Introduction
Stray dogs represent a significant reservoir for numerous intestinal helminth species, many of which are of zoonotic concern. The role of dogs in the spread of diseases that pose a potential risk to public health has been reported by many authors (Bentounsi et al. Reference Bentounsi, Meradi, Ayachi and Cabaret2009; Jenkins et al. Reference Jenkins, Lievaart, Boufana, Lett, Bradshaw and Armura-Fernandez2014; Ramos et al. Reference Ramos, Zocco, Medeiros Torres and Sinkoc2015; Amissah-Reynolds et al. Reference Amissah-Reynolds, Monney, Lucy and Agyemang2016; Geraili et al. Reference Geraili, Maroufi, Dabirzadeh, Noormohammadi and Khoshsima Shahraki2016; Ilić et al. Reference Ilić, Kulišić, Antić, Radisavljević and Dimitrijević2017; Dakkak et al. Reference Dakkak, El Berbri, Pétavy, Boué, Bouslikhane, Fassi Fihri, Welburn and Ducrotoy2017). Dogs are known definitive hosts of several helminths and as such contaminate the environment with infective and/or parasitic stages that undergo maturation in the soil. To minimize human exposure to zoonotic parasites, it is generally recommended to humanely manage and control populations of roaming dogs (FAO, 2014). However, in developing countries, dog population management is still based on the mass killing of unowned dogs, particularly following epidemics of human rabies. In Tunisia, this traditional approach is still widely practised although it is strongly contested by the public. Stray dogs in Tunisia are estimated to constitute 80% of the 509 000 rural dog populations (DGSV, 2011) and easily have access to condemned organs and abandoned livestock carcasses (Deplazes et al. Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017). Several surveys on dog intestinal helminths have previously been carried out for the central west, northwestern and southern regions of Tunisia (Lahmar et al. Reference Lahmar, Kilani and Torgerson2001, Reference Lahmar, Sarciron, Rouiss and Mensi2008, Reference Lahmar, Boufana, Lahmar, Inoubli, Guadraoui, Dhibi, Bradshaw and Craig2009; Oudni-M'rad et al. Reference Oudni-M'rad, Chaâbane-Banaoues, M'rad, Trifa, Mezhoud and Babba2017). To the best of our knowledge, this is the first study on the epidemiological parameters of intestinal helminths in stray dogs from two previously unexamined rural areas, Raoued and Soukra in northeastern Tunisia, with special reference to Echinococcus granulosus and other zoonotic helminths.
Materials and methods
Dogs and studied areas
Stray dogs (Canis familiaris) included in this study originated from Raoued and Soukra (Ariana Governorate) northeast of the capital Tunis, which have a population of 94 961 and 129 693 inhabitants, respectively (2014 census), and are located 20 and 6 km from the capital Tunis (Fig. 1). During the period between October and December 2014, a total of 271 stray dogs (130 males and 141 females) were shot as part of a rabies campaign conducted by the Ministry of Interior (MoI) to reduce stray dog numbers. During these campaigns, dog carcasses are normally disposed of in rubbish dumps and are subsequently burnt. Conscious of the relevance of such dog carcasses to scientific research and eager to implement strict carcass disposal measures, the Veterinary School of Sidi Thabet applied for and was granted special consent from the MoI to recover the animal carcasses directly following shooting. These were used for necropsy which, to date, remains the gold standard for exploring canine parasitic intestinal fauna in order to assess the potential risk of zoonotic infections. Carcasses were immediately transferred to the Veterinary School of Sidi Thabet where they were identified by place of origin, sex and age (estimated through the examination of teeth) (http://www.minpin.hu/health/teeth/teeth.htm). Dogs included in this study were between 3 months and 9 years old and were classified into four age groups, less than 1-year olds (<1), between 1 to less than 2 years (⩾1 to <2), from 2 years to less than 6 years (⩾2 to <6) and over ⩾6 years.
Fig. 1. Map of Ariana Governorate, Tunisia.
Parasitological procedures
Necropsies were carried out by qualified veterinarians at the Parasitology Department of Sidi Thabet Veterinary School. Ligatures were made at the cranial end of the duodenum and the end of the rectum thus allowing the removal of the entire intestinal tract. Intestines of necropsied animals were frozen at −80 °C for >15 days to inactivate infectious eggs of Echinococcus species. The intestine of each dog was defrosted, opened longitudinally and its contents and scrapings of the mucosa were washed with an isotonic saline solution (0·9% NaCl). The solution was allowed to stand and the sediment was carefully examined for the presence of helminth parasites. Differentiation of Taenia species and other species of cestodes was based on hook length and the size and morphology of the proglottids following staining with 1% acetocarmine solution (Khalil et al. Reference Khalil, Jones and Bray1994). The total number of Echinococcus tapeworms harboured by each dog was determined either directly by counting all the worms in the sediment (if worm burdens were small) or estimated by counting the worms in 20% of the sediment. Nematodes were clarified in chloral-lactophenol solution (44%) for morphological identification and enumeration (Anderson, Reference Anderson1992).
Molecular analysis
DNA-based molecular identification was carried out on retrieved Echinococcus adult tapeworms. Total genomic DNA was extracted using a commercial kit (Qiagen DNeasy Blood and Tissue DNA extraction Kit, Qiagen, Hilden, Germany) as per manufacturer's instructions. A fragment within the mitochondrial cytochrome c oxidase subunit 1 (cox 1) gene was amplified using published probes (Bowles et al. Reference Bowles, Blair and McManus1992). PCR products were viewed using UV illumination (Syngene G: Box gel documentation system, Cambridge Biosciences), purified using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) and commercially sequenced in both directions (Macrogen EZ- Sequence, Amsterdam, The Netherlands). The generated nucleotide sequences were compared to the Bowles et al. (Reference Bowles, Blair and McManus1992) G1-G3 genotype sequences as they appeared in the aforementioned publication.
Data analysis
The χ 2 test and Fisher's exact test were used to compare the prevalence of helminth species in the two studied regions, as well as prevalence according to host age and gender. All statistical analyses were performed using SPSS version 16. A P-value of ⩽0·05 denoted a statistically significant difference. The Student's t-test was also used to determine differences in prevalence and in abundance of each species according to host age and gender.
Results
Genotyping
DNA was extracted from E. granulosus adult tapeworms retrieved from 10 of the 14 infected dogs. A successful amplification of a 366 bp mitochondrial cox 1 fragment to identify species/genotypes of Echinococcus was achieved for worms removed from nine dogs. The alignment of the generated nucleotide sequences against those reported by Bowles et al. (Reference Bowles, Blair and McManus1992), showed that the nine Tunisian dogs were infected with E. granulosus sensu stricto (s.s.) (eight E. granulosus G1 and one E. granulosus G2 genotype).
General infection
Of the 271 examined stray dogs, 268 were infected with one or more helminth species, giving an overall prevalence of 98·89% (±0·006) and a mean intensity of 87·62 (95% CI 63·1–112·13) parasites per infected dog. Sixteen intestinal helminth species were identified, including three trematodes [Brachylaemus (Brachylaima) sp., Phagicola (Ascocotyle) italica, Heterophyes heterophyes], eight cestodes (E. granulosus s.s., Dipylidium caninum, Diplopylidium noelleri, Mesocestoides lineatus, Mesocestoides litteratus, Taenia hydatigena, Taenia pisiformis, Taenia multiceps), four nematodes (Toxocara canis, Ancylostoma caninum, Uncinaria stenocephala, Trichuris vulpis) and one acanthocephalan (Macracanthorhynchus hirudinaceus) (Table 1). Cestodes were the most prevalent parasites (97·04%), followed by nematodes (83·02%), trematodes (5·53%) and acanthocephalans (2·21%). The difference in the infection rate with each helminth group (P < 0·001) was highly significant. Nineteen dogs had a single infection, 61 had a double infection, 90 had a triple infection, 55 had four infections, 28 had five infections, 12 had six infections and three dogs had seven infections. A high percentage of infected dogs (95%) harboured zoonotic species including E. granulosus s.s., D. caninum, T. multiceps, T. canis, A. caninum and H. heterophyes.
Table 1. Prevalence, intensity and range of intestinal helminths of stray dogs from two northeastern areas in Tunisia
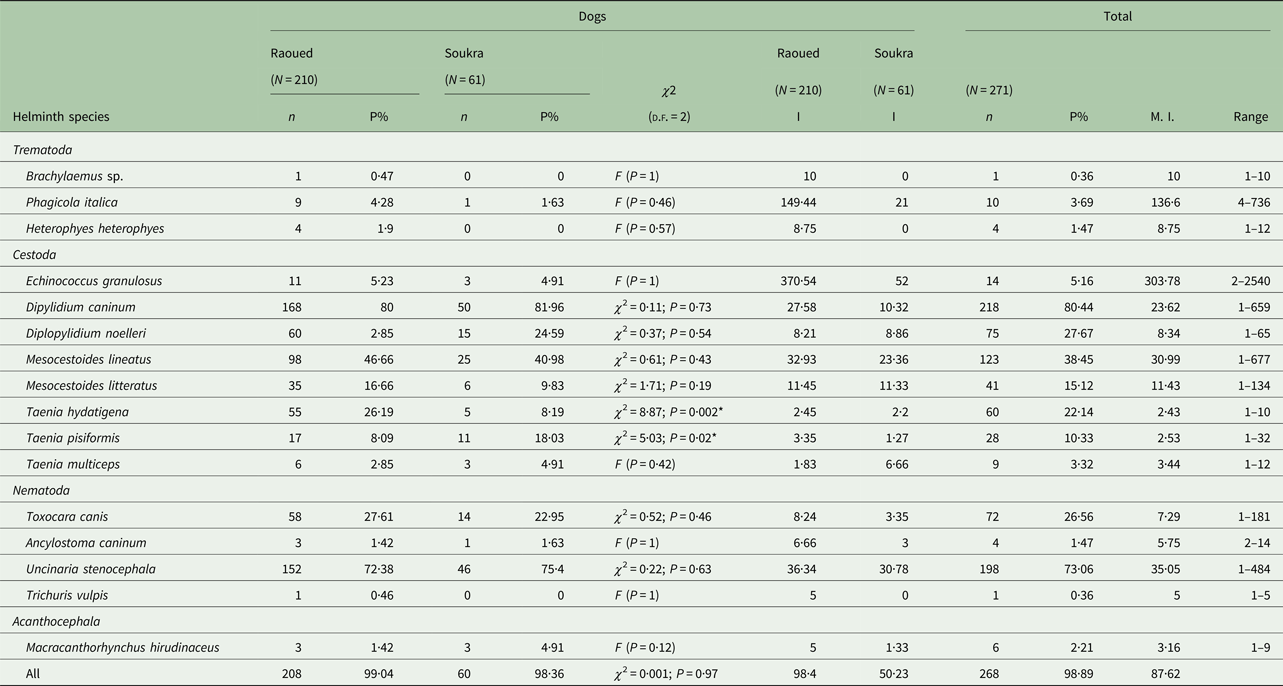
N, number of dogs examined; n, number of infected dogs; P, prevalence; I, intensity; M.I., mean intensity; *significant.
Prevalence and intensity of intestinal helminths
Prevalence rates for each helminth species are presented in Table 1. The highest worm intensity (303·7 worms) was observed for E. granulosus s.s. with the total number ranging from 2 to 2540 tapeworms. Another high mean intensity was recorded for the trematode P. italica (136·6 parasites/infected dog) with the number of worms varying between 4 and 736. Lower mean intensities (⩽10 worms) were detected for Brachylaemus sp., H. heterophyes, D. noelleri, T. hydatigena, T. pisiformis, T. multiceps, T. canis, A. caninum, T. vulpis and M. hirudinaceus (Table 1).
Prevalence and intensity of intestinal helminths in dogs by region
There was no significant difference in helminth prevalence observed between the two studied regions, Raoued (99·04% ± 0·007) and Soukra (98·36% ± 0·016) (χ 2 = 0·001; P = 0·97). However, the mean intensity of infection was significantly higher in dogs from Raoued than in those from Soukra (P = 0·01) (Table 1). Dogs from Raoued harboured all the helminth species described in this study while, Brachylaemus sp., H. heterophyes and T. vulpis were absent in dogs from Soukra. Multiple infections of dogs with each helminth species revealed no significant difference in prevalence between the two regions except for T. hydatigena (P = 0·002) and T. pisiformis (P = 0·02). Dipylidium caninum was the most prevalent helminth in the two regions with a higher intensity in Raoued (27·58 per dog) than in Soukra (10·32 per dog). Although E. granulosus s.s. infection rate was almost similar for the two regions, dogs from Raoued were more intensely infected (370·54 worms per infected dog), with one dog harbouring 2540 worms. However, in Soukra area, E. granulosus s.s. showed the highest helminth intensity followed by U. stenocephala. The trematode P. italica was present in dogs from Raoued with an intensity of 149·4 parasites per infected dog, while the lowest mean intensities of infection in the two regions were recorded for three Taenia species (T. hydatigena, T. pisiformis, T. multiceps) and M. hirudinaceus (Table 1).
Prevalence and intensity of intestinal helminths in relation to age and gender of dogs
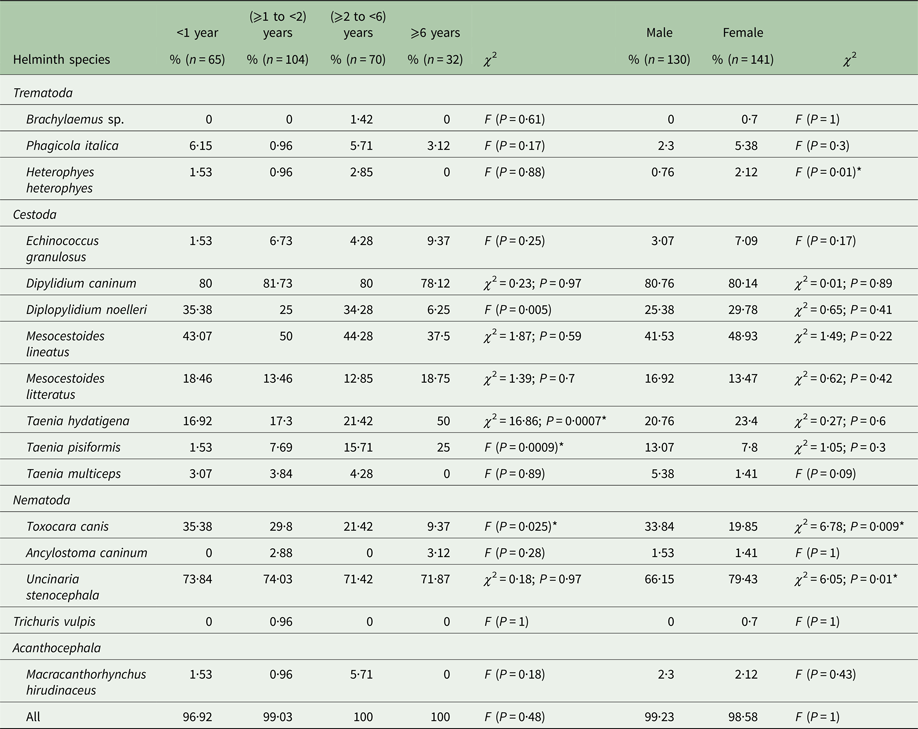
Age and gender distribution of intestinal helminths are represented in Tables 2 and 3. In all age groups, dogs were highly infected although a reduced prevalence was observed in animals aged <1 year (96·92%) and those ⩾1 to <2 years (99·03%) (P = 0·48). However, the abundance of infection was highest in dogs <2 years (P = 0·01). There was no significant difference in the prevalence (P = 1) observed between male (99·23% ± 0·008) and female dogs (98·58% ± 0·01), although the mean abundance of infection was significantly higher in males (P = 0·049). All helminth species were identified in both sexes except Brachylaemus sp. and T. vulpis, which were not found in any of the examined male dogs. There was no difference in prevalence between the sexes for any helminth species except for T. canis (P = 0·009), U. stenocephala (P = 0·01) and H. heterophyes (P = 0·01) (Table 2). For A. caninum there was no significant difference in prevalence between the sexes; however, females were more intensely infected (nine worms per infected dog) than males (2·5 parasites/infected dog). Ancylostoma caninum was found in two dog age groups (⩾1 to <2 and ⩾6 years) (P = 0·26), whereas U. stenocephala was retrieved from dogs of all age groups with no significant difference in prevalence (P = 0·97) but with a high difference in abundance (P = 0·004) (Table 3).
Table 2. Prevalence of intestinal helminths of stray dogs from two northeastern areas in Tunisia by host age and gender
n, number of dogs; *significant.
Table 3. Abundance and intensity of intestinal helminths of stray dogs from two northeastern areas in Tunisia by host age and gender
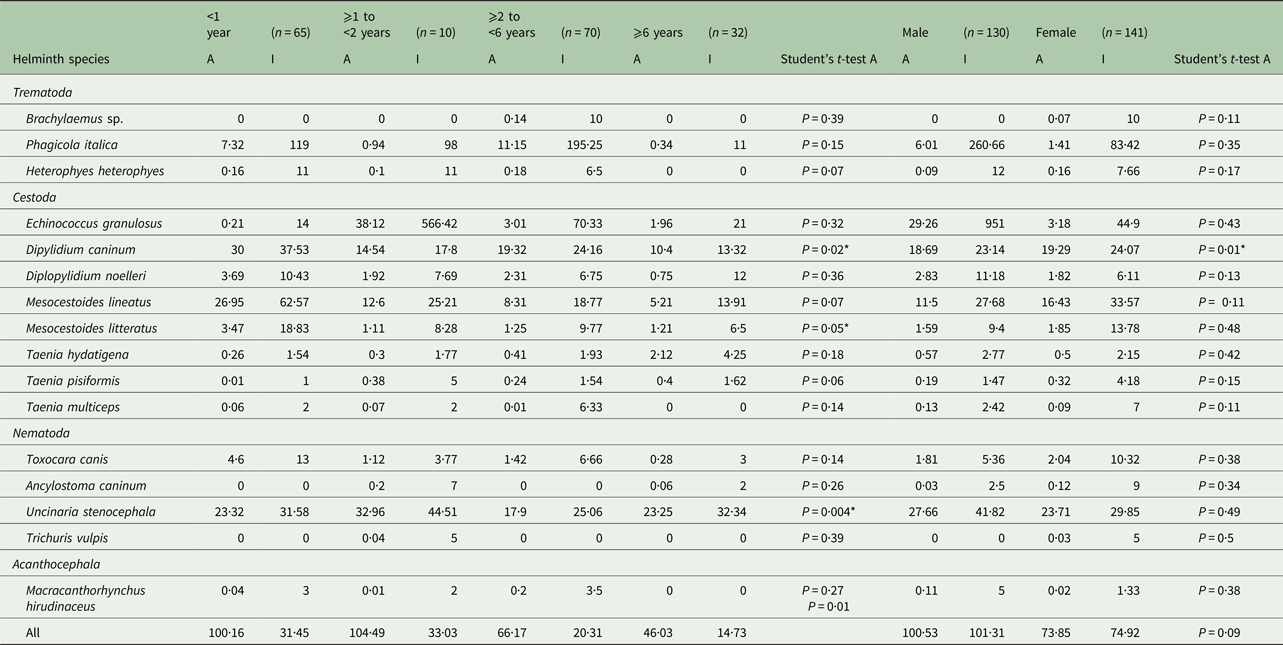
n, number of examined dogs; A, abundance; I, intensity; *significant.
The prevalence of E. granulosus s.s. was higher in female dogs (7·09%) than males (3·07%) (P = 0·17), but the abundance of infection was lower in females (3·18) than in males (29·26) (P = 0·43). The increase of E. granulosus s.s. infection rate with host age was not significant (P = 0·25), although abundance and intensity were highest for the second (⩾1 to <2) dog age group (Table 3). The mean intensity of infection with E. granulosus s.s. varied from 14 to 566·42 worms/infected dog. Young dogs (⩾1 to <2 years) had the highest worm burdens including two massively infected dogs harbouring 1200 and 2540 worms each; the remaining 12 dogs had 2, 9, 12, 14, 16, 26, 38, 58, 65, 67, 86 and 120 E. granulosus s.s. adult tapeworms, respectively. The distribution of E. granulosus s.s. in dogs examined in this study was overdispersed and the data for the intensity of infection gave a significant fit to the negative binomial model. Dipylidium caninum was abundant in male and female dogs (P = 0·01) with the highest abundance observed in young animals (P = 0·02). Brachylaemus sp. and T. vulpis infection were only recorded in dogs aged ⩾2 to <6 and ⩾1 to <2 years, respectively. Heterophyes heterophyes and T. multiceps were not found in older dogs (⩾6 years) (Table 2).
Discussion
Stray dogs across all age groups were highly and intensely infected with intestinal helminths. A total of 23 423 helminths were retrieved from 268 infected dogs corresponding to 16 species. The majority of helminth species described in this study (11/16, 68·8%) were previously recorded in stray dogs, golden jackals and red foxes from the northwest and centre west of Tunisia (Lahmar et al. Reference Lahmar, Kilani and Torgerson2001, Reference Lahmar, Sarciron, Rouiss and Mensi2008, Reference Lahmar, Boufana, Lahmar, Inoubli, Guadraoui, Dhibi, Bradshaw and Craig2009). This epidemiological status is not surprising. To the best of our knowledge, no public or private measures and/or initiatives are known to have been undertaken in recent years to control zoonotic and veterinary parasites within the explored areas (Raoued and Soukra). Furthermore, significant levels of environmental contamination with canine helminth eggs, as indicated by a soil contamination index of 55% for 1270 dog faecal samples collected from different Tunisian regions, were recently reported (Oudni-M'rad et al. Reference Oudni-M'rad, Chaâbane-Banaoues, M'rad, Trifa, Mezhoud and Babba2017). In the examined dogs, cestodes (97·04%) were more common than nematodes (83·02%), whereas trematodes (5·53%) and acanthocephalans (2·21%) were rare. The predominance of cestodes has previously been described (Bajalan, Reference Bajalan2010; Geraili et al. Reference Geraili, Maroufi, Dabirzadeh, Noormohammadi and Khoshsima Shahraki2016), and the prevalence of intestinal helminths observed in this study is similar to that reported for stray dogs from other parts of the world (Mateus et al. Reference Mateus, Castro, Ribeiro and Vieira-Pinto2014; Emamapour et al. Reference Emamapour, Borji and Nagibi2015; Ramos et al. Reference Ramos, Zocco, Medeiros Torres and Sinkoc2015; Rehbein et al. Reference Rehbein, Kaulfuß, Visser, Sommer, Grimm and Silaghi2016).
Dipylidium caninum, the most frequent cestode species identified in the present study (80·44%) was previously reported from necropsied stray dogs in Tunisia with prevalence rates ranging from 27·5 to 43·6% (Lahmar et al. Reference Lahmar, Kilani and Torgerson2001, Reference Lahmar, Boufana, Lahmar, Inoubli, Guadraoui, Dhibi, Bradshaw and Craig2009). Due to the absence of regular anthelminthic treatments and anti-ectoparasitic drugs for dogs, D. caninum can occasionally pose a threat to children through the accidental ingestion of flea intermediate hosts harbouring the infective cysticercoid stage (Szwaja et al. Reference Szwaja, Romański and Zabczyk2011). A report on the infection of a 17-month-old boy from China with D. caninum was recently published (Jiang et al. Reference Jiang, Xi Zhang, Liu, Wang and Cui2017).
The overall prevalence of E. granulosus s.s. seen in this study (5·16%) was lower than that reported from central and western parts of Tunisia (21%) (Lahmar et al. Reference Lahmar, Kilani and Torgerson2001). In Tunisia, E. granulosus s.s. is prevalent in domestic and wild intermediate hosts, golden jackals, stray dogs and humans (Boufana et al. Reference Boufana, Lahmar, Rebaï, Ben Safta, Jebabli, Ammar, Kachti, Aouadi and Craig2014, Reference Boufana, Lett, Lahmar, Griffiths, Jenkins, Buishi, Engliez, Alrefadi, Eljaki, Elmestiri, Reyes, Pointing, Al-Hindi, Torgerson, Okamoto and Craig2015; Deplazes et al. Reference Deplazes, Rinaldi, Alvarez Rojas, Torgerson, Harandi, Romig, Antolova, Schurer, Lahmar, Cringoli, Magambo, Thompson and Jenkins2017). Although three Echinococcus species namely, E. granulosus s.s., Echinococcus canadensis and Echinococcus equinus, have been identified from various intermediate hosts in Tunisia, to date, E. granulosus s.s. is the only species reported from definitive hosts (dogs and wild canids) (Lahmar et al. Reference Lahmar, Boufana, Lahmar, Inoubli, Guadraoui, Dhibi, Bradshaw and Craig2009, Reference Lahmar, Boufana, Ben Boubaker and Landolsi2014; Boufana et al. Reference Boufana, Lahmar, Rebaï, Ben Safta, Jebabli, Ammar, Kachti, Aouadi and Craig2014, Reference Boufana, Lett, Lahmar, Griffiths, Jenkins, Buishi, Engliez, Alrefadi, Eljaki, Elmestiri, Reyes, Pointing, Al-Hindi, Torgerson, Okamoto and Craig2015; Chaâbane-Banaoues et al. Reference Chaâbane-Banaoues, Oudni-M'rad, Cabaret, Mezhoud and Babba2015). We speculate that stray dogs in the studied regions may have acquired infection through feeding on hydatid ridden-viscera from rural clandestine sheep slaughtering and abandoned ruminant carcasses in the environment following natural mortality of livestock; however, the variation in dog echinococcosis prevalence depends largely on human behaviour and hygiene (El Berbri et al. Reference El Berbri, Ducrotoy, Pétavy, Fassi Fihri, Shaw, Bouslikhane, Boué, Welburn and Dakkak2015). Interestingly, Raoued and Soukra are not important sheep raising regions, with no livestock transhumance. Cystic echinococcosis annual surgical rate for this region was estimated to be 4·8/100 000 inhabitants, while the mean annual surgical rate for the whole country is 12·6 cases/100 000 inhabitants (Chahed et al. Reference Chahed, Bellali, Ben Alaya, Aoun and Zouari2015).
Our data showed that the age of dogs was not independent of E. granulosus s.s. infection (P = 0·25). The non-linear age-prevalence profile observed in this study suggested that older dogs acquired immunity under the E. granulosus s.s. endemic steady-state equilibrium in Tunisia (Lahmar et al. Reference Lahmar, Kilani, Torgerson and Gemmell1999, Reference Lahmar, Trifi, Ben Naceur, Bouchhima, Lahouar, Lamouchi, Maâmouri, Selmi, Dhibi and Torgerson2013) where dogs, submitted to repeated numbers of challenge infections become resistant (Gemmell, Reference Gemmell1990). Thus, the highest worm burdens were found in younger dogs compared with older ones that had lower parasite abundances. Similar findings were previously reported from Tunisia (Lahmar et al. Reference Lahmar, Kilani and Torgerson2001), Kazakhstan (Torgerson et al. Reference Torgerson, Shaikenov, Rysmukhambetova, Ussenbayev, Abdybekova and Burtisurnov2003) and Morocco (Azlaf et al. Reference Azlaf, Dakkak, Chentoufi and El Berrahmani2007; Dakkak et al. Reference Dakkak, El Berbri, Pétavy, Boué, Bouslikhane, Fassi Fihri, Welburn and Ducrotoy2017). In addition, this study revealed similarities in E. granulosus s.s. prevalence between male and female dogs (P = 0·17), which may be due to equal free roaming opportunities in search of offal.
In this survey, T. hydatigena was the most frequently encountered taeniid (22·14%). Epidemiological studies on dogs, the definitive hosts, ruminants and wild boars, the intermediate hosts, indicate that T. hydatigena is endemic in Tunisia (Maâmouri, Reference Maâmouri2005; Lahmar et al. Reference Lahmar, Sarciron, Rouiss and Mensi2008; Lahmar, Reference Lahmar2012; Lamouchi, Reference Lamouchi2009). However, no official data are available on the cost of condemnation of infected livers due to traumatic hepatitis caused by the metacestode larval stage, Cysticercus tenuicollis. Similar T. hydatigena infection rates to those seen here were reported in dogs from Albania (16·2%; Xhaxhiu et al. Reference Xhaxhiu, Kusi, Rapti, Kondi, Postoli, Rinaldi, Dimitrova, Visser, Knaus and Rehbein2011) and Sardinia (11%; Scala et al. Reference Scala, Pipia, Dore, Sanna, Tamponi, Marrosu, Bandino, Carmona, Boufana and Varcasia2015), whereas higher prevalence in older dogs were recorded from Algeria (43%; Bentounsi et al. Reference Bentounsi, Meradi, Ayachi and Cabaret2009), north-east (43%; Emamapour et al. Reference Emamapour, Borji and Nagibi2015) and southeastern Iran (53·3%, Geraili et al. Reference Geraili, Maroufi, Dabirzadeh, Noormohammadi and Khoshsima Shahraki2016). The second most frequently found taeniid was T. pisiformis (10·33%) whose prevalence increased with age from 1·53 to 25% (P = 0·0009). This may be related to the greater ability of older dogs to hunt rabbits and hares in which the larval stage, Cysticercus pisiformis, develops. In a previous study from Tunisia, 6·36% of dogs eliminated T. pisiformis worms following arecoline purgation (Lahmar et al. Reference Lahmar, Sarciron, Rouiss and Mensi2008). Infection rates similar to those seen here for this parasite were reported from stray dogs in Spain (Benito et al. Reference Benito, Carmena, Postigo, Estíbalez and Guisantes2003) and Algeria (Bentounsi et al. Reference Bentounsi, Meradi, Ayachi and Cabaret2009). Taenia multiceps was the least frequent taeniid (3·32%) observed in this study, which is consistent with the infection rates of 5·05 and 4·77% for this parasite previously reported from Tunisian dogs (Lahmar et al. Reference Lahmar, Kilani and Torgerson2001, Reference Lahmar, Sarciron, Rouiss and Mensi2008). Taenia multiceps eggs excreted into the environment by the definitive host are ingested by herbivorous, mainly sheep intermediate hosts, causing a coenurosis with neurological symptoms and death in young animals (Scala et al. Reference Scala, Cacedda, Varcasia, Liqios, Garippa and Genchi2007). Coenurosis is a rare zoonosis (Sabbatani et al. Reference Sabbatani, Zucchelli and Calbucci2004; El-On et al. Reference El-On, Shelef, Cagnano and Benifla2008); however, more than 100 human cases have been reported worldwide (Dhaliwal and Juyal, Reference Dhaliwal and Juyal2013). The occurrence of E. granulosus s.s., T. multiceps, and T. hydatigena in rural stray dogs confirms the role they play in the transmission of these taeniid infections. The persistence of these species in Tunisian dogs may be related to the large number of dogs, lack of control measures, extensive livestock husbandry, home slaughtering and the inadequate disposal of infected carcasses and offal.
This epidemiological investigation indicated that stray dogs are a potential source of T. canis infection to humans and other animals and are responsible for environmental contamination of rural areas. A contamination index of 27·08% for T. canis eggs in the environment was recently estimated (Chaâbane-Banaoues, Reference Chaâbane-Banaoues2016). The overall prevalence of T. canis in dogs observed in this study (26·56%) was higher than that previously reported from Tunisian dogs (Lahmar et al. Reference Lahmar, Kilani and Torgerson2001) but is comparable to that recorded from stray dogs in Iran (Emamapour et al. Reference Emamapour, Borji and Nagibi2015). Toxocara canis is the causative agent of visceral and ocular larva migrans in humans (Fillaux and Magnaval, Reference Fillaux and Magnaval2013; Chia-Kwung et al. Reference Chia-Kwung, Celia, Loxton and Barghouth2015) who become infected through the inadvertent ingestion of eggs and/or larvae present in soil and shed in dog feces (Benito et al. Reference Benito, Carmena, Postigo, Estíbalez and Guisantes2003). Importantly, cases due to T. canis larva migrans have recently been diagnosed in Tunisian patients (Hamrouni et al. Reference Hamrouni, Boussetta, Dhahri, Sayhi, Gharsallah, Metoui, Louzir, Ajili and Othmani2015; Lajmi et al. Reference Lajmi, Boussetta, Sayhi, Dhahri, Abid, Batikh, Louzir, Ajili and Othmeni2015).
Although U. stenocephala was identified as the most prevalent intestinal nematode in this study (198; 73·06%), A. caninum infection is highly pathogenic to dogs with evident zoonotic potential, as infective larvae can penetrate human skin and lead to eosinophilic enteritis and a possible sub-acute neuro-retinitis (Bowman et al. Reference Bowman, Montgomery, Zajac, Eberhard and Kasacos2010). From a total of 271 examined dogs, only four (1·47%) were found to be positive for A. caninum, while several studies including those from northwestern Tunisia and other parts of the world, have shown A. caninum to be the most widespread of all hookworms (Lahmar et al. Reference Lahmar, Kilani and Torgerson2001; Bajalan, Reference Bajalan2010; Mateus et al. Reference Mateus, Castro, Ribeiro and Vieira-Pinto2014; Emamapour et al. Reference Emamapour, Borji and Nagibi2015; Ramos et al. Reference Ramos, Zocco, Medeiros Torres and Sinkoc2015; Ilić et al. Reference Ilić, Kulišić, Antić, Radisavljević and Dimitrijević2017; Pumidonming et al. Reference Pumidonming, Salman, Gronsan, Abdelbaset, Sangkaeo, Kawazu and Igarashi2017). Few cases of presumed human infection with T. vulpis causing visceral larva migrans syndrome and/or intestinal infections have been reported (Díaz-Anaya et al. Reference Díaz-Anaya, Pulido-Medellín and Giraldo-Forero2015; Ilić et al. Reference Ilić, Kulišić, Antić, Radisavljević and Dimitrijević2017). However, the zoonotic potential of this parasite remains controversial (Traversa, Reference Traversa2011). Levels of dog infection with T. vulpis are very heterogeneous in several parts of the world and are influenced by host and environmental factors (Traversa, Reference Traversa2011). In the present study, T. vulpis infection was rare (0·36%), while in a previous Tunisian survey, a 10·6% infection rate in necropsied stray dogs was detected (Lahmar et al. Reference Lahmar, Kilani and Torgerson2001) and the environmental contamination index with whipworm eggs from rural dog feces was estimated to be 4·8% (Oudni-M'rad et al. Reference Oudni-M'rad, Chaâbane-Banaoues, M'rad, Trifa, Mezhoud and Babba2017).
Three intestinal trematodes, Brachylaemus sp., P. italica and H. heterophyes were reported here as first records from Tunisian dogs. The three species were found in Raoued, whereas only P. italica was identified in Soukra region. The occurrence of P. italica and H. heterophyes in dogs is probably related to the presence of large bodies of brackish water within the studied regions. These two species are transmitted in marine lagoons and saline inland waters, where first (snails) and second intermediate hosts (fish: Mugilidae) are abundant (Simões et al. Reference Simões, Barbosa and Santos2010). Dogs serve as the major definitive hosts. They become infected by consuming raw fish with muscle-encysted metacercariae. Euryhaline and marine fishes infected with H. heterophyes from Tunisia and Egypt (Chai, Reference Chai and Motarjemi2014; Hegazi et al. Reference Hegazi, Hassan, Al-Nashar, Abo-Elkheir and El-Lessi2014), and with H. heterophyes and P. italica from Sardinia have previously been reported (Masala et al. Reference Masala, Piras, Sanna, Chai, Jung, Sohn, Garippa and Merella2016). Other studies reported the occurrence of P. italica in dogs and foxes from Italy (Nardi, Reference Nardi1959) and in dogs from Turkey (Tinar, Reference Tinar1976), while H. heterophyes was detected in dogs from Greece (Himonas, Reference Himonas1964) and western India (Sen, Reference Sen1965). Heterophyes heterophyes is the causative agent of human heterophyiasis, an emerging fish-borne disease contracted through the consumption of raw mullet (Balozet and Callot, Reference Balozet and Callot1939; Collomb et al. Reference Collomb, Deschiens and Demarchi1960; Rousset and Pasticier, Reference Rousset and Pasticier1972; Taraschewski, Reference Taraschewski1984). The discovery of P. italica and H. heterophyes in Tunisia represents an indication of the potential impact of these helminths on public health. Further investigations are required to determine intermediate hosts and elucidate transmission modalities for these trematodes within the studied areas. Brachylaemus sp. is known to occur in dogs from northern Spain (Guisantes et al. Reference Guisantes, Benito, Estibalez and Mas-Coma1994; Benito et al. Reference Benito, Carmena, Postigo, Estíbalez and Guisantes2003), red foxes from Italy (Fiocchi et al. Reference Fiocchi, Gustinelli, Gelmini, Rugna, Renzi, Fontana and Poglayen2016) and Tunisian wild boars (Lahmar, Reference Lahmar2012). In these few reports, canids serving as definitive hosts of brachylaimid digeneans show consistently low burdens with very few parasites per host, which is similar to the prevalence rate (0·36%) reported in the present study.
The significant prevalence observed for M. lineatus (38·45%) and M. litteratus (15·12%) in the present survey could be due to predation by stray dogs on intermediate or paratenic hosts. These two cestodes are common taeniids of mammals and have previously been reported in dogs by several authors (Benito et al. Reference Benito, Carmena, Postigo, Estíbalez and Guisantes2003; Bentounsi et al. Reference Bentounsi, Meradi, Ayachi and Cabaret2009; Bajalan, Reference Bajalan2010; Nabavi et al. Reference Nabavi, Manouchehri Naeini, Zebardast and Hashemi2014; Emamapour et al. Reference Emamapour, Borji and Nagibi2015; Geraili et al. Reference Geraili, Maroufi, Dabirzadeh, Noormohammadi and Khoshsima Shahraki2016). An acanthocephalan species, M. hirudinaceus, was identified in this investigation (2·21%) as a first record in Tunisian dogs. It is common in red foxes, golden jackals and wild boars in Tunisia and elsewhere (Dalimi et al. Reference Dalimi, Sattari and Motamedi2006; Zare-Bidaki et al. Reference Zare-Bidaki, Mobedi, Ahari, Habibizadeh, Naddaf and Siavashi2010; Lahmar, Reference Lahmar2012, Lahmar et al. Reference Lahmar, Boufana, Ben Boubaker and Landolsi2014) and has also been reported in dogs from Iran (Dalimi et al. Reference Dalimi, Sattari and Motamedi2006; Zare-Bidaki et al. Reference Zare-Bidaki, Mobedi, Ahari, Habibizadeh, Naddaf and Siavashi2010). The presence of beetles and scarabs in the environment with probable encysted larval stages of these parasites could explain the infection seen in dogs in the current study. Diplopylidium noelleri recorded here for the first time in Tunisian dogs may represent an accidental infection as previous reports describe this parasite from stray cats (Schuster et al. Reference Schuster, Thomas, Sivakumar and O'Donovan2009; Waap et al. Reference Waap, Gomes and Nunes2014; El-Azazy et al. Reference El-Azazy, Abdou, Khalil, Al-Batel, Henedi and Tahrani2016) and wild canids (Lahmar et al. Reference Lahmar, Boufana, Ben Boubaker and Landolsi2014). Definitive hosts become infected through the ingestion of the cysticercoid larvae encysting in reptiles, which serve as second intermediate hosts.
In conclusion, this study confirmed that stray dogs from the studied northeastern Tunisian regions may play an important role in contaminating the environment with various parasites some of which may potentially influence human health. To decrease the incidence of parasitic cestodes, intermediate hosts would need to be inspected at slaughter, and organs containing hydatid cysts of Echinococcus, Coenurus cerebralis of T. multiceps and C. tenuicollis of T. hydatigena should be removed and destroyed to prevent consumption by dogs. Canine echinococcosis could be detected by screening E. granulosus in dogs using arecoline purgation and/or molecular approaches (Craig et al. Reference Craig, Hegglin, Lightowlers, Torgerson and Wang2017). In addition, the combination of EG95 lambs’ vaccination associated with regular praziquantel treatments of owned dogs could reduce the infection pressure of E. granulosus in the environment and decrease soil contamination (Torgerson and Heath, Reference Torgerson and Heath2003).
To control helminth contamination in the environment, a close collaboration between sanitary and agriculture authorities is needed for public health education, screening of intestinal parasites in free-roaming owned dogs, regular dog dosing and dog population management. Unfortunately, dog management in many developing countries usually translates into indiscriminate population killing and/or poisoning of roaming dogs. This is ethically unacceptable as well as being ineffective (FAO, 2014). Other procedures such as humane euthanasia, fertility control and use of praziquantel baits could be efficient but require huge funds and are economically and logistically difficult to implement in developing countries (Kachani and Heath, Reference Kachani and Heath2014).
Acknowledgements
We thank Dr Sana Oueslati from Raoued Municipality and the administration of Ariana Governorate for facilitating retrieval of dog carcasses. The European Union Reference Laboratory for Parasites (EURLP) is gratefully acknowledged for support provided.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.





