Introduction
Real-world data (RWD) are patient and health system data collected in settings outside an experimental study design for regulatory approval (Reference Berger, Sox and Willke1–Reference Gomes, Turner and Sammon3). Different types of RWD are needed and increasingly available for use in health technology assessments (HTAs) for reimbursement purposes and pricing negotiations for innovative health products (IHPs). In economic evaluation, RWD are often needed to populate the model parameters for cost-effectiveness analyses (CEAs) that assess the effectiveness, safety, quality of life (QoL), and related health sector costs. Although good practices related to the incorporation of RWD in the economic evaluation of health products have been proposed, they are still being refined and may require further conceptual structuring and standardization. Although regulatory and HTA recommendations focus on specifying real-world evidence (RWE) that assesses clinical evidence on the benefits and risks of health technology derived from RWD and addresses the use of RWD in estimating relative effectiveness and safety (4–8), few specific recommendations focus on the use of RWD for some other methodological choices required in prospective economic modeling.
In France, the economic evaluations of innovative IHPs (i.e., new medicinal products and medical devices (MDs)) developed by manufacturers are assessed by the French National Authority for Health (Haute Autorité de la Santé, HAS). This process is performed in accordance with regulatory criteria (9) and is approved by the Committee of Economic Evaluation and Public Health (CEESP) for negotiating prices between the French Economic Committee of Health Products (CEPS) and manufacturers. As of today, there are no specific recommendations related to the incorporation of RWD in the HAS doctrine for economic evaluation (10) or in the guidelines for CEAs or Budget Impact Analyses (11–Reference Ghabri, Autin, Poullié and Josselin12). Only a few general good practices for RWD use are mentioned in a guide dedicated to the assessment of health products (13).
Our objectives are: (i) to review how RWD studies have been incorporated in the CEAs of IHPs submitted by manufacturers and assessed by the HAS from January 2016 to May 2023; (ii) to identify methodological issues related to the use of these studies; and (iii) to suggest ideas for improving the use of RWD studies in the HAS assessments of economic evaluations.
Data and methods
Study design
We conducted a retrospective analysis on the use of RWD in the CEAs of IHPs that were submitted by product manufacturers to the HAS from January 2016 to May 2023, and that fulfilled the HAS eligibility criteria for the economic evaluation of IHPs updated in 2022 (9;14). We focused on this 7-year period to identify the temporal trends related to the use of RWD, and because the 2015–2017 period corresponded to debates on the extent to which RWD may be useful in the requirements of the French HTA (Reference Bégaud, Polton and von Lennep15).
Sources of RWD studies
The following data sources were reviewed: assessments of CEAs by the HAS and technical reports submitted by manufacturers and associated clarification letters when necessary (i.e., requests for further information sent to manufacturers after receipt of the submission and manufacturers’ responses to these requests). For simplicity, information on BIAs was not included, and because the same types of RWD used in CEAs (e.g., analyzed population, comparators, and categories of costs) are also considered in BIAs, which also need some additional RWD (i.e., project uptake at the population level). All the data were anonymized. No product or manufacturer is mentioned in our study.
Inclusion and exclusion criteria
All assessments of the CEAs of IHPs (drugs, MDs, and vaccines) were included within the considered period. Analysis of the use of RWD in the assessment of IHPs for their potential inclusion on the list of reimbursable drugs and MDs does not fall within the scope of our study because these assessments are approved by two other parallel HAS committees.
Classification of types of RWD study
The RWD were classified according to the following eight types of studies: prospective cohort studies, retrospective cohort studies, pragmatic studies, medico-administrative studies, registries, prescription surveys, early access programs, and other types. An RWD study was categorized as relying on the basis of the type of study that had been identified in the manufacturer’s submissions. If it was not available, the research team identified it on the basis of its design and characteristics as reported in the technical reports and publications provided by the manufacturers. Finally, if the type of study was not identifiable to the research team, owing to a lack of details reported in the study, then it was classified as “not detailed.”
We used the following definitions for the study of different categories of RWD:
-
- Prospective and retrospective cohorts are longitudinal studies (i.e., they follow up with patients over time); for retrospective studies, the data are already collected, and the outcome is retrospectively analyzed; for prospective studies, the data are collected from a certain time.
-
- Medico-administrative studies involve data routinely collected through patient care in health care system databases such as the “Système national des données de santé” (SNDS). The SNDS is the most important national healthcare dataset that gathers and links reimbursement records, and medical information such as diagnosis codes, hospital stays, and causes of death (Reference Maillard, Bun and Laanani16).
-
- Registries are defined based on the description of the authors of the publication mentioned in the submitted CEA.
-
- Prescription surveys are cross-sectional studies conducted by manufacturers that specifically describe the breakdown of the comparators available in the French clinical practice setting.
-
- Early access program studies discuss a specific early access mechanism for drugs in France, during which data can be collected.
-
- Pragmatic studies (i.e., pragmatic trials) evaluate effectiveness and safety in “real-world” clinical settings.
-
- The category “other” refers to a range of heterogeneous data sources that do not adhere to the above definitions. For example, vignette studies (that estimate health state utilities) were classified within this category.
This classification of the types of RWD studies is globally consistent with the definitions used in HTA agency guidelines, such as those of the HAS, National Institute for Health and Care Excellence (NICE) (5;Reference Faria, Hernandez Alava, Manca and Wailoo7;Reference Duffield and Jónsson17), and the International Society of Pharmacoeconomics and Outcome Research Good Practices on RWD (Reference Berger, Sox and Willke1). However, although medico-administrative studies could be considered retrospective studies, it has been decided to classify those studies in a separate category as “medico-administrative studies” without double-counting. This choice was made to highlight the use of the medico-administrative database in economic evaluation, which is particularly of interest in the French context, regarding the SNDS, for example.
Selected examples extracted from the assessments of CEAs and related publications that illustrate the categories of RWD studies are reported in Supplementary Table 1.
Outcomes and analysis of extracted data
We extracted the main characteristics of the CEAs (e.g., type of health product, therapeutic area, and drug status). In addition, to assess the quality of the included CEAs (that used RWD) we extracted the main methodological aspects identified in the HAS assessments of the CEAs: (1) characterization of the analyzed population considered in the economic decision model; (2) characterization of the included comparators; (3) aspects of modeling (e.g., estimation of transition probabilities, disease progression, or risk equations); (4) external model validation (e.g., survival outcomes of the standard of care); (5) estimation of health state utilities; and (6) cost estimations (i.e., specific RWD studies implemented by manufacturers beyond the publicly available sources of costs as well as the framework of their valuation).
Other statistical aspects included in this checklist were extracted (i.e., type of RWD study, sample size, reporting, and management of missing data).
We measured the proportions and percentages of RWD studies, the types of RWD use (i.e., aspects of the economic models integrating RWD), and the trends in the types of RWD studies over the period from January 2016 to May 2023. We calculated the proportions and percentages of the types of RWD studies by the type of RWD use. We also analyzed the data in terms of the involved drugs (including vaccines) or MDs or the state of the health product (such as the orphan state for drugs). The results were also organized by type of inscription and model type. A specific focus on oncology and hematology was also provided.
We calculated the results in terms of the number of CEAs (N), the number of types of RWD use (N1), or the number of RWD studies among the economic evaluations containing RWD data (N2). We reported whether methodological limitations related to the use of RWD were identified in the HAS assessments of the CEAs (cf. paragraph 3.4). The gradation of the methodological limitations is described in the doctrine of the CEESP (10).
Quality control
The items described in Section “Classification of types of RWD study” were extracted in an Excel template (cf. Supplementary Table 2) by the authors. MS extracted the data, and SG checked the extracted information. Any disagreements or ambiguities were resolved through discussions and additional rechecking of the data sources.
Results
General characteristics of the CEAs
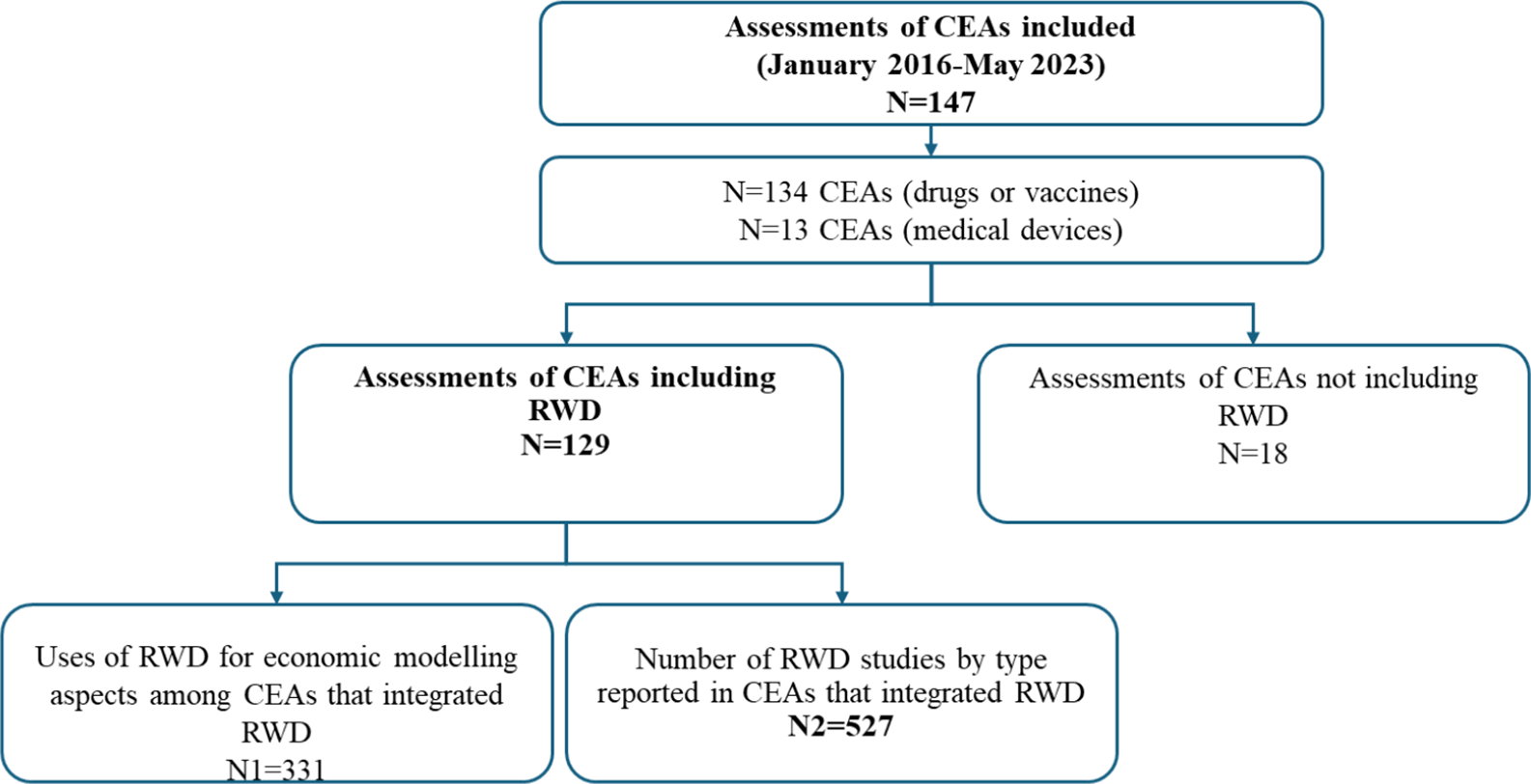
The flowchart of the CEAs included in the analysis is shown in Figure 1. The HAS assessments of the CEAs submitted within the analyzed period are listed in Supplementary Table 3. A total of 147 CEA assessments were performed; of these, 91 percent (134/147) concerned drugs or vaccines. One CEA potentially involved many RWD studies, and 88 percent (129/147) of the CEAs integrated RWD. In one CEA, RWD could be used for six aspects of economic modeling. Among the CEAs that integrated RWD, 331 used RWD for at least one aspect of economic modeling. Several RWD studies can be used for one aspect of economic modeling; in this regard, 527 RWD studies have been reported. Most of the studies concerned the first inclusion on the list of reimbursable drugs and MDs (50 percent, 64/129), whereas others concerned extensions of these inclusions to other indications (48 percent, 62/129), and only a few studies concerned reassessment of health products for the same indication (2 percent, 2/129). The main therapeutic area was oncology. Among the CEAs that did not integrate RWD (18/147), 10 CEAs were of drugs, and 8 were of MDs. Cardiology (5/18) and oncology (4/18) were the main therapeutic areas.

Figure 1. Flowchart of the CEAs included in the analysis.
Main aspects of economic modeling
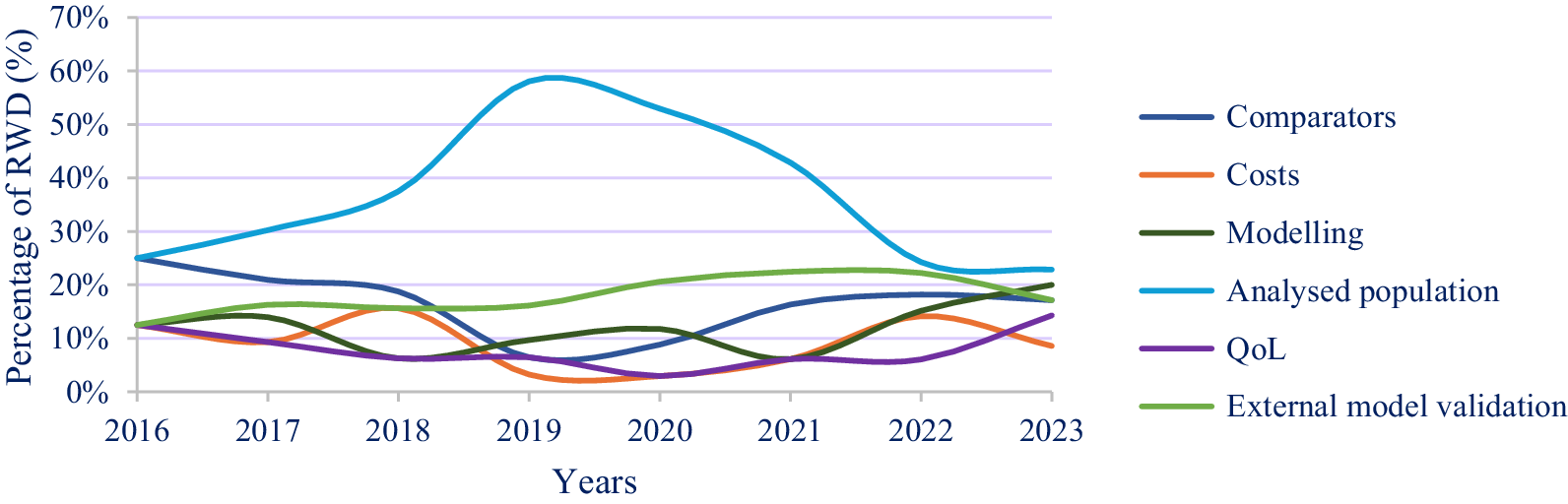
Evolution of the use of RWD studies in the CEAs submitted by manufacturers
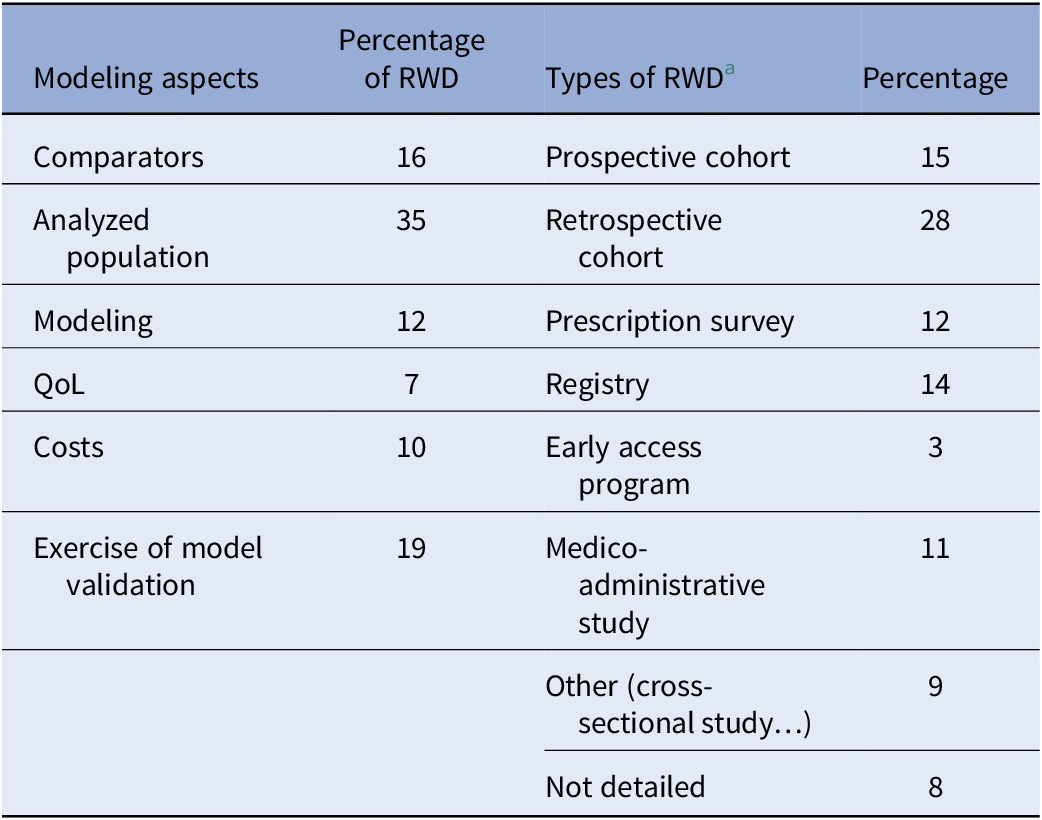
The use of RWD to populate economic decision models has generally increased since 2019. RWD studies have been used mainly to document the inclusion of the following methodological aspects in CEAs: description of the analyzed population, comparator breakdown, and external validation (Table 1).
Table 1. Percentages of RWD studies (N1 = 331) used for methodological aspects and the types of RWD study (N2 = 527) between January 2016 and May 2023

a Pragmatic studies were reported in <0.5 percent of the cases; thus, this category is not reported in the table for ease of readability.
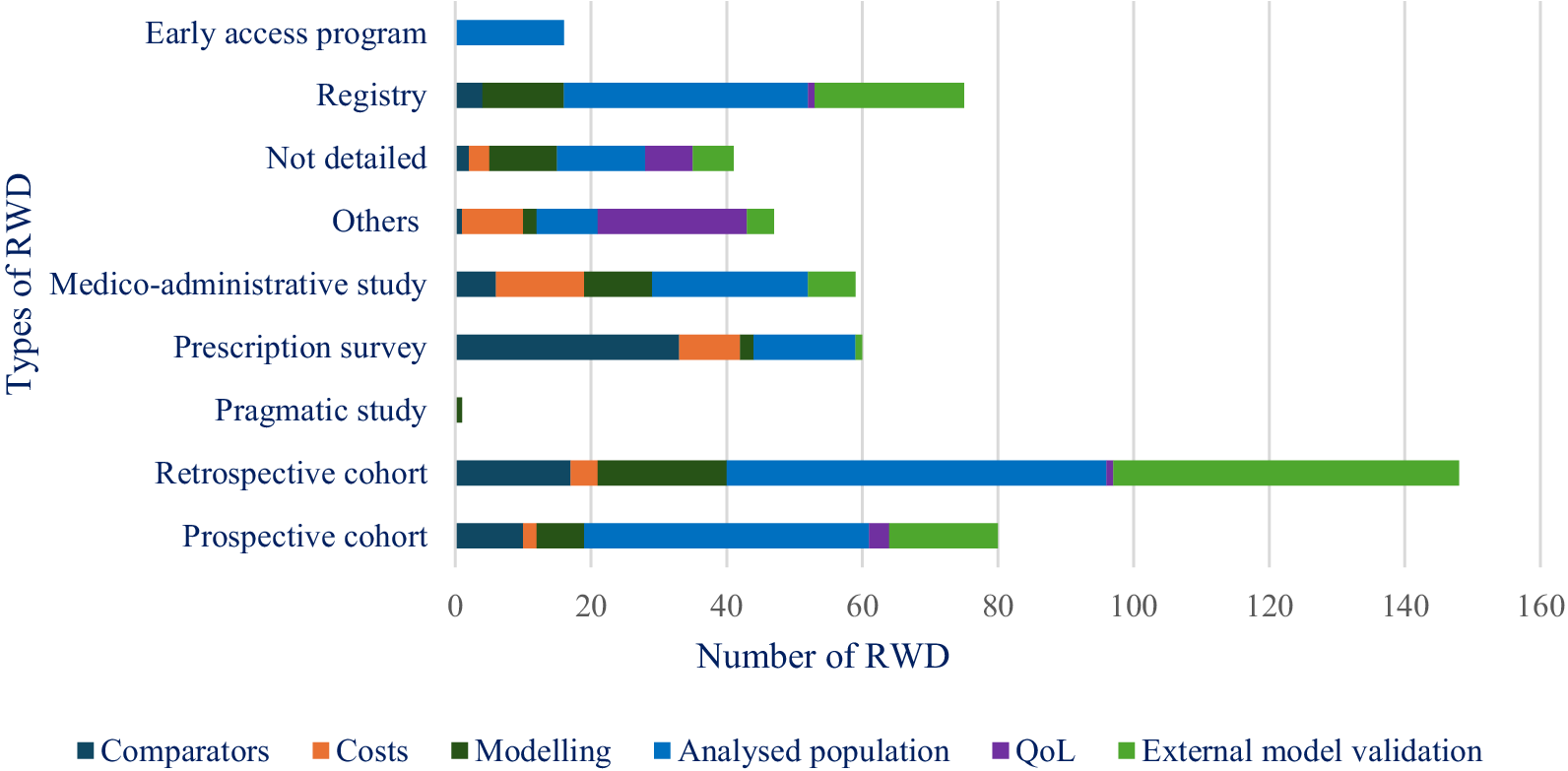
Retrospective cohorts were the most common type of RWD study used in the CEAs submitted by the manufacturers (Table 1), whereas prospective cohorts were most often used for documenting the analyzed population and for external validation (Figure 2). Among the other types of RWD studies, prescription surveys were used for documenting comparators and analyzed populations, whereas registries were used for other aspects of modeling, external validation, and comparisons of the analyzed population to the French population regarding product indication. Medico-administrative studies were especially used to describe the analyzed populations and the breakdown of cost categories. The small percentage of RWD studies used to populate costs is justified by the fact that most of the RWD data mobilized to identify resources were based on expert opinion or aggregate estimates of costs extracted from publicly available statistics or from literature (not RWD studies). These data sources did not correspond to RWD studies implemented by manufacturers that met the criteria of RWD categories defined in the Methods section.

Figure 2. Breakdown of the types of RWD used by methodological aspects between January 2016 and May 2023.
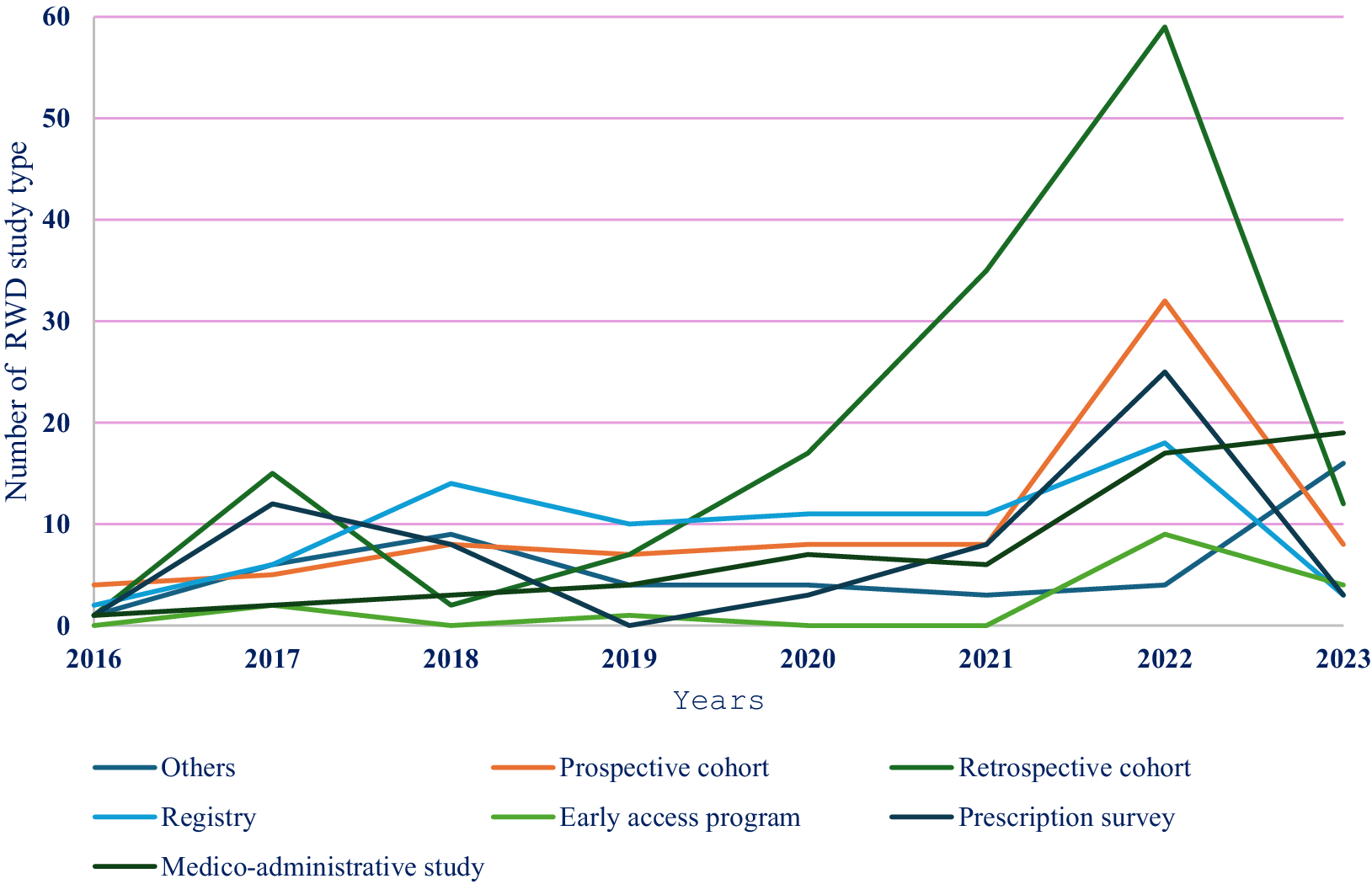
Analysis of the evolution of the use of RWD studies by type revealed opposite trends for the use of cohorts and registries, but these categories of studies remained the main RWD studies used. Specifically, the following trends were observed (Figures 3 and 4):
-
- Prescription surveys were regularly used for analyzing comparators and analyzed populations from 2016 to 2023.
-
- RWD were increasingly used to characterize the analyzed populations from 2016 to 2021. After 2021, the use of RWD for this purpose began decreasing but remained relatively high. A weak but growing trend in the use of RWD for justifying comparators and model validation has been observed since 2019.
-
- QoL data were used very little from 2016 to 2022, but this use increased after 2022.

Figure 3. Evolution of the use of different RWD study types between January 2016 and May 2023 (N2 = 527).
NOTE: For ease of readability, “not detailed” studies and pragmatic studies are not shown in the figure.

Figure 4. Changes in the RWD study for methodological aspects.
In these figures, the interpretation of the results is based mainly on the 2016–2022 period, as 2023 was not fully included.
Analysis by type of assessment and decision economic model
Among the CEAs that integrated RWD, there were a total of 161 (49 percent, 161/331) uses of RWD for an economic modeling aspect for the assessments of first inclusion on the list of reimbursable drugs, vaccines, and MDs; 165 (50 percent, 165/331) for the extension of indication; and 5 (2 percent, 5/331) for the reassessment of the same indication. RWD studies have been mainly used to describe the analyzed population (17 percent, 56/331), comparator breakdown (8 percent 25/331) for the first inclusion dossiers and the analyzed population (17 percent, 57/331), and model validation (12 percent, 40/331) for the extension of indication dossiers (Supplementary Table 4 and Supplementary Figure 1).
Among the CEAs that integrated RWD, there were a total of 154 (47 percent, 154/331), 125 (38 percent, 125/331), and 30 (9 percent, 30/331) uses of RWD for an economic modeling aspect in partitioned survival models (PSMs), Markov models (MMs), or decision tree + Markov models, respectively. RWD data were mainly used to provide data for informing the analyzed population (38 percent, 58/154) and the comparators (23 percent, 36/154) in PSMs, and to document the analyzed population (32 percent, 40/125) and assumptions modeling (21 percent, 26/125) in MMs (Supplementary Table 5).
Analysis of the main therapeutic area
Among the CEAs that integrated RWD, there were a total of 171 (52 percent, 171/331) and 51 (15 percent, 51/331) uses of RWD for an economic modeling aspect in oncology and hematology, respectively. In oncology, RWD were mainly used to document the analyzed population (37 percent, 64/171) and validation (23 percent, 40/171). In hematology, RWD were used to document the analyzed population (31 percent, 16/51) and the comparators (22 percent, 11/51) (Supplementary Table 6).
CEAs on orphan drugs and MDs
In all, 24 percent of the CEAs (36/147) were for orphan drugs. As with the overall sample of health products, 25 percent (9/36) of the orphan drug CEAs were related to oncology, and 94 percent (34/36) of these CEAs employed RWD studies. In addition, the main use of these RWD studies was regarding comparisons of the analyzed populations and external validation of survival outcomes.
Nine percent of the CEAs (13/147) were for MDs, among which 15 percent (2/13) were oncology-related devices, and 39 percent (5/13) involved RWD studies. However, the small number of CEAs regarding the current eligibility criteria for economic evaluation of MDs did not allow a detailed analysis.
Reporting of other methodological aspects
Missing data (28 cases among all RWD studies) and information on their imputation (18 cases among all RWD studies) were rarely provided. Similarly, potential confounding factors were generally insufficiently documented (26 cases among all RWD studies).
Methodological issues related to the use of RWD reported in the HAS assessments of CEAs
We identified several methodological limitations related to the use of RWD in the HAS assessments of CEAs reported for the entire analysis period. Whereas “important” methodological limitations induce high uncertainty regarding the variability of the Incremental cost-effectiveness ratio (icer), “major” methodological limitations invalidate the icer.
Several important limitations related to the use of RWD, mainly related to the analyzed population, validation, and other aspects of modeling, were reported in the assessments. The main important methodological issues were: (i) a lack of comparability between the registries or cohorts used and the analyzed French population regarding drug indications. In some cases, the RWD data that were used to assess the transposability underlined some notable differences between the analyzed population and the French population of the indication; (ii) insufficient documentation of the external validation of the long-term effectiveness of the comparators. For example, in some cases, the RWD reported for external validity were not sufficient to document the extrapolation of survival outcomes (e.g., standard of care).
Approximately the ICERs of 8 percent (10/129 of the CEAs submitted by the manufacturers 3.4), including RWD, were considered invalid because of major methodological limitations or uncertainties related to the use of RWD. We provided two examples of major limitations highlighted in the conclusions of CEA assessments, including RWD studies: The first concerns the lack of transparency and data on the dimension of Qol, which makes it difficult to assess the robustness of the obtained health state utilities scores (e.g., using vignettes and retrospective RWD studies to validate mapping studies in onco-hematology CEAs). The second concerns the methodology limitations related to the use of an external comparator arm based on RWD when the pivotal study is a single-arm trial (e.g., orphan drugs).
Discussion and perspectives
Main findings
To our knowledge, this is the first French study to evaluate the use of RWD in CEAs submitted by manufacturers and assessed by the HAS between January 2016 and May 2023. The most common types of RWD studies involved were retrospective and prospective cohort studies, and the use of RWD in economic decision models has generally increased since 2019. This is partly explained by (1) the awareness of healthcare decision-makers and HTA bodies and stakeholders (e.g., manufacturers) about the importance of the use of RWD (Reference Bégaud, Polton and von Lennep15) and the availability of RWD databases (e.g., French medico-administrative databases such as those provided by SNDs) (Reference Maillard, Bun and Laanani16;Reference Bouée-Benhamiche, Bousquet and Ghabri18) and RWD guidelines (5;Reference Faria, Hernandez Alava, Manca and Wailoo7;13;Reference Duffield and Jónsson17) and (2) the growing requests of HAS assessors and committees to conduct clinical and economic assessments of health products.
Comparison with published HTA studies
We summarized the principal characteristics of similar reviews in Western Europe, the United States, Canada, Australia, and China (Supplementary Table 7), which include the main therapeutic area of submissions, including RWD studies, the main study design and sources of RWD, and the economic aspects included in the submissions. With respect to the findings of these reviews, we highlight two points. The first concerns the results related to the type of RWD studies considered in reviews focusing on assessments of CEAs performed by HTA organizations (e.g., NICE, Canadian Drug Agency (CDA), and the US Institute for Clinical and Economic Review (ICER)). As with our findings, the most frequently used study design was a retrospective cohort, and RWD are mostly used in oncology submissions (Reference Shephard, Ting and Arora19–Reference Wang, Tan and Wu26). From January 2014 to June 2019, Lee et al. (2021) (Reference Lee, Dayer and Jiao27) assessed the use of RWE in 33 economic assessments of drugs by ICER. They reported that a retrospective cohort was the most frequently used study design, that registry data were the predominant data source used, as in the case of CDA (Reference Shephard, Ting and Arora19–Reference Guggenbickler, Barr, Hoch and Dewa21), and that RWD were often incorporated into CEAs to document disease progression. Che et al. (Reference Che, Duffield and Gomes28) included 64 NICE HTA submissions and reported that the main sources of RWD used in the CEAs were disease registries and electronic health records. Their aim was to investigate the current use of RWD for estimating relative treatment effects in manufacturers’ submissions to NICE. In a recent report produced by the decision support unit of the University of Sheffield (2025) (Reference Metry, Latimer, Wailoo and Tappenden29), 8 cases on technology appraisals were analyzed, and demonstrated how the retrospective systemic anticancer therapy datasets provided for interventions considered in the Cancer Drugs Fund could be used to produce cost-effectiveness estimates and compare them to those based on clinical trials.
Second, concerning the results related to the RWD use for the chosen aspects of economic evaluations, given that a few similar findings related to the estimation of costs or QoL frequently included in reviews described other HTA practices (Reference Tunaru, Robinson, MacDougall and Carpenter22;Reference Che, Duffield and Gomes28;Reference Makady, van and Jonsson30), our analysis enriched recent and published reviews. Whereas our extracted data focused on large-choice items used in economic evaluations and the principal structuring aspects of the HAS assessments of CEAs (11;Reference Drummond, Sculpher, Claxton, Stoddart and Torrance31) (i.e., analyzed population, comparators, aspects of modeling, estimations of health state utility, estimations of health product-related costs, and external validation), previous reviews focused on (i) specific economic items such as related drug costs (Reference Che, Duffield and Gomes28; HYPERLINK \l “B30” 30), (ii) the use of RWD in the estimation of relative effectiveness (Reference Che, Duffield and Gomes28; HYPERLINK \l “B32” 32), and (iii) therapeutic area (e.g., oncology (Reference Tunaru, Robinson, MacDougall and Carpenter22;Reference Bullement, Podkonjak and Robinson32;Reference Kang and Cairns33)). Other reviews focused on specific HTA issues, such as the use of RWE in health technology reassessment. For example, a study (Reference Jaksa, Arena, Hanish and Marsico34) across HTA agencies (CDA, NICE, HAS, G-BA/IQWIG, ZIN, and PBAC) from January 2018 through October 2023 showed that RWE should be optimized since no de novo comparative effectiveness studies were evaluated.
Strengths and limitations
Our material included many sources of data: the 147 assessments of CEAs published on the HAS website and, when relevant, the technical reports of the CEAs submitted by the manufacturers and associated clarification letters from January 2016 to May 2023. Unlike the above-mentioned studies, which are based on publicly available HTA reports, the large number of data sources incorporated in this study allows additional checks of the RWD study categories and accurate documentation of the tendencies in the use of RWD. Second, our template for data extraction is dedicated mainly to analyzing RWD use following the principal aspects of the economic evaluation of IHPs. It includes new items such as characterization of the analyzed population and aspects of economic modeling (e.g., probabilities of transition) and external validation of decision models not included in previous reviews (Reference Shephard, Ting and Arora19–Reference Wang, Tan and Wu26;Reference Che, Duffield and Gomes28;Reference Makady, van and Jonsson30;Reference Bullement, Podkonjak and Robinson32). Third, our classification of RWD studies likely includes all potential types of studies beyond only cohort studies, registries, and claims data (e.g., the growing use of early access programs and medico-administrative databases (Reference Maillard, Bun and Laanani16)).
Our analysis has several limitations. First, our classification of RWD studies was based on the technical reports of CEAs submitted by the manufacturers and was not always obvious for special cases of RWD studies lacking a description of the study design (e.g., retrospective vs. prospective cohorts). To address these issues, we checked the references of interest provided in the manufacturers’ technical reports in some cases and defined the “other” category when the study type could ultimately not be determined for a small number of studies. Second, we included CEAs up to May 2023. The results of the 2023 data should therefore be interpreted with caution, as information from the full year is not included. Third, the analyses of the use of RWD studies in the economic evaluation of orphan and MDs should also be interpreted with caution because of the small number of manufacturer submissions meeting the criteria of HAS eligibility.
Perspectives
Today, RWD studies undoubtedly play an important role in the economic modeling of innovative health interventions in France. However, potential actions can be taken to develop good recommendations and strengthen and improve their use.
Standardize the definitions of the types of RWD studies and develop transparent reporting guidance to assess their quality and transposability to the studied disease condition in the French context
A straightforward and standardized typology of RWD studies in terms of design and data sources should be clearly defined whenever possible, as well as its use in the CEAs submitted by manufacturers. For example, a framework including the following elements might be developed: the type of evidence needed and its rationale (disease epidemiology, treatment patterns, comparative effectiveness, safety, disease progression, resource use, QoL instrument, and treatment cost), the likelihood of RWD, and the types of data sources and their designs) (Reference Wang, Tan and Wu26;Reference Kc, Lin and Bayani35;Reference Fitzke, Fayzan, Watkins, Galimov and Pierce36). The reporting guidance may focus on identifying specific bias affecting observational studies and the emergent use of powerful artificial intelligence tools in the analysis and automation of RWD (e.g., deep learning and large language machines) (Reference Fitzke, Fayzan, Watkins, Galimov and Pierce36;Reference Ghabri37).
Building a transparent framework to improve the documentation of economic model inputs and assumptions
Such a framework should specify how several methodological aspects related to comparative effectiveness, drug adherence, and tolerability in a real clinical setting may be justified and presented. It may also aid in the external validation of the long-term survival outcomes of comparators and the collection of utilities. More specifically, a well-designed prospective cohort can be useful in some circumstances, such as for (i) estimating health state utilities when quality-of-life questionnaires cannot be administered in clinical trials (Reference Wolowacz, Briggs and Belozeroff38); (ii) for calibrating transmission models used in the economic evaluation of vaccines (Reference Pitman, Fisman and Zaric39); and (iii) for improving the model goodness of fit and expert elicitations regarding the extrapolation of survival outcomes included in the external validation of the economic model for anticancer products (Reference Bouée-Benhamiche, Bousquet and Ghabri18;Reference Soares, Colson and Bojke40).
Perform reassessments of the CEAs of a prechosen IHPs to address huge uncertainties
Healthcare decision bodies (e.g., the CEPS) or manufacturers may ask HAS to perform reassessments of economic evaluations of IHPs (e.g., cases of rare diseases or expensive advanced therapy medicinal products) that are characterized by important uncertainty in the first HAS assessments. Such an opportunity should facilitate the evaluation of the extent to which the required RWD studies may address these uncertainties (e.g., a lack of robust effectiveness and safety available at the time of marketing authorization) and, at the same time, improve economic modeling by increasing the flexibility of the simulated patient pathway, especially where the history of disease indicates heterogeneous patient profiles (Reference Ghabri, Dawoud and Drummond41). Another example of the usefulness of incorporating RWD studies in economic evaluations for HTA bodies and healthcare decision-makers lies in the reassessment of cost-effective therapeutic classes of drugs when additional evidence in clinical settings becomes available (e.g., the economic evaluations of biological treatments in the management of rheumatoid arthritis performed by the NICE (Reference Stevenson, Archer and Tosh42), HAS (43), and ICER (44), and in aiding CEPS to set up managed entry agreements (MEAs) with manufacturers (e.g., frameworks requiring the specific outcomes in real-life settings or financial clauses of risk sharing or performance-based agreements). De Pouvourville et al. (Reference Cunningham and Fricke45) highlighted the potential use of RWD in MEAs among European healthcare decision-makers (e.g., France, Sweden, and Germany).
Enhance the coherence of HAS IHP guidelines and appraisals and collaboration between scientific committees
Strengthening collaboration among the three committees (Commission de transparence, CT; Commission nationale d’évaluation des dispositifs médicaux et des technologies de santé, CNEDIMTS; Commission d’évaluation économique et de santé publique, CEESP) is essential when they recommend, for example, a certain QoL questionnaire on the basis of RWE. For instance, the common use of generic instruments for the adult population should harmonize and reinforce the assessment of the benefit of QoL in HAS assessments dedicated to reimbursement and price negotiation purposes for innovative pharmaceuticals, vaccines, and MDs.
Strengthen dialogue between national and international HTA bodies, and between the academic and private sectors to facilitate the production of high-quality RWD
Dialogue among HTA bodies (e.g., European countries involved in the European Union framework of Joint Clinical Assessment and the academic and private sectors (Reference Claire, Elvidge and Hanif46)) should continue to promote the development and standardization of cost and RWD databases (QoL, conditions, and disease management) and the sharing of successful international experiences, especially regarding rare disease databases. An ongoing project (“Méthodes et Outil de Valorisation en médico-économie) (Reference Castelli, Mounié and Costa47) is being undertaken by a French academic team to develop a reference for database unit costs of health care similar to those of European countries (e.g., the United Kingdom and the Netherlands).
Establish a transparent framework suggesting deliberative decision rules to interpret the results of CEAs
It is important to emphasize the need to improve the decision rules in the current French guidelines for economic evaluation (10–11) of IHPs to first provide more incentives for the optimal use of RWD and, second, to assess the extent to which RWD contribute to reducing the uncertainties regarding, for example, health outcomes in the submissions of CEAs. The current framework indicates only whether the results are methodologically acceptable and does not provide recommendations on the basis of decision rules (e.g., a benchmark for cost-effectiveness thresholds).
Conclusion
Our study identified key patterns in the use of RWD in the HAS assessments of the CEAs of IHPs submitted by manufacturers from January 2016 to April 2023. Retrospective cohort studies were the most commonly used source of RWD to populate CEA parameters, and their use has increased over time. There is a need (i) to improve the use of registries and cohort studies to increase the comparability between the analyzed populations and the French population regarding the studied indications, and (2) to sufficiently document the exercise of external validation and the collection of QoL. Improving the French decision rules of economic evaluation, as well as the development of good methodological practices for specific aspects of RWD modeling, should help to create incentives for the collection of high-quality RWD to reduce the sources of uncertainty in CEAs.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0266462325103425.
Acknowledgements
The authors would like to appreciate valuable comments on earlier drafts of this manuscript provided by Louis Garrison (University of Washington) and Andrew Partington (Flinders University). The authors would like to thank Karen Facey (University of Edinburgh and FIPRA), Yasmine Fahfouhi (former project manager at HAS), Isabelle Poullié (HAS), and the former committee of economic evaluation and public health (Commission d’évaluation économique et de santé publique, CEESP) for providing insightful comments on the first preliminary results presented at HAS in 2024.
Funding statement
This research received no specific grant from any funding agency, commercial or nonprofit sector. The opinions expressed in this article are those of the authors and do not necessarily represent the views of the HAS. The authors contributed to the article in the following way: Salah Ghabri wrote the first draft of this manuscript. The data were extracted and analyzed by Marine Sion and Salah Ghabri. Marine Sion commented on the draft in different versions. All the authors have approved the finalized version of the article.
Competing interests
The authors declare none.