INTRODUCTION
Infections with Toxoplasma gondii are distributed globally in people and animals such as rats, dogs, cats, sheep, hens and pigs [Reference Bojar and Szymanska1, Reference Pezzoli2]. Contamination with this parasite is usually long-lasting and asymptomatic [Reference Silva3]. However, it causes serious clinical symptoms such as congenital ocular and neurotoxoplasmosis and affects immunosuppressed patients [Reference Silva3]. Particularly dangerous are primary infections in pregnant women, causing transmission of T. gondii through the placenta to the fetus and congenital infections with a severe, sometimes fatal course [Reference Nowakowska4]. The consumption of raw or undercooked meat and unwashed raw vegetables or fruits were reported as eating habits being important risk factors for toxoplasmosis [Reference Bojar and Szymanska1, Reference Gao5]. Enhanced risk of T. gondii infection is also associated with older gestational age at the time of maternal infection, although the risk of serious consequences then decreases [Reference Remington, Remington and Klein6]. Of socioeconomic risk factors determined were occupational exposure to parasite tissue cysts and oocysts, health status, living in urban or rural areas, frequent exposure to feline faeces, and low educational level [Reference Bojar and Szymanska1, Reference Alvarado-Esquivel, Estrada-Martinez and Liesenfeld7].
Prevalence of T. gondii varies between and within different countries depending on both region and ethnicity [Reference Pappas, Roussos and Falagas8, Reference Ramos9]. Prevalence rates in seropositive pregnant women have been reported from 4% to 100% with values over 60% in Central and South America, Africa and Asia [Reference Pappas, Roussos and Falagas8, Reference Ramos9]. Serological analyses conducted in people living in Polish regions showed high prevalence rates in women of Przodkowo commune from Pomorskie province (54·4%), forestry workers from Pomorskie and Warmińsko-Mazurskie provinces (62·5%) and in pregnant women (41·3%) [Reference Nowakowska10–Reference Holec-Gasior12]. The past few decades brought a considerable decrease in prevalence of T. gondii observed in different countries worldwide [Reference Pappas, Roussos and Falagas8]. In Poland a similar trend in seroprevalence was found, with a significant decrease from 45·4% in 1998 to 39·4% in 2003, with an annual decline of 1% observed in pregnant women [Reference Nowakowska10]. Of 1920 pregnant women treated in 1998 the prevalence of T. gondii IgG without IgM antibodies was 43·4% [Reference Nowakowska13].
Toxoplasmosis is severe threat during pregnancy. Seroepidemiological monitoring of pregnant women is undoubtedly needed to develop educational programmes on risk of T. gondii infections in pregnant women and strategies to prevent congenital toxoplasmosis [Reference Gollub14, Reference Benard15]. In this study, we investigated the prevalence of specific T. gondii IgG antibodies in a group of pregnant women from Poland admitted to the Polish Mother's Memorial Hospital Research Institute in Lodz between 2004 and 2012.
MATERIALS AND METHODS
During a 9-year period (2004–2012) a total of 8281 pregnant women treated at the Polish Mother's Memorial Hospital Research Institute in Lodz, participated in this study. The patients came from Lodzkie province and other Polish regions, as the Institute is a specialist perinatal care centre. Currently, the Polish Society of Gynecology recommends serological testing for T. gondii infections for pregnant women within the first trimester and in negative individuals in the third trimester. However, screening for T. gondii is still not mandatory in Poland.
Serological tests
Blood samples were taken from all pregnant women participating in the study by venepuncture during the first visit to the Institute. Serum fractions were gained by centrifugation and stored at 4°C until analysis within 2 days. Serological tests were performed in the Department of Clinical Microbiology at the Institute.
Screening for T. gondii IgG antibodies was performed with an enzyme-linked fluorescent assay (ELFA) (Vidas Toxo IgG II, bioMérieux, France), using 4 IU/ml as the cut-off point. Detection of IgM antibodies was performed with an ELFA assay (Vidas Toxo IgM, bioMérieux). Samples with indexes ⩾0·65 were considered as positive. Determination of T. gondii IgG avidity was performed using an ELFA assay (Vidas Toxo IgG Avidity, bioMérieux). Samples with indexes <0·200 were interpreted as low avidity, 0·200–0·300 as borderline avidity, and ⩾0·300 as high avidity.
Statistical analysis
The yearly seroconversion rate for T. gondii IgG antibodies was estimated using a mathematical model to determine the dependency between age and prevalence. The linear trend for age was calculated by binomial regression with identity link. Departures from the linear trend for age were performed using quadratic orthogonal trend for age. For prevalence, 95% confidence intervals (CIs) were estimated, assuming binomial distribution. We tested the interaction between age and period for prevalence between 2004 and 2012. We included main effect for age group (18–25, 26–35, 36–47 years) and continuous period, and their interaction. Differences in prevalence rates and age of pregnant women, observed between 1998–2003 and 2004–2012 periods were tested using interaction term in binomial regression. Period variable was categorical and age was continuous.
All results were determined as statistically significant at the significance level P ⩽ 0·05 for main effects and P ⩽ 0·1 for interaction terms. Data were analysed using Stata v. 11 software (StataCorp., USA).
RESULTS
Prevalence rates in pregnant women of different age
T. gondii IgG antibodies were identified in 40·6% (3364/8281, 95% CI 39·6–41·7) of pregnant women aged between 18 and 47 years (mean age 28·7 years).
The prevalence of IgG antibodies related to age is indicated in Figure 1. The incidence rate of T. gondii IgG increased significantly with a yearly seroconversion rate of 0·8% (95% CI 0·6–1·0, P ⩽ 0·001). Departure from the linear trend was not significant (P = 0·117). The highest mean prevalence was observed for women aged 45 years (80%, 95% CI 30·9-97·3); however, the number of pregnant women aged >40 years was smaller compared to other studied patients (114 vs. 8167) (Fig. 1). In the group of older pregnant women (n = 114), the wider 95% CIs for prevalence ranged from 24·3% to 72·8%. For women aged <40 years (n = 8167) the mean prevalence values for particular age groups were between 25·7% and 48·6% and for prevalence the 95% CIs ranged from 7·0% to 19·8% (Fig. 1).
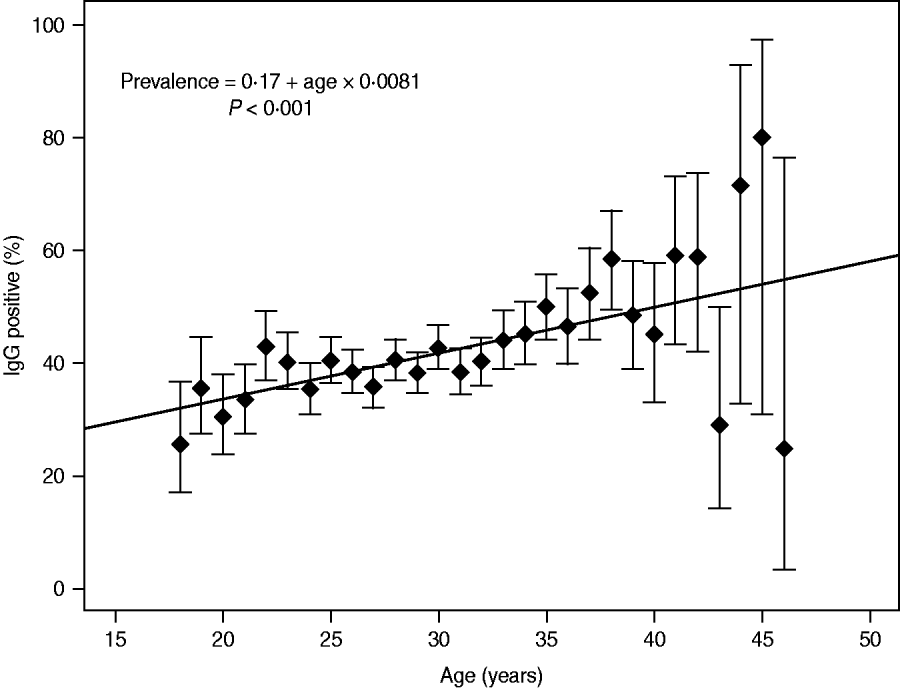
Fig. 1. Incidence rate of Toxoplasma gondii IgG in 8281 pregnant women; estimation model based on the dependency between age and prevalence, with a yearly seroconversion rate of 0.8%.
The estimation of morbidity
Taking into account a yearly seroconversion rate of 0·8%, the estimated risk of primary infection with T. gondii was 0·6% (0·8% × (9/12)). Hence, the risk of acquiring infection during pregnancy was 6/1000 pregnancies. For the whole studied cohort of pregnant women we estimated 49·7 cases to be primary infections during pregnancy.
It is estimated that about 14%, 29% and 59% of newborns of mothers with T. gondii primary infection within the first, second and third trimester of pregnancy, respectively, would be infected [Reference Remington, Remington and Klein6]. Hence, in the analysed population seven (0·85/1000), 14·4 (1·74/1000) and 29·3 (3·54/1000) neonates would be infected in utero during the first, second and third trimester of pregnancy, respectively. Considering that the reported materno-fetal adjusted mean transmission rate of 19·5% occurred from 3 to 34 weeks of pregnancy, 9·7 neonates (1·17/1000) would be expected to have congenital toxoplasmosis in the studied group [Reference Remington, Remington and Klein6]. However, the transmission rate after 34 weeks of pregnancy should also be considered, therefore we took into account the mean materno-fetal transmission rate of 30% as the most accurate coefficient and calculated that 14·9 neonates (1·80/1000) would be congenitally infected [Reference Remington, Remington and Klein16].
Assuming 95% CIs for a yearly seroconversion rate of 0·8%, the calculated rate ranged from 0·6 to 1·0. Considering this range the risk for primary infection during pregnancy would be 0·45-0·75% that means that 4·5–7·5 primary-infected pregnant women per 1000 treated individuals. Hence, in the analysed group from 37·3 to 62·2 pregnant women would have a primary infection. Considering a materno-fetal transmission rate of 30%, about 11·2 (1·4/1000) to 18·7 (2·3/1000) newborns would be infected in utero.
Prevalence rates between 2004 and 2012
Between 2004 and 2012 the prevalence of T. gondii decreased from 41·8% (95% CI 37·7-46·1) to 37·8% (95% CI 32·3-43·5) (Table 1). A decreasing, but not significant trend in prevalence of T. gondii was observed (4%, P = 0·103). It was observed in particular for pregnant women aged 20–35 years. After adjustment for age the annual decline was higher and significant (5%, P = 0·025). For particular age groups the trend ranged from −0·010 to −0·001. However, the yearly trend was homogenous across age groups (P = 0·490). A continuously increasing age of pregnant women (from 28·0 to 29·7 years, mean 28·7 years) was observed.
Table 1. Prevalence of Toxoplasma gondii IgG antibodies in pregnant women tested in Lodz, Poland, between 2004 and 2012
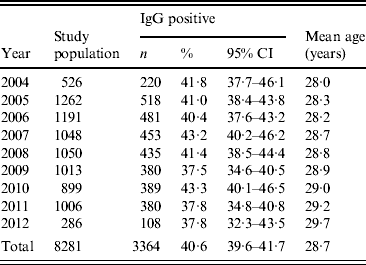
n, Number of women; CI, confidence interval.
Prevalence of IgM antibodies
T. gondii IgM antibodies were identified in 9·7% of studied pregnant women (95% CI 9·1–10·3). Of these women 0·3% were IgM positive without IgG and 9·4% were IgM positive with IgG.
Prevalence rates occurring between 1998–2003 and 2004–2012
Compared to our earlier results, obtained for pregnant women treated between 1998 and 2003 at the same Institute and admitted to the study based on the same criteria, the current yearly seroconversion rate of T. gondii IgG antibodies between 2004 and 2012, was a little higher (0·8% vs. 0·7%) [Reference Nowakowska10]. These small discrepancies were not significant (P = 0·528). Simultaneously, we observed a significantly higher prevalence of T. gondii IgM antibodies (9·7 vs. 4·9%, P ⩽ 0·001). The mean age of pregnant women treated between 2004 and 2012 was also significantly higher compared to patients from the earlier period (28·7 vs 26·7 years, P ⩽ 0·001, data not shown).
DISCUSSION
In women at childbearing age T. gondii prevalence differs from 9% in the UK, 12% in Eastern Spain, 21% in Italy, 24% in the North of Portugal to 41% in Poland, 44% in France and 49% in Albania [Reference Ramos9, Reference De17–Reference Villena22]. In this study we observed the prevalence rate to be 40·6% of 8281 pregnant women. Taking into account data obtained for pregnant women treated at the same Institute from 1998 to 2003 (41·3%), current prevalence rates were slightly lower, but still high compared to other European countries [Reference Nowakowska10]. A similarly low decreasing trend was observed in Croatia, where a prevalence of 29·1% was observed for pregnant women treated from 2005 to 2009 was similar to that of 31·4% described in 2002 [Reference Vilibic-Cavlek23]. However, for the period between 2004 and 2012, we observed a decreased IgG prevalence from 41·8% to 37·8%. The decreasing trend was not significant, but after adjustment for age of pregnant women, the annual decline was higher (5% vs. 4%) and significant. Decrease in toxoplasmosis seropositivity was also observed for other European countries like Austria (from 48% to 50% at the end of the 1970s to 31-35% in recent years), North of Portugal (31·4% in 2005 to 24·4% in 2010), Italy (31·4% in 2001 to 21·4% in 2005) and The Netherlands (35·2% in 1995/1996 to 18·5% in 2006/2007) [Reference Lopes18, Reference Pinto21, Reference Edelhofer and Prossinger24–Reference Sagel, Kramer and Mikolajczyk26].
Considering the age of the studied pregnant women, we calculated a yearly T. gondii IgG seroconversion rate of 0·8%. Based on the estimation model, the prevalence rates increased with age in a linear manner. A similar association between age and seroprevalence was also observed in our earlier study (seroconversion rate of 0·7%) and in other countries [Reference Pappas, Roussos and Falagas8, Reference Nowakowska10]. The linear trend remained in accordance with changes observed for pregnant women treated in Upper Austria between 2000 and 2007 [Reference Sagel, Kramer and Mikolajczyk26]. However, in that population, the seroconversion rate was lower than observed in our study (0·5% vs. 0·8%). It should be noted that the median age of Austrian women (28·3 years) was comparable to the mean age of the Polish pregnant women (28·7 years) [Reference Sagel, Kramer and Mikolajczyk26]. A similar mean age of pregnant women was also observed in other populations including Spaniards (29·9 years for native-born, 28·4 for migrants), French (29·5 years), Hungarians (27·7 years), and Albanians (27 years) [Reference Ramos9, Reference Maggi19, Reference Beke27, Reference Cornu28]. Compared to our study from 2006, the currently observed mean age of pregnant women was significantly higher (28·7 vs. 26·7 years) [Reference Nowakowska10]. Hence, it is plausible that in the Polish population the effect of falling prevalence rates is abolished by the elevated age of pregnant women.
Simultaneously to a decrease in the IgG prevalence rate, we observed significantly elevated prevalence of IgM antibodies (9·7% vs. 4·9%). This is similar to that described in pregnant women in Austria and Kosovo [Reference Edelhofer and Prossinger24, Reference Dentico29]. In pregnant women from Austria the IgG prevalence decreased from 48% to 50% and then to 35% which was accompanied by an increase in IgM prevalence from 0·4% to 0·83% [Reference Edelhofer and Prossinger24]. In pregnant women in Kosovo the IgG seroprevalence of 29·4%, lower than found in other European countries, was observed in parallel with a relatively high IgM seropositivity of 4·1% [Reference Dentico29]. The IgM prevalence observed in Polish pregnant women is much higher compared to the incidence from other countries including rates of 2% or 4·1% in North of Portugal, 0·8% in central Italy and 0·9% or 1·2% in Legnano in Italy [Reference Lopes18, Reference Pinto21, Reference De30]. The much lower estimated incidence of primary infection compared to the observed IgM prevalence (9·7% vs. 0·6%) probably results from the persistence of IgM antibodies for at least 1 year after contamination [Reference Nowakowska10]. The frequency of IgM-positive women without specific IgG was much lower than for those with IgG (0·3% vs. 9·4%). In the population studied in this work, the unexpected increased prevalence rate of IgM antibodies observed simultaneously with the increasing age of pregnant women, and the IgG prevalence elevated with age, indicate increasing age of pregnant women as the most important factor affecting the prevalence rate in pregnant women from Poland. High prevalence of IgM antibodies probably results from their persistence in the host organism for several years after primary infection. We also think that discrepancies between our current and earlier studies might result from differential serological tests used to identification of IgM antibodies. Before March 2000, the serological status of IgM was determined with Platelia Toxo-M assay (Diagnostics Sanofi Pasteur, France). Of four different tests, including two used in our studies, the currently applied Vidas Toxo IgM (bioMérieux) was shown to provide the highest positive predictive value (80·8%) and specificity (99·3%) [Reference Hofgartner31].
We calculated that for the studied Polish pregnant women about 14·9 neonates would be congenitally infected, that means 1·80 infected newborns for every 1000 live births. To date, various mother-to-child transplacental T. gondii transmission rates have been reported, ranging from 0% in case of maternal infection acquired before gestation to 67%, when infection was acquired between weeks 31 and 34 [Reference Remington, Remington and Klein6]. Hence, similar to our earlier study, we assumed the mean transmission rate of 30% as the most accurate coefficient for calculation of congenital infection incidence. Compared to limited data from other European countries, the estimated incidence rate of congenital toxoplasmosis in Polish neonates is lower than that found in Italy (9–35/1000), The Netherlands (2·2/1000) or Kosovo (0·2–2·0/1000), but higher than in France (0·3/1000), Austria (0·1/1000) or Ireland (0·1/1000) [Reference Villena22, Reference Edelhofer and Prossinger24, Reference Hofhuis25, Reference Dentico29, Reference De30, Reference Ferguson32, Reference Ricci33]. Based on our results, the incidence of congenital toxoplasmosis in neonates in Poland is still unfortunately high. The current calculated higher morbidity rate in neonates compared to values from our earlier study (1·80% vs. 1·50%) might be reasoned from the origin of samples, which were collected at a referral centre in Poland as well as from the altered sensitivity of applied serological tests.
T. gondii prevalence in pregnant women in Poland is still high, although a decreasing linear trend in age-adjusted IgG prevalence was found. The observed increased prevalence of IgM antibodies is of particular concern, as it might reflect the elevated level of primary infections in pregnant women during recent years. Both the use of primary prophylactics to increase the awareness of the risk of T. gondii infections in Polish women of childbearing age and secondary prevention by serological testing are absolutely necessary to prevent further spread of the pathogen in future mothers and the development of congenital infections in their babies.
DECLARATION OF INTEREST
None.



