prolonged apparent half-lives, their delayed release (particularly with risperidone) and the risk of post-injection syndrome (olanzapine). The use of test doses appears not to mitigate this complexity nor to minimise tolerability difficulties. Moreover, the lack of robust dose–response data for most preparations makes for rather approximate dosing in practice. Even with risperidone and haloperidol LAIs (for which fixed-dose studies have established the dose–response relationship) doubts persist over the clinical value of doses at the lower end of the dose range.
First-generation antipsychotic LAIs are clearly linked to high rates of extrapyramidal effects and tardive dyskinesia, among other adverse effects. There appear to be few differences between individual FGA–LAIs, although fluphenazine decanoate may be associated with a relatively higher rate of movement disorder and pipotiazine palmitate a relatively lower rate. Risperidone LAI has, on the face of it, important advantages over FGA drugs in respect of movement disorders, but its use is complicated – and clinical utility compromised – by excessively delayed release and by the requirement for cold storage of the product. Olanzapine LAI also appears to have advantages over FGA formulations but its use is complicated by the risk of post-injection syndrome and by the risk of metabolic adverse effects. The apparent advantages of SGA–LAIs need to be confirmed in large-scale randomised controlled trials. It is notable that the claimed advantages of oral SGAs become less apparent when these drugs are directly compared with oral FGAs in well-conducted trials. Reference Lieberman, McEvoy, Swartz, Rosenheck, Perkins and Keefe83,Reference Jones, Barnes, Davies, Dunn, Lloyd and Hayhurst84
Appendix 1
Adverse effects
Extrapyramidal symptoms
Symptoms are tremor, rigidity, mask-like face, reduced arm swing, drooling, dystonia (muscular spasm), oculogyric crisis, akathisia (severe inner restlessness).
Tardive dyskinesia
Tardive dyskinesia is characterised by:
-
(a) abnormal masticatory movements (lip-smacking, licking);
-
(b) tongue protrusion;
-
(c) vermicular (worm-like) movement;
-
(d) abnormal postures (tardive dystonia): opisthotonos (arching of back and neck), retrocollis (backward arching of head and neck), torticollis (sideways turn of head and neck);
-
(e) digital abnormalities (‘pill-rolling’, ‘piano playing’, ‘guitar playing’);
-
(f) grimacing.
Metabolic adverse effects
Effects include impaired glucose tolerance/diabetes mellitus, weight gain, increased triglyceride levels, increased cholesterol levels.
Appendix 2
Factors complicating long-acting injection dosing
-
(a) The effect of any dose is likely to be prolonged. Test doses are usually required but may not properly assess tolerability in longer-term use.
-
(b) Some long-acting injections (LAIs) show delayed as well as prolonged release. Cover with oral antipsychotics may be necessary.
-
(c) Attainment of steady-state plasma levels is usually delayed for up to 2–3 months. During this time, plasma levels are likely to rise substantially even when dosages are not increased.
-
(d) Dose–response relationships are poorly defined for most LAIs.





eLetters
No eLetters have been published for this article.