1. Introduction
Personality disorders are a group of psychological conditions characterized by enduring, maladaptive patterns of thinking, feeling, and behavior. These patterns are inconsistent with social and cultural expectations and result in significant impairments across various domains of life, including interpersonal relationships, work, and education (Leichsenring et al., Reference Leichsenring, Fonagy, Heim, Kernberg, Leweke, Luyten and Steinert2024). Personality disorders are typically classified into three main clusters: Cluster A, which includes odd and eccentric disorders; cluster B, encompassing dramatic, emotional, and erratic disorders; and cluster C, involving anxious and fearful conditions such as avoidant and obsessive-compulsive personality disorders (Furnham and Grover, Reference Furnham and Grover2022). Cluster B disorders are relatively prevalent yet highly disruptive, with borderline personality disorder (BPD) and antisocial personality disorder (ASPD) being the most prominent. These disorders impose a substantial burden on healthcare, judicial, and social systems (Fonagy et al., Reference Fonagy, Simes, Yirmiya, Wason, Barrett, Frater and Bateman2025).
ASPD is defined by a persistent pattern of disregard for the rights of others, emotional detachment, and lack of empathy. Individuals with ASPD often exhibit callousness and indifference toward others (Douzenis et al., Reference Douzenis, Tsopelas and Tzeferakos2012) and frequently violate social norms and rules (van Dam et al, Reference van Dam, Rijckmans and van den Bosch2022). These individuals typically present with emotional coldness and an inability to empathize, which severely impairs their interpersonal functioning. Common behaviors include chronic deceitfulness, manipulation, aggression, impulsivity, and an absence of guilt or remorse. Although such individuals may appear charming and sociable on the surface, they often lack genuine concern for others’ emotions or rights (Delisi et al., Reference DeLisi, Drury and Elbert2019).
In contrast, BPD – one of the most complex personality disorders – is marked by severe emotional instability, impulsive behavior, and tumultuous interpersonal relationships (Leichsenring et al., Reference Leichsenring, Fonagy, Heim, Kernberg, Leweke, Luyten and Steinert2024). Individuals with BPD often struggle with emotion regulation, which can lead to self-injurious behavior (Beeney et al., Reference Beeney, Levy, Gatzke-Kopp and Hallquist2014). Emotional dysregulation may contribute to identity disturbances and a pervasive sense of emptiness (Kramer et al., Reference Kramer, Sander, Bertsch, Gescher, Cackowski, Hegerl and Herpertz2019). Rapid mood shifts and unstable attachments often result in intense relational conflicts and frequent interpersonal crises (Fonagy et al., Reference Fonagy, Luyten and Bateman2017).
As evident, these disorders share common features, including difficulties with emotion regulation, impaired empathy, dysfunctional relationships, and poor adherence to social norms, leading to significant consequences on individual and societal levels. However, knowledge about their underlying etiology and optimal treatment remains limited. For decades, the conceptualization of antisocial and borderline disorders has been entangled in definitional, philosophical, and ethical debates, resulting in overlapping or comorbid diagnoses that complicate both assessment and treatment (Leichsenring et al., Reference Leichsenring, Fonagy, Heim, Kernberg, Leweke, Luyten and Steinert2024). This complexity is further exacerbated by gender biases, whereby women exhibiting traits typically associated with men are often diagnosed with BPD, while men are more likely to be diagnosed with ASPD.
Personality disorders have both biological and environmental origins (Schermer et al., Reference Schermer, Colodro-Conde, Grasby, Hickie, Burns, Ligthart and Boomsma2020). Identifying these factors and understanding the underlying neurobiological mechanisms can facilitate the development of more accurate diagnoses and innovative treatment approaches. One of the most important biological mechanisms involves neuro-metabolites and biomarkers, which can be investigated using proton magnetic resonance spectroscopy (¹H-MRS).
¹H-MRS is a unique, noninvasive, and radiation-free technique that enables the measurement of metabolite levels in specific brain regions in vivo. This method provides more detailed information about neuronal abnormalities at the cellular and metabolic levels compared to broader structural imaging techniques. The key neuro-metabolites detectable by ¹H-MRS include N-acetylaspartate (NAA), choline (Cho), myo-inositol (mI), and creatine (Cr), among others (Caetano et al., Reference Caetano, Olvera, Hatch, Sanches, Chen, Nicoletti and Soares2011; Zhong et al., Reference Zhong, Wang, Zhao, Xiang, Ling, Liu and Jia2014).
By utilizing ¹H-MRS, researchers can gain valuable insights into neurochemical alterations associated with personality disorders, potentially uncovering distinct biological pathways involved in their etiology and aiding the development of targeted therapeutic interventions. This approach promotes a deeper understanding of how these disorders manifest at the metabolic level, thus enhancing diagnostic precision and treatment efficacy.
In a study on patients with BPD, MRS was used to measure levels of NAA, Cr, and Cho in the hippocampus. Results showed that NAA/Cr and NAA/Cho ratios in the hippocampus of BPD patients were significantly lower than those in the control group, indicating a reduction in NAA levels and suggesting potential neuronal degeneration in this region (Atmaca et al., Reference Atmaca, Karakoc, Mermi, Gurkan Gurok and Yildirim2014).
In another study by Wang et al. (Reference Wang, van Eijk, Demirakca, Sack, Krause-Utz, Cackowski and Ende2017) titled “GABA Levels in the Anterior Cingulate Cortex (ACC) and Its Relationship with Activation and Functional Connectivity in the Frontostriatal Network during Interference Inhibition in Patients with Borderline Personality Disorder (BPD)”, it was found that BPD patients exhibited lower functional connectivity between the ACC and the caudate nucleus during interference inhibition tasks compared to healthy individuals. Moreover, GABA levels in the ACC were positively correlated with activation in frontostriatal regions and the strength of ACC-caudate connectivity during interference inhibition. This relationship was particularly associated with impulsivity-driven behavior in BPD patients. Mediation analyses revealed that caudate activation and ACC-caudate connectivity mediated the relationship between GABA levels and impulsivity (Robbins et al., Reference Robbins, Banca and Belin2024).
In a separate study examining metabolic changes in the amygdala of BPD patients using ¹H-MRS, it was observed that total N-acetylaspartate (tNAA) and total creatine (tCr) levels in the amygdala were significantly lower in BPD patients compared to healthy controls. Furthermore, BPD patients with comorbid post-traumatic stress disorder (PTSD) had even lower tCr levels than BPD patients without PTSD (Hoerst et al., Reference Hoerst, Weber-Fahr, Tunc-Skarka, Ruf, Bohus, Schmahl and Ende2010).
In a different investigation using MRS, individuals with ASPD demonstrated a significant reduction in the Glu-to-GABA ratio in the striatum compared to control subjects (Tully et al., Reference Tully, Pereira, Sethi, Griem, Cross, Williams and Blackwood2024). This imbalance in Glu/GABA regulation within the striatum may be linked to impulsive and aggressive behaviors in individuals with ASPD.
In a study by Basoglu et al. (Reference Basoglu, Semiz, Oner, Gunay, Ebrinc, Cetin and Sonmez2008) examining antisocial behavior, psychopathy, and violent crime among military conscripts using MRS, it was found that, although no significant differences in metabolic ratios (NAA/Cr and Cho/Cr) were observed between the groups in the dorsolateral prefrontal cortex (dlPFC), ACC, and amygdala–hippocampus regions, the NAA/Cr ratio in the ACC showed a significant negative correlation with overall psychopathy scores (PCL-R) and interpersonal/emotional deficits.
In another study investigating differences in glutamate and glutamine (Glx) biomarkers associated with aggressive behaviors in individuals with bipolar disorder and ASPD, it was reported that the ASPD group exhibited significantly higher Glx levels in the dlPFC compared to both the bipolar disorder group and healthy controls. No significant differences in Glx levels were found between the bipolar group and the control group. These findings suggest that elevated Glx levels in individuals with ASPD may be linked to the neurochemical underpinnings of aggression, potentially explaining structural abnormalities observed in this population (Smaragdi et al., Reference Smaragdi, Chavez, Lobaugh, Meyer and Kolla2019).
As these findings indicate, brain cellular metabolites may serve as indicators of specific behavioral problems and psychiatric conditions. Given that BPD and ASPD share numerous behavioral characteristics, this study aims to explore whether similarities also exist at the neurochemical level and whether such metabolic assessments can aid in differential diagnosis when conventional methods fall short.
In this semi-quantitative study, we focused on two key brain regions: the left ACC and the left orbitofrontal cortex (OFC) – areas critically involved in emotional regulation, behavioral control, and reward/punishment processing. Previous research has identified potential dysfunctions in these regions in relation to both disorders. Additionally, we examined the ratios of NAA/Cr, GABA/Cr, and Glu/Cr using a multi-voxel spectroscopy approach.
2. Methods
2.1 Participants
A total of 120 individuals participated in the study: 60 with BPD and 60 with ASPD. The two groups were matched by age and gender. Initially, 180 individuals were recruited from psychiatric clinic clients who, based on clinical interviews and the Millon Clinical Multiaxial Inventory-III (MCMI-III), met the diagnostic criteria for either BPD or ASPD without any comorbid psychiatric conditions. Of these, 157 individuals met the inclusion criteria. From this group, 60 individuals who met only the criteria for BPD and consented to participate were assigned to the BPD group. From the remaining 97 individuals, 60 who met only the criteria for ASPD and were willing to participate were assigned to the ASPD group. The inclusion criteria were as follows: A clinical diagnosis of BPD or ASPD confirmed by structured interview and a Millon test score above 85, no comorbid psychiatric diagnoses, no history of psychiatric hospitalization or electroconvulsive therapy, no history of head trauma, brain tumors, or chemotherapy, and absence of claustrophobia. The exclusion criteria included: refusal to undergo magnetic resonance imaging (MRI) scanning, use of alcohol, drugs, or stimulants within two weeks prior to evaluation, and having received more than five psychotherapy sessions. To ensure ethical compliance, written informed consent was obtained from all participants. Ethical approval was granted by the University of Mohaghegh Ardabili (Approval Code: IR.UMA.REC.1403.030).
2.2 Imaging
Both MRI and ¹H-MRS were performed using a discovery MR 750 scanner (General Electric) operating at 3.0 Tesla, with a standard gradient system and an 8-channel head coil for signal transmission and reception. Participants lay in the supine position, with the nasion used as the reference point. Earplugs and foam padding were provided to minimize noise and head movement. Routine T1-weighted fluid-attenuated inversion recovery (FLAIR) images were acquired [repetition time (TR) = 1800 ms, echo time (TE) = 24 ms], along with T2-weighted fast spin-echo images (TR = 4500 ms, TE = 120 ms), to rule out any structural or signal abnormalities in the brain. Axial imaging was conducted using T2-weighted fast spin-echo (FSE) sequences (TR = 5000 ms, TE = 113 ms, NEX = 2, slice thickness = 5 mm, no interslice gap, 18 slices, FOV = 24 cm, matrix = 256 × 256) to obtain an anatomical reference for voxel placement. The volume of interest (VOI) was determined by an experienced spectroscopy specialist using anatomical landmarks in the left ACC and OFC, ensuring accurate and consistent voxel placement (see Figure 1). All VOIs were placed at a safe distance from the lateral ventricles, cerebrospinal fluid (CSF) spaces, and skull. Each VOI encompassed approximately 50 nominal voxels (7.5 × 7.5 × 10 mm3).

Figure 1. a) Placement of the ¹H-MRS voxel in the left orbitofrontal cortex (OFC) based on anatomical landmarks. b) Placement of the ¹H-MRS voxel in the anterior cingulate cortex (ACC). T1-weighted MRI images were used for localization of VOIs.
2.3 Imaging parameters
The spectroscopy imaging parameters were as follows:
-
TR = 1000 milliseconds
-
TE = 144 milliseconds
-
Number of excitations (NEX) = 1
-
Spatial matrix = 18 × 18
-
Field of view (FOV) = 240 × 240 mm2
-
Slice thickness = 10 millimeters
-
Nominal voxel size = 7.5 × 7.5 × 10 mm³
Additional saturation bands were applied outside the VOI to minimize lipid contamination from the scalp. Before each spectroscopy scan, an automated pre-scan was performed to optimize spectral quality, ensuring a full width at half maximum (FWHM) of 10 Hz or less. The total acquisition time for the ¹H-MRS sequence was 5 minutes and 28 seconds.
2.4 Spectral analysis
Spectral data were analyzed using the GE 3.0 T platform on a sun advantage windows ADW4.5 workstation. Metabolite distribution maps were generated and overlaid with anatomical MRI images to produce the ¹H-MRS curves. The software automatically performed phase correction, frequency encoding, and baseline correction. The scanner’s internal system automatically calibrated the baseline, averaged signal spectra, and identified metabolites, calculating peak areas for NAA, GABA, and Glu in the ACC and OFC. Ratios of NAA/Cr, GABA/Cr, and Glu/Cr were subsequently computed.
All voxel placements and spectral analyses were conducted by a trained radiologist blinded to participants’ diagnostic groupings, ensuring objective and unbiased data collection.
2.5 Statistical Analysis
Statistical analyses were performed using SPSS version 20, with a two-tailed significance level set at p < 0.05. The normality of the variables was assessed using the Kolmogorov–Smirnov test. Depending on the distribution of the data, parametric tests (independent-samples t-test or one-way ANOVA) were used for normally distributed continuous variables, and non-parametric tests (Mann–Whitney U test or Kruskal–Wallis test) were applied for non-normally distributed variables. One-way ANOVA followed by the Bonferroni post hoc test was used to identify specific group differences when overall significance was found. Correlation coefficients between neurochemical metabolite ratios and clinical variables were calculated using Pearson’s correlation analysis for normally distributed variables and Spearman’s rank-order correlation for non-normally distributed variables.
3. Results
The gender distribution between the two groups did not differ significantly, as assessed by the chi-squared (χ²) test. Therefore, gender was not considered an influencing factor in the outcomes of this study. The mean age was also similar between the two groups, with no statistically significant difference, as determined by the independent-samples t-test. However, the difference in educational attainment between the groups approached statistical significance, as evaluated using an independent-samples t-test (see Table 1).
Table 1. Demographic and clinical characteristics of participants in the BPD and ASPD groups

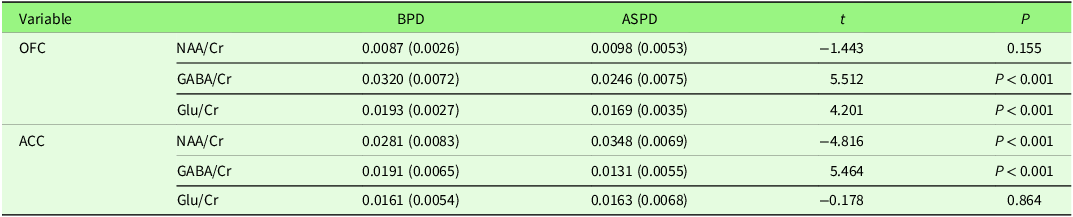
The comparison of neurochemical metabolite ratios between individuals with BPD and ASPD in the ACC and left OFC is presented in Table 2, focusing on the ratios of NAA/Cr, GABA/Cr, and Glu/Cr.
Table 2. Comparison of neurochemical metabolite in ACC and OFC between BPD and ASPD groups

The comparison of metabolite concentrations in the two brain regions – the left OFC and the left ACC – between the BPD and ASPD groups revealed the following results: In the left OFC, GABA/Cr and Glu/Cr ratios were significantly higher in the BPD group compared to the ASPD group (P < 0.001). In the left ACC, the GABA/Cr ratio was also significantly higher (P < 0.001) in the BPD group, while the NAA/Cr ratio was lower compared to the ASPD group. However, the results showed no significant difference between the two groups in NAA/Cr levels in the OFC and Glu/Cr levels in the ACC.
4. Discussion
According to our data, this is the first study to investigate neurochemical metabolite levels and biological distinctions between BPD and ASPD within the same diagnostic category. To date, no prior research has directly compared these two personality disorders in these specific brain regions using ¹H-MRS with a 3.0 Tesla multi-voxel approach. This study aims to differentiate the two disorders at the biochemical level, thereby contributing to a more accurate conceptualization and clinical distinction between them. Such differentiation may support the development of more targeted psychotherapeutic and pharmacological interventions. As such, this research serves as a preliminary investigation in this direction.
Given the lack of direct comparative studies, and the fact that existing literature has often focused on either disorder in isolation or on unrelated brain areas, we will briefly discuss some of the most relevant prior findings. One of the closest studies examined Glu and GABA levels in the ACC among female patients with BPD and attention-deficit/hyperactivity disorder (ADHD), reporting elevated Glu and reduced GABA levels in both groups relative to healthy controls. Notably, ADHD patients exhibited markedly lower GABA levels (Ende et al., Reference Ende, Cackowski, van Eijk, Sack, Demirakca, Kleindienst and Schmahl2016). Considering the frequent overlap between antisocial behavior and childhood ADHD, these results may be partially consistent with our findings.
Another study investigated metabolite concentrations (tNAA, Cr, and Glx) in the ACC and left cerebellum of 14 women with BPD and ADHD and 18 healthy controls. The results revealed significantly higher levels of tNAA and Glu in the ACC of the clinical group, while glutamine (Gln) levels were reduced, and no significant changes were observed in the cerebellum (Rüsch et al., Reference Rüsch, Boeker, Büchert, Glauche, Bohrmann, Ebert and Tebartz van Elst2010). These findings may indicate alterations in energy metabolism or delayed neurodevelopment.
In a study by Wang et al. (Reference Wang, van Eijk, Demirakca, Sack, Krause-Utz, Cackowski and Ende2017), GABA concentrations in the ACC were found to be significantly lower in BPD patients compared to healthy individuals, partially aligning with the current study. Although our study did not include a healthy control group, comparisons with existing literature suggest that GABA levels in both BPD and ASPD may be substantially lower than in non-clinical populations. Supporting this pattern, Tully et al. (Reference Tully, Pereira, Sethi, Griem, Cross, Williams and Blackwood2024) reported reduced GABA and Glu levels in individuals with ASPD compared to healthy controls. Similarly, Basoglu et al. (Reference Basoglu, Semiz, Oner, Gunay, Ebrinc, Cetin and Sonmez2008) found a notable reduction in Glu levels in the ACC among antisocial individuals, consistent with our findings.
Taken together, previous studies have primarily compared psychiatric disorders to healthy control groups. Our analysis also suggests that average metabolite levels in both disorders deviate from those found in healthy individuals – with Glu levels increased, and both GABA and NAA levels decreased. However, the distinctive neurochemical patterns observed between BPD and ASPD in our study highlight meaningful biological differences that may correspond to fundamentally different symptomatology and behavioral profiles. These distinctions underscore the potential need for disorder-specific treatment strategies in future clinical practice.
In this study, the levels of GABA/Cr and Glu/Cr in the OFC – a brain region associated with emotional processing and decision-making – were significantly higher in individuals with BPD compared to those with ASPD. This finding suggests greater GABAergic and glutamatergic activity in BPD patients, although prior research has shown that both groups typically exhibit lower levels of these metabolites compared to healthy controls.
Since GABA is an inhibitory neurotransmitter, its elevated levels in BPD patients may reflect increased internal effort to regulate emotional responses and impulsive behaviors. This interpretation aligns with clinical observations, where individuals with BPD often demonstrate a strong desire for behavioral change and frequently engage in treatment voluntarily. They commonly report feelings of guilt following impulsive actions and actively pursue self-correction and emotional stability (Chapman et al., Reference Chapman, Dixon-Gordon, Layden and Walters2010).
In contrast, Glu plays a central role in mood regulation and impulse control. The higher Glu levels observed in BPD patients may help explain their mood instability and rapid emotional shifts. While prior studies have generally shown that individuals with ASPD display greater impulse control than those with BPD, the current findings may be influenced by the higher education levels observed in the ASPD group. Many participants with ASPD in this study held advanced academic degrees and did not typically present with overt aggression. Instead, their antisocial traits were expressed through more covert, sophisticated behaviors, such as deception, fraud, and manipulation. These individuals often lack remorse and may even view their behavior as a mark of intelligence or strategic thinking, taking pride in their ability to exploit others.
Although no significant differences in NAA levels were found between the BPD and ASPD groups in the OFC, the mean levels in both groups were lower than those reported in healthy individuals in previous studies. Given that NAA is associated with emotional and cognitive functions and serves as a marker of neuronal integrity, these reduced levels reflect underlying neural dysfunction and energy regulation deficits, consistent with the behavioral patterns observed in both disorders. Although the group difference was not statistically significant, the slightly lower NAA levels in the BPD group may suggest greater cognitive impairments, particularly in memory, learning, and emotional regulation, compared to those with ASPD.
The study also revealed significantly higher GABA/Cr levels in the ACC among BPD patients compared to ASPD patients, while NAA/Cr levels in the same region were lower. The elevated GABA levels in the ACC of BPD individuals may reflect greater activity of inhibitory mechanisms in this brain region. This may correspond to the relatively greater emotional control and internal regulation efforts observed in BPD patients, as opposed to the impulsive and explosive anger more characteristic of ASPD individuals. Nonetheless, it is important to note that average GABA levels in both groups remain lower than those of healthy controls, consistent with prior research.
The reduction in NAA/Cr levels in the ACC among BPD patients compared to ASPD may signal compromised neuronal viability or disturbances in cellular metabolism. As a widely recognized biomarker of neuronal health, a decrease in NAA may point to neurodegeneration or metabolic dysfunction, particularly relevant to the emotional instability and cognitive difficulties commonly observed in BPD. These findings may underlie frequent mood swings and impaired decision-making processes in this population.
Meanwhile, no significant differences were found in Glu/Cr levels in the ACC between the two groups, suggesting that glutamatergic functioning in this region may be similarly affected. Given that Glu is a primary excitatory neurotransmitter involved in learning, memory, and affective processing, this similarity may imply share vulnerabilities in these domains. Although Glu levels were slightly lower in BPD patients, the difference did not reach statistical significance.
Overall, this study provides evidence of distinct neurochemical differences between BPD and ASPD, particularly in the OFC and ACC regions. However, several limitations must be acknowledged. A key limitation was the lack of a healthy control group, which was due to time and access constraints. Additionally, the relatively small sample size may have limited the statistical power and reduced the generalizability of the findings.
Furthermore, the cross-sectional nature of the study prevents the inference of causal relationships, restricting interpretations to correlational findings only. Another limitation concerns the use of Cr as a reference metabolite instead of reporting absolute metabolite concentrations. Although Cr is typically considered a stable internal reference, recent research has questioned its consistency across individuals (Liu et al., Reference Liu, Wang, Zhong, Wang, Liao, Lai and Jia2017). In this study, no significant differences in Cr levels were observed across groups, suggesting that the use of Cr as a reference was unlikely to bias the results.
For future research, it is recommended to include a healthy control group, increase sample sizes, and when possible, conduct longitudinal or process-oriented assessments of neurochemical changes. Moreover, educational backgrounds should be examined as a potential mediating variable, as it may meaningfully influence behavioral and neurobiological outcomes. Such expanded and methodologically rigorous studies will contribute to the development of more targeted and effective psychotherapeutic and pharmacological treatments tailored to each disorder.
5. Conclusion
The results of this study reveal significant differences in neurochemical metabolite concentrations within the OFC and ACC between individuals diagnosed with BPD and ASPD. Specifically, the elevated GABA/Cr and Glu/Cr ratios in the left OFC among BPD patients may indicate increased neural activity and greater engagement in emotional regulation processes in this group. In addition, the higher GABA/Cr and lower NAA/Cr ratios in the left ACC in BPD patients suggest both emotional dysregulation and potential neuronal impairment in this region. By contrast, the absence of significant group differences in NAA/Cr levels in the OFC and Glu/Cr levels in the ACC suggests that certain metabolic characteristics may be shared between the two disorders, potentially reflecting common underlying neurobiological factors. Overall, these findings provide new insights into the neurobiological distinctions between personality disorders, contributing to a more nuanced understanding of brain function in BPD and ASPD. Such insights may support the development of more accurate diagnostic tools and disorder-specific therapeutic interventions in clinical practice.
Data Availability
Data will be available upon request.
Financial Support
None.
Competing interests
The authors declare that there is no conflict of interests.