White matter abnormalities constitute one element of the pathogenesis of major depressive disorder (MDD). Reference Drevets, Price and Furey1,Reference Taylor, Hsu, Krishnan and MacFall2 Diffusion tensor imaging (DTI) is a reliable magnetic resonance technique for examining white matter microstructure in vivo and has provided information that can estimate the integrity of pathways within relevant neural networks. A commonly used DTI-based quantitative measure is the fractional anisotropy value, which estimates the degree to which tissue organisation limits diffusion of water molecules in white matter fibre tracts. For example, the fractional anisotropy value is thought to provide a useful marker of white matter fibre tract integrity, with high values of fractional anisotropy indicating intact healthy neurons. Recently, tract-based spatial statistics (TBSS), a novel voxel-wise approach, was introduced. Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols and Mackay3 This method has superior objectivity and interpretability of DTI analyses than the regions of interest method. In addition, TBSS can analyse the entire brain for group differences or correlations, so it is not restricted to specific brain areas. Previous DTI studies using TBSS methods have found abnormalities (i.e. reduced fractional anisotropy) of various white matter fibre tracts in people with MDD, which include the anterior thalamic radiation, Reference Bessette, Nave, Caprihan and Stevens4,Reference Lagopoulos, Hermens, Hatton, Battisti, Tobias-Webb and White5 inferior fronto-occipital fasciculi, Reference Bessette, Nave, Caprihan and Stevens4,Reference Lagopoulos, Hermens, Hatton, Battisti, Tobias-Webb and White5 uncinate fasciculi, Reference Bessette, Nave, Caprihan and Stevens4–Reference Zuo, Fang, Lv, Zhou, Hong and Li6 sagittal stratum, Reference Korgaonkar, Grieve, Koslow, Gabrieli, Gordon and Williams7,Reference Kieseppa, Eerola, Mantyla, Neuvonen, Poutanen and Luoma8 cingulum, Reference Bessette, Nave, Caprihan and Stevens4,Reference Korgaonkar, Grieve, Koslow, Gabrieli, Gordon and Williams7,Reference Henderson, Johnson, Vallejo, Katz, Wong and Gabbay9 longitudinal fasciculi, Reference Bessette, Nave, Caprihan and Stevens4,Reference Zuo, Fang, Lv, Zhou, Hong and Li6,Reference Cole, Chaddock, Farmer, Aitchison, Simmons and McGuffin10,Reference Versace, Almeida, Quevedo, Thompson, Terwilliger and Hassel11 internal and external capsule, Reference Bessette, Nave, Caprihan and Stevens4,Reference Korgaonkar, Grieve, Koslow, Gabrieli, Gordon and Williams7,Reference Guo, Liu, Xue, Gao, Wu and Ma12 corpus callosum, Reference Bessette, Nave, Caprihan and Stevens4,Reference Kieseppa, Eerola, Mantyla, Neuvonen, Poutanen and Luoma8,Reference Cole, Chaddock, Farmer, Aitchison, Simmons and McGuffin10,Reference Guo, Liu, Xue, Gao, Wu and Ma12 and corona radiate. Reference Bessette, Nave, Caprihan and Stevens4,Reference Lagopoulos, Hermens, Hatton, Battisti, Tobias-Webb and White5,Reference Korgaonkar, Grieve, Koslow, Gabrieli, Gordon and Williams7,Reference Cole, Chaddock, Farmer, Aitchison, Simmons and McGuffin10–Reference Guo, Liu, Xue, Gao, Wu and Ma12 Since these fibre tracts form the important anatomical connectivity or circuits, they may be directly relevant to the pathophysiology of MDD. Reference Sexton, Mackay and Ebmeier13
Many theories exist regarding the pathophysiological basis of MDD; genes, the environment and endocrine dysfunction are all considered to be factors influencing MDD. Reference Northoff14 A previous study provided evidence of a hyperactive hypothalamic–pituitary–adrenal (HPA) axis in people with MDD and elevated cortisol levels have been observed in most patients with MDD. Reference Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen and van Dyck15 Thus, deregulation of the HPA axis is thought to play a role in the aetiology of MDD. Reference Vreeburg, Hoogendijk, van Pelt, Derijk, Verhagen and van Dyck15 Several magnetic resonance studies demonstrated a negative correlation between the hippocampus and anterior cingulate cortex volume and cortisol levels in people with MDD. Reference Sapolsky16–Reference Treadway, Grant, Ding, Hollon, Gore and Shelton18 However, there have been no imaging studies that have evaluated the relationship between high levels of cortisol and white matter damage. We hypothesised that a prolonged exposure to glucocorticoids might have an impact not only on the hippocampus and anterior cingulate cortex, Reference Sapolsky16–Reference Treadway, Grant, Ding, Hollon, Gore and Shelton18 but also extend into the white matter of other regions in people with MDD. This may be supported by an animal study, in which the authors demonstrated that elevated glucocorticoids inhibited the proliferation of astrocytes and oligodendrocytes, which are responsible for myelinating the axons of white matter fibre tracts. Reference Rajkowska and Miguel-Hidalgo19 Therefore the purpose of the current study was to evaluate the relationship between white matter abnormalities and serum cortisol levels in the first depressive episode of patients with MDD who are drug-naive using TBSS.
Method
Participants
In total, 29 right-handed, drug-naive individuals with MDD in their first depressive episode were recruited (the MDD group). A psychiatrist (K.H., with 7 years of experience in psychiatry) who did not know the serum cortisol levels or imaging data for the patients, diagnosed them as having MDD using a structured clinical interview according to the DSM-IV-TR criteria. 20 The severity of depression was evaluated using the 17-item Hamilton Rating Scale for Depression (HRSD-17). Reference Hamilton21 Only those with a HRSD-17 score ⩾14 were eligible for the study. The exclusion criteria included any history of neurological diseases or other physical diseases, and the presence of other disorders (i.e. the participants had no evidence of schizoaffective disorder, bipolar disorder, Axis II personality disorder or intellectual disability). The age of the participants in the MDD group ranged from 22 to 67 years (mean = 45.7, s.d. = 12.5), and 17 were men and 12 women.
In addition, 47 right-handed, healthy participants (the control group) were recruited via an interview conducted by the same psychiatrist using the Structured Clinical Interview for the DSM-IV-TR, non-patient edition (SCID-I-NP). Reference First, Spitzer, Gibbon and Williams22 None of them had a history of serious medical or neuropsychiatric illness or a family history of major psychiatric or neurological illness among their first-degree relatives, and all were matched with the patients in terms of age and gender. The age of the control group ranged from 20 to 65 years (mean = 41.8, s.d. = 11.0), 37 were men and 10 women. This study was approved by our institutional review board. Written informed consent was obtained from each participant after they had been given a detailed description of the study.
Serum cortisol evaluation
Morning (09.00–10.00 h) blood samples were obtained from all participants in both groups for cortisol measurement. All the blood samples were immediately centrifuged, and the serum was stored at −20°C until it was assayed. The precipitation of proteins with ethanol was followed by a direct radioimmunoassay using a highly specific antibody. Reference Heckmann, Wudy, Haack and Pohlandt23
MRI acquisition
Magnetic resonance imaging (MRI) scans were performed on the same day as cortisol measurement (09.00–17.00 h). All magnetic resonance examinations were performed using a 3T MR system (Signa EXCITE 3T; GE Healthcare, Waukesha, Wisconsin, USA) with an eight-channel brain-phased-array coil. DTIs were acquired by a single-shot, spin-echo planar sequence with the following parameters: repetition time (TR)/echo time (TE) = 12000/83.3 ms; 4 mm slice thickness; no gap; field of view (FOV) = 26 cm; number of excitations 1 and spatial resolution 1.02 × 1.02 × 4 mm. Diffusion gradients (b-value of 1000 s/mm2) were always applied for each of the three axes simultaneously around the 180° pulse. The diffusion properties were measured in 25 non-collinear directions.
Image processing
The structural distortion of the diffusion-weighted magnetic resonance images was corrected based on each T 2-weighted echo-planar image (b = 0 s/mm2) by using eddy current correction in the FMRIB Diffusion Toolbox software program (parts of the FMRIB Software Library; FSL v5.0.4). Non-brain tissue of each magnetic resonance image was deleted by using the brain extraction tool. The voxel-wise statistical analysis of the DTI data was performed by using the TBSS version 1.1 software program. The fractional anisotropy volumes were aligned to a target image as follows: (a) apply non-linear registration of each participant's fractional anisotropy into the FMRIB58_FA_1 mm standard-space image as the target image; (b) the target image was affine transformed to 1 × 1 × 1 mm MNI 152 (Montreal Neurologic Institute, Montreal, Canada) space. A mean fractional anisotropy image was created by averaging the aligned individual fractional anisotropy images, and was then thinned to create a fractional anisotropy skeleton representing white matter tracts common to all participants. Reference Smith, Jenkinson, Johansen-Berg, Rueckert, Nichols and Mackay3 The fractional anisotropy skeleton was thresholded at 0.2 to exclude voxels with low fractional anisotropy values, which are likely to include grey matter or cerebrospinal fluid. Individual fractional anisotropy data and voxel-wise statistical results were projected onto this fractional anisotropy skeleton. Subsequently, the axial diffusivity and radial diffusivity were projected onto the mean fractional anisotropy skeleton and also compared between groups in the same spatial location.
Statistical analysis
For the analysis of the demographic and clinical characteristics of the participants, two-tailed t-tests and the χ2-test were performed to compare the differences in the serum cortisol levels, age and gender between the MDD group and control group using the SPSS v16.0 software program. The voxel-wise statistical analysis was performed using a permutation-based inference tool. The comparisons of the fractional anisotropy, axial diffusivity and radial diffusivity values between the two groups were performed using a two-sample t-test. The correlations between the serum cortisol levels and fractional anisotropy values were analysed using a single-group average with additional covariates. Age and gender were included as covariates of no interest to control for confounding variables. The number of permutations in all voxel-wise analyses was set at 5000. Values of P<0.05 and >50 voxels were considered to indicate a statistically significant difference and a correlation after family-wise error (FWE)-correction for multiple comparisons at the cluster level, using the threshold-free cluster enhancement option. The anatomical location of significant clusters was detected using the Johns Hopkins University white matter tractography atlas and the International Consortium of Brain Mapping (ICBM)-DTI-81 white matter labels atlas.
Results
Participants
Demographic and clinical information is shown in Table 1. Compared with the control group, significantly higher cortisol levels were found in the MDD group (P<0.05). There were no significant differences in terms of age and gender in the groups.
Table 1 Demographic information and serum cortisol data

| Control group (n = 47) |
Major depressive disorder group (n = 29) |
P | |
|---|---|---|---|
| Age, years: mean (s.d.) | 41.8 (11.0) | 45.7 (12.5) | 0.16 |
| Women, n | 10 | 12 | 0.60 |
| Hamilton Rating Scale for Depression-17 score, mean (s.d.) | 21.4 (5.2) | ||
| Serum cortisol, nmol/l: mean (s.d.) | 9.3 (3.4) | 12.4 (5.1) | <0.01 |
Comparison between the two groups
Significant fractional anisotropy differences were observed between the two groups. Online Fig. DS1 shows the spatial distribution of the brain regions, indicating a reduction of fractional anisotropy values in the MDD group compared with the control group. The MDD group had significantly reduced fractional anisotropy values (P<0.05, FWE-corrected) in the bilateral corticospinal tracts, left inferior fronto-occipital fasciculus, left uncinate fasciculus, left anterior thalamic radiation, left external capsule and right superior corona radiata (Fig. DS1). No significant differences were found in axial diffusivity and radial diffusivity.
Correlations between fractional anisotropy values and serum cortisol levels
A significant negative correlation of the fractional anisotropy values with the serum cortisol levels was observed in the MDD group. There were no regions that showed a positive correlation between the fractional anisotropy values and serum cortisol levels. For the control group, we found no regions that showed a significant correlation between the fractional anisotropy values and the serum cortisol levels.
Online Fig. DS2 shows the areas in which the fractional anisotropy values were associated with the cortisol levels in the MDD group. The fractional anisotropy values of the bilateral inferior fronto-occipital fasciculus, bilateral anterior thalamic radiation, left uncinate fasciculus, left cingulum, left anterior corona radiata and right external capsule had significantly negative correlations with the cortisol levels (P<0.05; FWE-corrected). Therefore, the fractional anisotropy values of the left inferior fronto-occipital fasciculus, anterior thalamic radiation and uncinate fasciculus in the MDD group were significantly decreased compared with those in the control group, and also had a significant inverse correlation with the cortisol levels (P<0.05; FWE-corrected). The cluster voxel size and significant MNI coordinates are presented in Table 2.
Table 2 The results of the image analyses

| Anatomical region | Cluster size |
P a | MNI coordinates, mm | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Fractional anisotropy values, between-group comparison (control group>MDD group) b | |||||
| Right corticospinal tract | 53 | 0.049 | 70 | 109 | 119 |
| Right superior corona radiata | 0.049 | 70 | 105 | 112 | |
| Left inferior fronto-occipital fasciculus | 283 | 0.039 | 114 | 147 | 72 |
| Left uncinate fasciculus | 0.042 | 115 | 144 | 71 | |
| Left external capsule | 0.049 | 119 | 140 | 69 | |
| Left corticospinal tract | 1221 | 0.036 | 111 | 106 | 111 |
| Left anterior thalamic radiation | 0.040 | 111 | 104 | 116 | |
| Negative correlation with the cortisol levels in MDD group c | |||||
| Right inferior fronto-occipital fasciculus | 96 | 0.047 | 56 | 113 | 73 |
| Right external capsule | 0.049 | 58 | 103 | 75 | |
| Left anterior thalamic radiation | 706 | 0.044 | 112 | 156 | 88 |
| Left uncinate fasciculus | 0.045 | 111 | 169 | 88 | |
| Left inferior fronto-occipital fasciculus | 0.047 | 113 | 151 | 90 | |
| Left cingulum | 1036 | 0.031 | 107 | 144 | 100 |
| Left anterior corona radiata | 0.037 | 106 | 155 | 97 | |
| Right anterior thalamic radiation | 3921 | 0.031 | 65 | 161 | 78 |
MNI, Montreal Neurologic Institute, MDD, major depressive disorder.
a. Family-wise error-corrected.
b. See online Fig. DS1.
c. See online Fig. DS2.
Discussion
Main findings and comparison with other studies
Hyperactivity of the HPA axis and elevated cortisol levels are considered to be characteristics of the pathophysiology of MDD. Reference Carroll, Curtis and Mendels24–Reference Pfohl, Sherman, Schlechte and Winokur26 We also found significantly higher serum cortisol levels in the MDD group compared with the control group. In the current study, the fractional anisotropy values of the left inferior fronto-occipital fasciculus, anterior thalamic radiation and uncinate fasciculus in the MDD group were significantly decreased compared with those in the control group, and also had a significant inverse correlation with the cortisol levels.
Our findings may be supported by the results of several DTI studies that demonstrated the presence of white matter abnormalities in the inferior fronto-occipital fasciculus, uncinate fasciculus and anterior thalamic radiation in people with MDD. Reference Kieseppa, Eerola, Mantyla, Neuvonen, Poutanen and Luoma8,Reference Zhu, Wang, Xiao, Zhong, Liao and Yao27–Reference Cullen, Klimes-Dougan, Muetzel, Mueller, Camchong and Houri29 Several studies reported decreased fractional anisotropy values in the inferior fronto-occipital fasciculus in MDD. Reference Bessette, Nave, Caprihan and Stevens4,Reference Lagopoulos, Hermens, Hatton, Battisti, Tobias-Webb and White5,Reference Liao, Huang, Wu, Yang, Kuang and Du30 The inferior fronto-occipital fasciculus are considered to be involved in reading, attention and visual processing. Reference Fox, Iaria and Barton31,Reference Catani and Mesulam32 Zhang et al found decreased fractional anisotropy values and an increased radial diffusivity of the right uncinate fasciculus in mid-life patients with MDD, and concluded that this reflected the demyelination of the right uncinate fasciculus, which interrupted the functional connectivity of the frontal–limbic circuits. Reference Zhang, Leow, Ajilore, Lamar, Yang and Joseph33 Cullen et al also found lower fractional anisotropy values in the bilateral uncinate fasciculus in adolescents with MDD. Reference Cullen, Klimes-Dougan, Muetzel, Mueller, Camchong and Houri29
Interpretation of our findings
The inferior fronto-occipital fasciculus, uncinate fasciculus and anterior thalamic radiation are considered to play key roles in the frontal–subcortical circuits and frontal–limbic circuits. The frontal–subcortical circuits contains five discrete parallel loops that connect specific areas of the frontal cortex (motor area, supplementary motor area, dorsolateral prefrontal cortex, anterior cingulate cortex and orbitofrontal cortex) through the striatum (caudate nucleus, putamen, ventral striatum), globus pallidus, substrantia nigra and thalamus, back to the frontal cortex (Fig. 1). Reference Tekin and Cummings34 Anatomically, the inferior fronto-occipital fasciculus, uncinate fasciculus and anterior thalamic radiation have a projection into the frontal cortex from the thalamus. A ‘disconnection’ of the frontal–subcortical circuit was proposed as one of the pathogenic elements associated with MDD. Reference Sexton, Mackay and Ebmeier13 The frontal–limbic circuits are ‘open’ loops incorporated into the functional connectivity between frontal areas (anterior cingulate cortex and orbitofrontal cortex) and other cortex, thalamus and limbic system areas (amygdala and hippocampus) (Fig. 2). Reference Bonelli and Cummings35 Anatomically, the uncinate fasciculus has a projection into the anterior cingulate cortex and orbitofrontal cortex from limbic system areas. An association between dysregulation of the frontal–limbic circuits and MDD has been postulated based on functional imaging studies. Reference Drevets, Price and Furey1,Reference Drevets36,Reference Wakana, Jiang, Nagae-Poetscher, van Zijl and Mori37
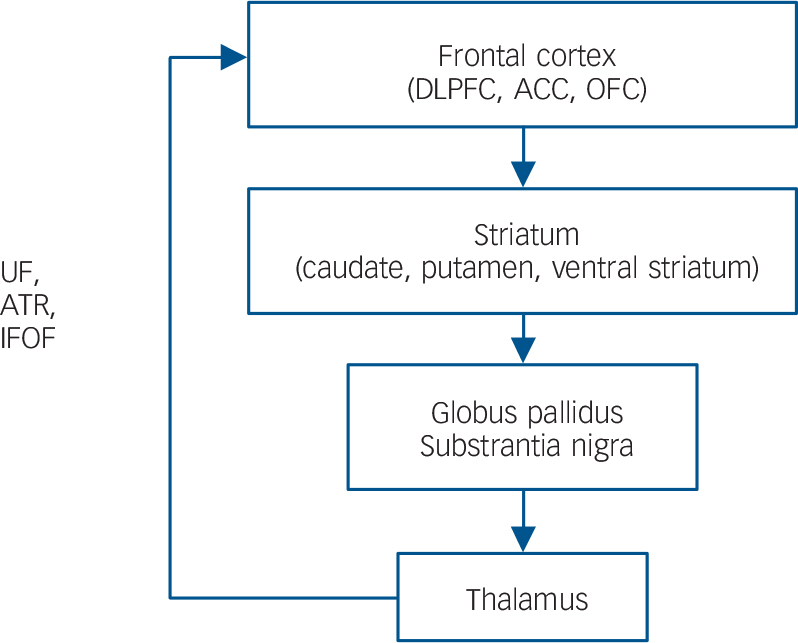
Fig. 1 General organisation of the frontal–subcortical circuits.
Inferior fronto-occipital fasciculus (IFOF), uncinate fasciculus (UF) and anterior thalamic radiation (ATR) have a projection into the frontal cortex from the thalamus. DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; OFC, orbitofrontal cortex.
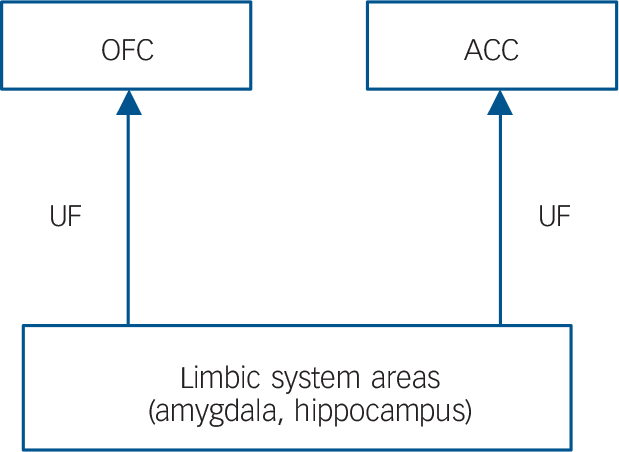
Fig. 2 General organisation of the frontal–limbic circuits.
Uncinate fasciculus (UF) has a projection into the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC) from limbic system areas.
Therefore, in the current study, the observation of fractional anisotropy reductions in the inferior fronto-occipital fasciculus, uncinate fasciculus and anterior thalamic radiation may support the theory that the disconnection in the frontal–subcortical circuits and/or frontal–limbic circuits is one of the pivotal issues associated with the pathogenesis of MDD. Furthermore, our results suggest that the high levels of cortisol in people with MDD might injure the microstructures in these specific white matter circuits.
Reduced fractional anisotropy values are thought to reflect reduced organisation of the white matter, reduced axonal density and/or reduced myelination. Reference Basser and Pierpaoli38,Reference Beaulieu39 Although the exact cause of the fractional anisotropy reduction in the cortisol-associated regions (i.e. inferior fronto-occipital fasciculus, uncinate fasciculus and anterior thalamic radiation) remains unclear, we speculate that there may be two possible mechanisms: (a) direct damage to the white matter caused by the high levels of cortisol; (b) a secondary effect on the white matter mediated by grey matter damage because of the high levels of cortisol.
With regard to the direct damage, an animal study demonstrated that elevated glucocorticoid levels inhibited astrocyte and oligodendrocyte proliferation. Reference Rajkowska and Miguel-Hidalgo19 Because white matter fibre tracts are myelinated by oligodendrocytes our results might indicate that high levels of cortisol can alter the myelination of the white matter fibre tracts in people with MDD. In the current study, there were no significant differences in the fractional anisotropy values in other regions that showed significantly negative correlations with the cortisol levels (the right external capsule, left cingulum and left anterior corona radiata, between the MDD and control groups. In other words, our findings suggest that the integrity of the white matter in the left anterior thalamic radiation, inferior fronto-occipital fasciculus and uncinate fasciculus might be more sensitive to hypercortisolism than other regions, and the microstructural changes in these white matter tracts might occur even in the early stages of MDD.
In terms of grey matter damage because of the high levels of cortisol, previous animal studies have demonstrated that the hippocampus, anterior cingulate cortex and frontoparietal cortex (sensory and motor) contained high concentrations of glucocorticoid receptors and were vulnerable to the noxious effects of glucocorticoids, consequently impairing neuronal plasticity and neurogenesis. Reference Sapolsky17,Reference Ahima and Harlan40 Preclinical studies revealed that exposure to high levels of cortisol was associated with brain atrophy of the hippocampus Reference Sapolsky16 and the medial prefrontal cortex in patients with MDD. Reference Diorio, Viau and Meaney41 In another study, patients with MDD showed a decreased rostral anterior cingulate cortex volume compared with controls, and the anterior cingulate cortex volume reduction was inversely correlated with the cortisol levels. Reference Treadway, Grant, Ding, Hollon, Gore and Shelton18 Since the inferior fronto-occipital fasciculus, uncinate fasciculus and anterior thalamic radiation are all connected to the anterior cingulate cortex or the hippocampus (Figs 1 and 2), our observation of fractional anisotropy reduction in the cortisol-associated regions could be the result of secondary damage because of the grey matter abnormalities.
Although, in the current study, the MDD group had significantly reduced fractional anisotropy values in the bilateral corticospinal tracts, left external capsule and right superior corona radiata, the fractional anisotropy values of these regions had no correlation with the cortisol levels. Many investigators have found a reduced fractional anisotropy value in these regions using TBSS. Reference Versace, Almeida, Quevedo, Thompson, Terwilliger and Hassel11,Reference Song, Korgaonkar, Armstrong, Eagles, Williams and Grieve42,Reference Benedetti, Yeh, Bellani, Radaelli, Nicoletti and Poletti43 Therefore, just like the effects of the high levels of cortisol, several other factors such as genotype, stress adverse developmental environment and biochemistry may also be linked to white matter microstructural abnormalities of tract fibres detected in this study. Reference Northoff14 A previous study reported that white matter microstructural abnormalities in superior corona radiata were associated with the brain-derived neurotrophic factor val66met polymorphism in remission of geriatric depression. Reference Alexopoulos, Glatt, Hoptman, Kanellopoulos, Murphy and Kelly44
Limitations
Our study is associated with some limitations that should be kept in mind when interpreting the results. The first limitation was the relatively small size of the MDD group, which might have affected our results. Second, in this study, the mechanism underlying how the elevated cortisol levels altered the myelination of the white matter fibre tract has not been elaborated. The relationship between the HPA axis and frontal–subcortical circuits and frontal–limbic circuits might be an interesting topic for further studies.
Implications
In conclusion, in patients with early-stage MDD, the fractional anisotropy values of the inferior fronto-occipital fasciculus, uncinate fasciculus and anterior thalamic radiation were significantly decreased compared with those in a control group, and also showed significant inverse correlations with the cortisol levels. Since the inferior fronto-occipital fasciculus, uncinate fasciculus and anterior thalamic radiation are essential elements of the frontal–subcortical circuits and frontal–limbic circuits, the high levels of cortisol in people with MDD might injure the microstructures in these specific white matter circuits. Furthermore, our findings also support the theory that the ‘disconnection’ of the frontal–subcortical and frontal–limbic circuits is one of the elements associated with the pathophysiology of MDD.
Funding
This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan.






eLetters
No eLetters have been published for this article.