Introduction
Beta-lactam antibiotic allergies are reported in up to 20% of hospitalized patients of which penicillin and cephalosporin allergies make up approximately 10%–15% and 1%–2% of these recorded allergies, respectively. Reference Lee, Zembower and Fotis1–Reference MacFadden, LaDelfa and Leen4 However, allergy documentation in medical records has been widely demonstrated to inaccurately document allergies. Only about 25%–45% of patients with a recorded penicillin allergy in the medical record, ie, a penicillin allergy label, report true immune-mediated allergic reactions. Reference MacFadden, LaDelfa and Leen4–Reference Blumenthal, Shenoy, Varughese, Hurwitz, Hooper and Banerji6 Also, since IgE-mediated reactions to penicillin have been shown to fade over time with 80% of patients losing hypersensitivity after 10 years, older documented allergies may no longer be clinically relevant. Reference Shenoy, Macy, Rowe and Blumenthal7,Reference Patterson and Stankewicz8 Facilities that have implemented penicillin allergy testing programs have identified roughly 95% of patients with a recorded penicillin allergy can safely receive penicillin and other beta-lactam antibiotics, such as cephalosporins. Reference Blumenthal, Kuper and Schulz3,Reference Rimawi, Cook and Gooch5,Reference Blumenthal, Shenoy, Varughese, Hurwitz, Hooper and Banerji6,Reference Lee9–Reference Ham, Sukerman, Lewis, Tucker, Yu and Joshi11 The high proportion of misreported allergy labels paired with waning sensitivity of IgE-mediated reactions results in many patients having penicillin allergy labels that improperly reflect their true allergy status.
Beta-lactam antibiotics such as penicillins and cephalosporins are the preferred treatment for many bacterial infections because they are safer, more effective, and more specific when compared to alternative antibiotics. Reference Lee9,Reference Holten and Onusko12–Reference Tamma, Avdic, Li, Dzintars and Cosgrove14 The presence of a beta-lactam allergy label can influence provider selection of antimicrobial treatment. This results in decreased usage of penicillins and cephalosporins and higher usage of second-line antibiotics such as vancomycin, clindamycin, fluoroquinolone, and carbapenem. Reference Huang, Cluzet, Hamilton and Fadugba2–Reference MacFadden, LaDelfa and Leen4,Reference Kaminsky, Ghahramani, Hussein and Al-Shaikhly15 Beta-lactam allergy labels have also been associated with worse patient outcomes, higher healthcare costs, and higher infection rates from multi-drug resistant organisms. Reference Huang, Cluzet, Hamilton and Fadugba2,Reference MacFadden, LaDelfa and Leen4,Reference Kaminsky, Ghahramani, Hussein and Al-Shaikhly15–Reference Blumenthal, Lu, Zhang, Li, Walensky and Choi20 One study of hospitalized patients with hematologic malignancies found patients with a beta-lactam allergy label were hospitalized on average 4 days longer than patients without a beta-lactam allergy label and were 1.6 times more likely to die. Reference Huang, Cluzet, Hamilton and Fadugba2 The negative effects observed in this population could be exaggerated for patients who require a bone marrow transplant procedure (BMT).
BMT is performed over 20,000 times per year in the United States. Reference Cusatis, Feng, Allbee-Johnson and Shaw21 Following transplant, patients suffer from severe neutropenia and are at a higher risk of serious infection. Within 100 days post-BMT, infections are responsible for an estimated 20% of the deaths. Reference Cusatis, Feng, Allbee-Johnson and Shaw21 A weakened immune system coupled with extended hospital exposure means BMT patients could suffer worse consequences from beta-lactam allergy labels and the associated lack of preferential antibiotic therapy that studies in other populations have shown. Reference Huang, Cluzet, Hamilton and Fadugba2,Reference MacFadden, LaDelfa and Leen4,Reference Kaminsky, Ghahramani, Hussein and Al-Shaikhly15,Reference Charneski, Deshpande and Smith18,Reference Trubiano, Leung, Chu, Worth, Slavin and Thursky19
While other studies have detailed the risks associated with allergy labels in general inpatient populations, this study expands on how beta-lactam allergy labels impact patients undergoing BMT. Our objective was to quantify the association between beta-lactam allergy labels and clinical outcomes in BMT patients. Additionally, we quantified the potential for allergy de-labeling in this population by (1) evaluating documentation of individual allergy labels to confirm presence of a true allergy and (2) identify potential for oral challenge or skin testing.
Methods
Study design and inclusion/exclusion criteria
We conducted a retrospective cohort study of inpatients that received a BMT procedure between April 2018 and March 2020. The study site is a 576-bed quaternary care, academic medical center. Patients under the age of 18 at the time of transplant were excluded. During the study period, an established hospital antimicrobial stewardship program was in place; a febrile neutropenia protocol, collaboratively developed with the BMT physician teams, called for meropenem in patients with severe Ig-E mediated reactions (anaphylaxis, angioedema) and cefepime in patients with less severe reactions (eg rash, hives). Approval for the study was obtained from the local Institutional Review Board.
Data collection
Eligible study subjects were identified using a research repository of continuously refreshed longitudinal data of inpatient and outpatient electronic health record (EHR) data. Repository data included patient demographics, ICD-10 diagnosis codes, antibiotic allergy labels, pharmacy data, and transplant procedure data. In addition, we manually reviewed patients’ EHRs and collected data on patient outcomes and allergy labels which were entered into a REDCap data collection form. Reference Harris, Taylor and Minor22,Reference Harris, Taylor, Thielke, Payne, Gonzalez and Conde23 Allergy label data included the specific drug allergy, date of reaction, reaction type, sufficient characterization, and eligibility for penicillin allergy testing per institutional protocol (appendix 1). Reference Ham, Sukerman, Lewis, Tucker, Yu and Joshi11
Variable definitions
Our primary exposure of interest was the presence of a beta-lactam allergy label in the patient’s EHR prior to the BMT. Allergy label reaction type was classified from the documented reaction as IgE-mediated, non-IgE-mediated, undifferentiated rash, adverse reaction, or unknown based on institutional protocol (appendix 1). Reference Ham, Sukerman, Lewis, Tucker, Yu and Joshi11 IgE- and non-IgE-mediated reactions were further classified as mild or severe based on reaction descriptions and classifications within the allergy label. The adverse reactions category primarily included common antibiotic side effects such as diarrhea, nausea, and vomiting. Allergies were classified as sufficiently characterized if both the reaction and date of reaction were described. Specific notes for when the allergy occurred were required to differentiate between the reaction date and when the allergy label was entered in the medical record. Eligibility for penicillin allergy testing was determined using an institutional protocol (appendix 1) and was based on the reaction type and when the reaction occurred. Reference Ham, Sukerman, Lewis, Tucker, Yu and Joshi11 Eligibility for cephalosporin testing was not evaluated. Two infectious disease pharmacists assisted with evaluating allergy type, severity, and eligibility for penicillin testing.
Our outcomes of interest were mortality, readmission, hospitalization days following transplant, intensive care unit (ICU) admission, C. difficile infection, development of fever, graft vs host disease, and antibiotic usage. All outcomes were assessed using healthcare-system wide data within the 100-day period following the BMT procedure except mortality which was additionally assessed within 30 days of BMT. ICU admission was only recorded for patients after receipt of their first antibiotic. Antimicrobial treatment data was recorded only for intravenous antibiotics to isolate treatment for suspected infections rather than prophylaxis. Median days of total and specific IV antibiotic use was calculated from patients that received at least one day of the measured antibiotic. Subsequent readmissions and health care encounters that occurred outside of the healthcare system following transplant could not be accounted for.
We also collected data on patient demographics and comorbidities present at time of admission for transplant. Transplant type was identified as autologous or allogeneic. Transplant indication was categorized as: acute lymphoid leukemia, acute myeloid leukemia, non-Hodgkin’s lymphoma, Hodgkin’s lymphoma, multiple myeloma, and other. Rare forms of cancer and nonmalignant diseases were included in the “other” category. Patients with myelodysplastic syndromes were included in the acute myeloid leukemia group due to similarities between the conditions.
Statistical analysis
BMT patients with and without a beta-lactam allergy label present in their EHR at the time of transplant were initially described using frequencies for categorical data and medians and interquartile ranges for continuous variables. Continuous data were compared using Wilcoxon-rank sum tests, while
![]() ${\chi ^2}$
or Fischer exact tests were used to compare categorical data. Statistical significance was defined as P < 0.05.
${\chi ^2}$
or Fischer exact tests were used to compare categorical data. Statistical significance was defined as P < 0.05.
Multivariable logistic regression models were used to evaluate the independent effect of beta-lactam allergy labels on clinical outcomes. Separate models were constructed for 100-day mortality, ICU admission, readmission, and carbapenem use, while adjusting for confounding variables. A stepwise model building approach was utilized. Confounders were retained in the model if they individually resulted in a greater than 10% change in the effect of allergy labels on the outcome of interest. All variables associated with the outcome of interest at the p < 0.2 level in bivariable analysis were considered for entry into the multivariable model. Reference groups varied between models with the most protective variable level being chosen as the reference. Variables remained in the model if they stayed below a significance level of p < 0.05 or were identified as a confounder. Allergy label was retained in all models. All statistical analysis was conducted using SAS v9.4 (Cary, NC).
Results
Demographics and comorbidities
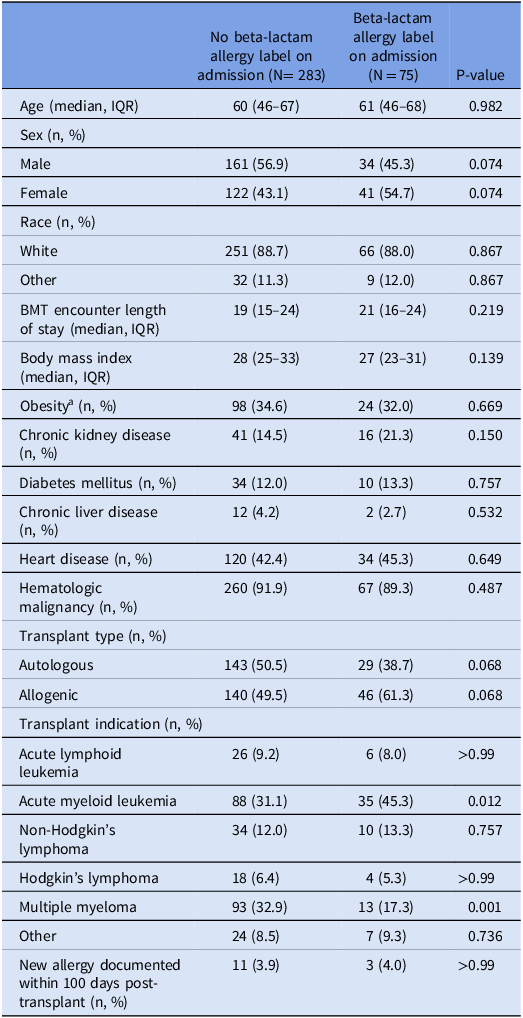
In total, 358 patients met our inclusion criteria and were included in this study (Table 1). Overall, 75 (20.9%) patients had at least one beta-lactam allergy label prior to transplant; 59 (16.5%) patients had a penicillin allergy label, and 21 (5.9%) patients had a cephalosporin allergy label. Note that 5 (1.4%) of these patients had reported an allergy to both penicillin and cephalosporin. Patients with and without allergy labels were comparable for age, body mass index, obesity, heart disease, diabetes, hematologic malignancy, and multiple cancer types. Patients with an allergy label had higher prevalence of chronic kidney disease (21.3% vs 14.5%), allogenic transplants (61.3% vs 49.5%), and acute myeloid leukemia (45.3% vs 31.1%) when compared to patients without an allergy label. Conversely, the non-allergy label group had more male patients (56.9% vs 45.3%), autologous transplants (50.5% vs 38.7%), and multiple myeloma (32.9% vs 17.3%).
Table 1. Characteristics of hospitalized patients receiving bone marrow transplant (BMT) stratified by presence of allergy label on admission
a
Obesity defined as a body mass index
![]() $ \ge $
30.
$ \ge $
30.
Characteristics of allergy labels
There were 92 individual beta-lactam allergy labels recorded from the 75 study patients with a beta-lactam allergy label prior to transplant. There were 66/92 (71.7%) penicillin allergy labels and 26/92 (28.3%) cephalosporin allergy labels (Table 2). Non-IgE-mediated allergic reactions were not documented in any of the 92 allergy labels. IgE-mediated allergic reactions were documented in 19/66 (28.8%) and 5/26 (19.2%) of penicillin and cephalosporin allergy labels. Of these IgE-mediated reactions, 9/66 (13.6%) penicillin labels and 3/26 (11.5%) cephalosporin labels were documented as severe. Undifferentiated rash was more common in the cephalosporin allergy group with 20/26 (76.9%) labels reporting this reaction compared to 38/66 (57.6%) in penicillin labels. Both penicillin and cephalosporin allergy groups shared a high proportion of insufficiently characterized allergy labels at 64/66 (97%) and 26/26 (100%), respectively. An unspecified date of reaction (93.9%, 100%) was a much more common reason for this than the reaction not being described (7.6%, 3.8%). Based on institutional protocol (Figure 1), 66/66 (100%) of the penicillin allergy labels were eligible to be tested for accuracy and delabeled if no true allergy is present.
Table 2. Characteristics of individual patient allergy labels prior to bone marrow transplant stratified by drug class
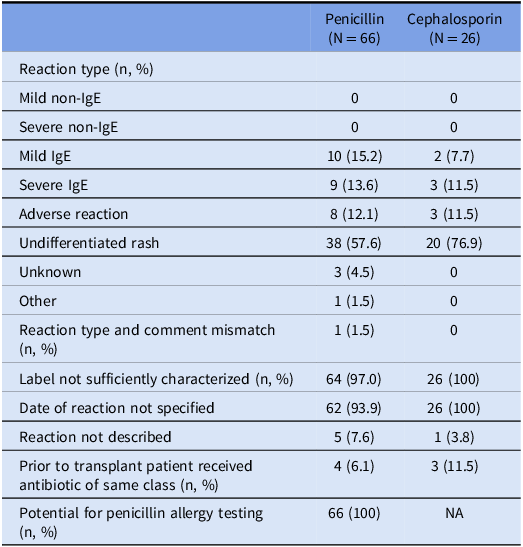
Table 2 is measured at the allergy level. Some patients have more than 1 allergy label in their medical chart.
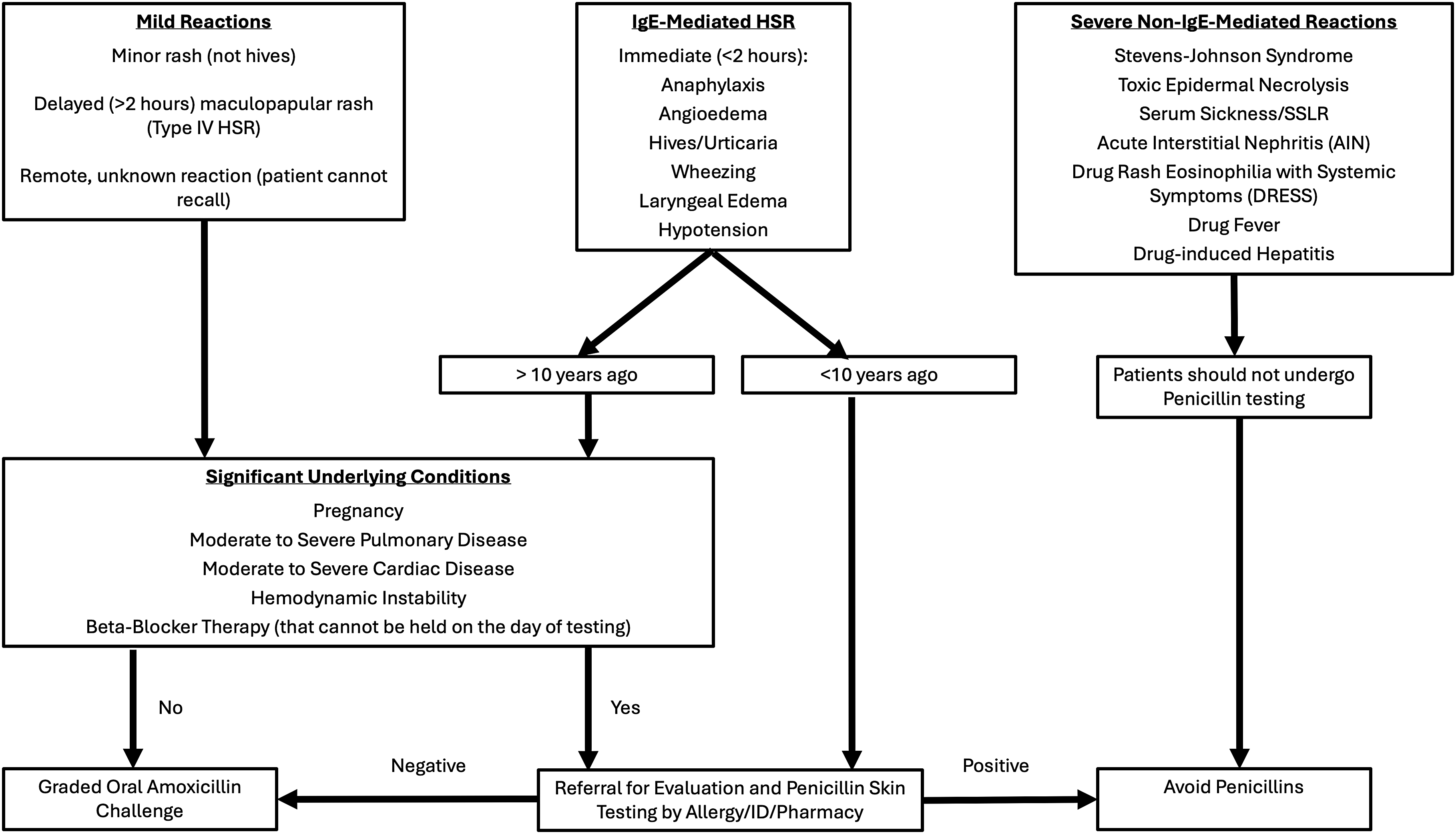
Figure 1. Institutional protocol for determining patient eligibility for Penicillin allergy testing.
Antibiotic usage
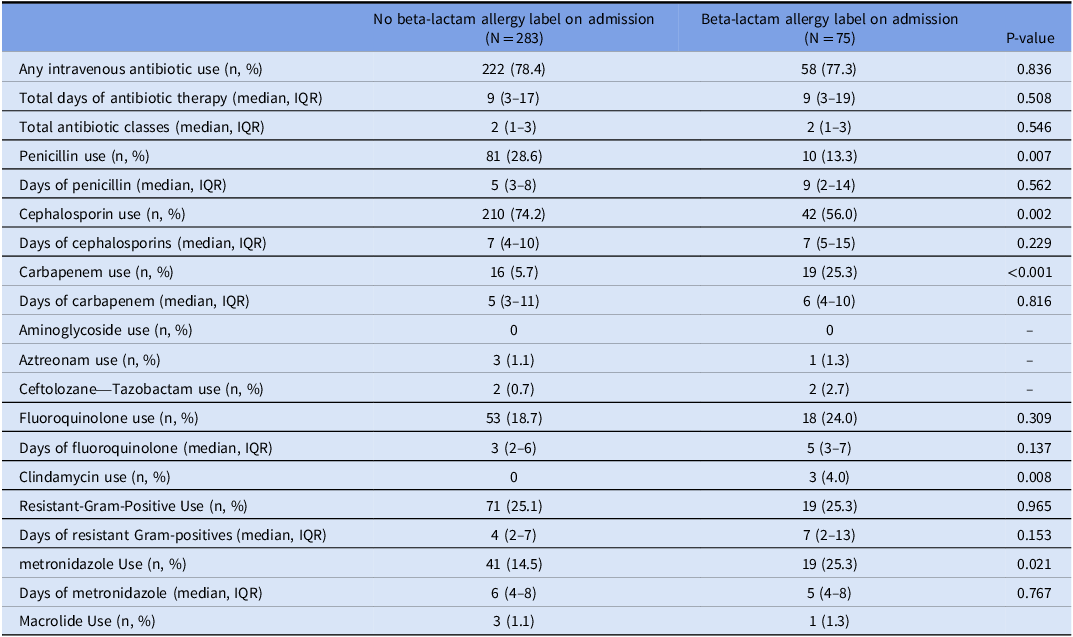
Antibiotic use varied significantly between the allergy label and non-allergy label groups (Table 3). Patients without a beta-lactam allergy label were more likely to receive penicillin (28.6% vs 13.3%, P = 0.007) and cephalosporin (74.2% vs 56%, P = 0.002) compared to patients with a beta-lactam allergy label. Conversely, beta-lactam allergy label patients were more likely to receive carbapenems (25.3% vs 5.7%, P < .001), metronidazole (25.3% vs 14.5%, p = 0.021), clindamycin (4% vs 0%, P < 0.01), and fluoroquinolone (24% vs 18.7%, P = 0.31). This group also had higher median days of fluoroquinolone therapy (5 vs 3, P = 0.137) and resistant gram-positive therapy (7 vs 4, P = 0.153), although these results were not statistically significant. No difference was observed between the two groups for total days of antibiotic therapy or median number of antibiotic classes used.
Table 3. IV antibiotic usage 100 days post-transplant stratified by presence of allergy label prior to transplant
The results of the multivariable logistic regression model identifying independent predictors of carbapenem usage is show in Table 4; antibiotic allergy label and gram-negative blood culture were identified as independent risk factors for receiving carbapenem antibiotics. Patients with an allergy label were 6.3 times more likely to receive a carbapenem (odds ratio [OR] = 6.27; 95% confidence interval [CI] = 2.81 – 13.98; P < 0.0001), while patients who had a gram-negative blood culture were 13.8 times as likely (OR = 13.79; 95% CI = 3.96 – 48.08; P = 0.019). Gram-negative blood culture and transplant indication were identified as confounders of allergy labels impact on carbapenem antibiotics. Non-Hodgkin’s lymphoma served as the reference group against other transplant indications.
Table 4. Independent predictors of carbapenem treatment in logistic regression analysis
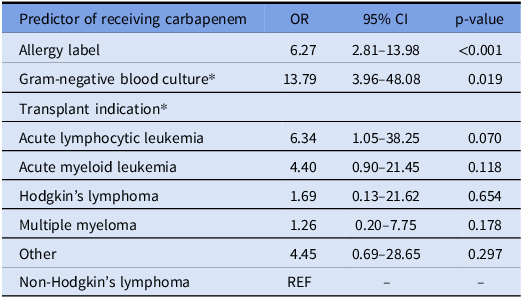
Independent predictors of receiving carbapenem antibiotics within 100 days following bone marrow transplant.
* Denotes confounding variables on the primary exposure variable allergy label.
Treatment-related outcomes
Both patient groups were comparable for several treatment related outcomes, and no outcomes yielded statistically significant differences below the P = 0.05 level (Table 5). The greatest difference between the two groups was for 100-day mortality where death occurred at a higher rate in the beta-lactam allergy label group (14.7% vs 7.8%, P = 0.067). The beta-lactam allergy label group also showed higher rates for ICU admission (14.7% vs 11%, P = 0.374), graft vs host disease (16% vs 13.1, P = 0.512), and readmission (25.3% vs 22.3%, P = 0.574). Medians for days with fever (3 vs 2, P = 0.472) and days with low absolute neutrophil count (15 vs 13, P = 0.451) were also higher in the beta-lactam allergy label group. The non-BL allergy group had a higher rate of C. difficile infection (5.7% vs 2.7%, P = 0.385). Little to no difference was observed between the groups for median days hospitalized after transplant, 30-day mortality, new acute kidney injury, and positive blood culture.
Table 5. Patient outcomes 100 days post-transplant stratified by presence of allergy label prior to transplant
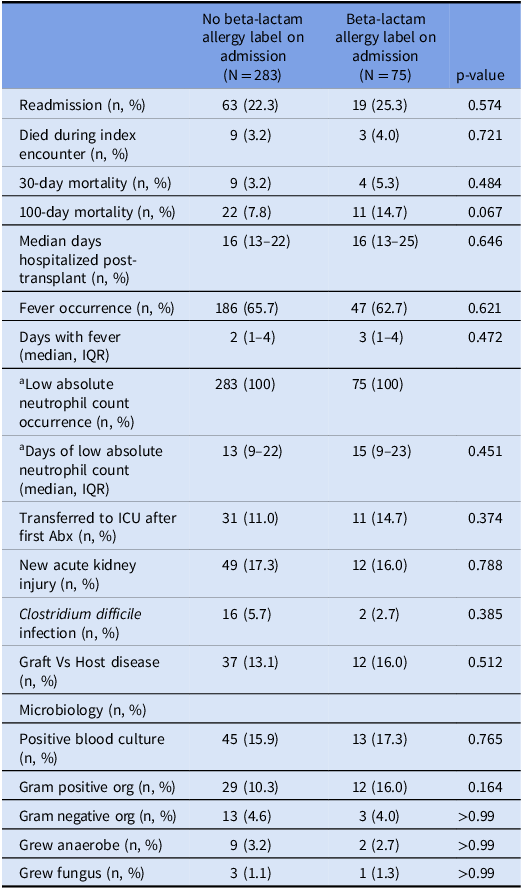
All variables are measured in the 100-day period following bone marrow transplantunless otherwise specified.
a Low absolute neutrophil count defined as < 500.
Independent Predictors of 100-day mortality are included in Table 6. Mortality within 100 days of transplant was 1.6 times more likely (OR = 1.60; 95% CI = 0.68 – 3.78; P = 0.28) in patients with a beta-lactam allergy label, although this relationship was not statistically significant. Chronic kidney disease was a strong predictor of 100-day mortality (OR = 8.78; 95% CI = 3.74 – 20.59; P < 0.0001). Chronic kidney disease, transplant indication, and transplant type were identified as confounders of allergy label’s effect on 100-day mortality. Autologous transplant was the most protective transplant type, and multiple myeloma was the most protective transplant indication for 100-day mortality. Multivariable analysis did not identify the presence of a beta-lactam allergy label to be an independent predictor of readmission (eTable 1) or ICU admission (eTable 2) within 100 days of transplant.
Table 6. Multivariable regression for 100-day mortality
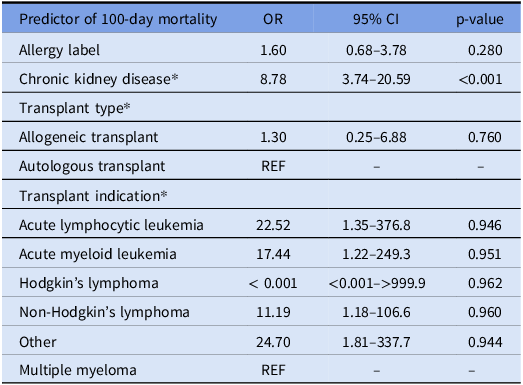
Independent predictors of mortality within 100 days following bone marrow transplant.
* Denotes confounding variables on the primary exposure variable allergy label.
Discussion
In this study, we did not observe a significant association between beta-lactam allergy labels and increased risk of poor patient outcomes among BMT patients. Although not statistically significant, mortality within 100 days of transplant occurred in 15% of the allergy label group which was nearly double the rate seen in patients without beta-lactam allergy labels (P = 0.067). Larger studies in other patient populations have previously demonstrated the negative impacts of antimicrobial allergy labels on patient outcomes. While our results were more inconsistent for outcomes such as length of stay, ICU admission, readmission, and C. difficile infection when compared to prior research, this may be due to either an insufficient sample size or broader inclusion criteria. Reference Huang, Cluzet, Hamilton and Fadugba2,Reference MacFadden, LaDelfa and Leen4,Reference Kaminsky, Ghahramani, Hussein and Al-Shaikhly15,Reference Charneski, Deshpande and Smith18,Reference Kaminsky, Ghahramani, Hussein and Al-Shaikhly15–Reference Charneski, Deshpande and Smith18
Intravenous antibiotic utilization varied significantly between patients with and without a beta-lactam allergy label in their medical record. After adjusting for transplant indication and gram-negative blood culture, patients with a beta-lactam allergy label were 6.3 times more likely to receive carbapenem antibiotics than patients without an allergy label. This marks a significant increase over what has been reported for non-BMT populations. Reference Blumenthal, Kuper and Schulz3,Reference Kaminsky, Ghahramani, Hussein and Al-Shaikhly15,Reference Al-Hasan, Acker, Kohn, Bookstaver and Justo24 Blumenthal et al. conducted a study of 11,000 general inpatients at over 100 hospitals and observed that patients with a penicillin allergy label were only 1.83 times more likely to receive carbapenem antibiotics. Reference Blumenthal, Kuper and Schulz3 The prevalence of beta-lactam allergy labels prior to transplant in our cohort was 21%, with penicillin and cephalosporin allergy labels present in 16.5% and 5.6% of patients, respectively. These values are higher than previously reported in general inpatient populations. Reference Lee, Zembower and Fotis1,Reference MacFadden, LaDelfa and Leen4,Reference Charneski, Deshpande and Smith18,Reference Baxter, Bethune, Powell and Morgan25 All patients with a documented allergy to penicillin in our study were eligible for either a graded oral amoxicillin challenge or penicillin skin test. Prior research suggests that as many as 95% of patients with a penicillin allergy label in their EHR test negative and could be de-labeled when subject to a penicillin skin or oral challenge. Reference Blumenthal, Kuper and Schulz3,Reference Rimawi, Cook and Gooch5,Reference Blumenthal, Shenoy, Varughese, Hurwitz, Hooper and Banerji6,Reference Lee9–Reference Ham, Sukerman, Lewis, Tucker, Yu and Joshi11 The higher frequency of penicillin allergy labels, coupled with the significantly increased likelihood of carbapenem use among those with the label, highlights the importance of de-labeling programs specifically within BMT patient populations.
In addition to the potential antimicrobial stewardship implications of de-labeling, the cost-savings benefits associated with the removal of allergy labels have also been previously demonstrated. Reference Rimawi, Cook and Gooch5,Reference Lee9,Reference Macy and Shu26 A UK study by Li et al. identified that at least 40% of patients could have penicillin allergy labels removed from patient medical records following structured interviews, and these patients had antibiotic expenditures that were 1.82 to 2.58-fold higher than if they had used first-line agents. Reference Li, Krishna, Razaq and Pillay27 The authors calculate that de-labeling could reduce antibiotic costs by £5,851.18 to £14,471.93 for each patient. Reference Li, Krishna, Razaq and Pillay27 If similar savings are observed in the United States, this represents meaningful savings for hospitals compared to the one-time cost of $145-$220 for penicillin allergy testing. Reference Blumenthal, Li, Banerji, Yun, Long and Walensky28
Several limitations should be considered when interpreting the validity of our study. First, the retrospective nature and lack of sufficient allergy history in our study made us unable to confirm the validity of patient allergies or know which allergy labels influenced clinical decision making. Although present, some providers may choose to disregard patient allergy labels if the risk of alternative antibiotic therapy poses a greater threat to the patient than a potential allergic reaction. Given this, some patients in the study were likely treated as if they did not have a beta-lactam allergy even though they were retained in the allergy label group. Second, the data in our study was confined to the healthcare system. Thus, we could fully capture historical antibiotic use, and any readmissions or subsequent healthcare utilization that occurred outside of our healthcare system in the 100-day window following the initial transplant encounter would not have been captured in our analysis and could result in our outcomes being under-reported. Finally, we classified allergy label status immediately prior to transplant. Our analysis did not allow for time-varying classification of exposure status, so a small number of patients who developed a new allergic reaction, and hence a new allergy label during the follow-up period, were retained in the no allergy group (Table 1). Due to our small sample size, we chose not to exclude these patients from the study, but their allergic reactions and subsequent changes in antibiotic therapy may have impacted our outcomes.
In conclusion, our study aimed to evaluate the impact of beta-lactam allergy labels on BMT patient outcomes. Additionally, we examined individual beta-lactam allergy labels to understand how they are characterized and evaluate patient potential for penicillin allergy testing. We observed a non-significant trend towards increased 100-day mortality rates among patients with a beta-lactam allergy label, potentially attributable to sub-optimal antimicrobial treatment following BMT. Patients with a beta-lactam allergy label were less likely to receive penicillin and cephalosporin class antibiotics while being more likely to receive alternative antibiotics such as carbapenems and clindamycin. All patients with a penicillin allergy label in this study were eligible for allergy testing. To decrease the number of patients that report beta-lactam allergies in EHRs, healthcare systems should focus on improved allergy documentation and implementation of penicillin allergy de-labeling programs. Past studies have shown that penicillin allergy testing can improve patient outcomes, optimize antibiotic utilization, and reduce healthcare costs. This could be especially true for vulnerable populations, such as those undergoing BMT, that are at a high risk of infection and would benefit from having as many antimicrobial treatment options as possible.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ash.2025.172.
Financial support
This project utilized core resources supported through the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (grant no. UL1TR002369).
Competing interests
All authors have no conflicts of interest to report relevant to this work.









