INTRODUCTION
Non-typhoidal salmonellosis usually causes mild, self-limiting gastroenteritis [Reference Heymann1]. Symptoms usually appear 12–36 h after eating and last up to 7 days. The disease can be severe and life-threatening in vulnerable populations like children, the elderly and people with underlying comorbidities [Reference Hunter and Francois Watkins2]. Salmonellosis ranks second after campylobacteriosis as the most commonly reported food-borne disease across the European Union (EU) [3, 4]. Despite a significant downward trend in Salmonella infections since 2008, a 15·3% increase in the overall EU notification rate was noted in 2014 compared with 2013, followed by a further 1·9% increase in 2015 [3, 4]. Most European cases of non-typhoidal salmonellosis are currently caused by Salmonella enterica serovar Enteritidis [5]. S. enterica ser. Enteritidis is the predominant serovar associated with Salmonella outbreaks [4]. In the EU, in 2015, S. enterica ser. Enteritidis was implicated in 63% of 953 food-borne outbreaks caused by Salmonella. A Europe-wide large outbreak of S. enterica ser. Enteritidis PT8, with multiple locus variable-number tandem repeat analysis (MLVA) profiles 2-9-7-3-2 and 2-9-6-3-2, occurred from May 2016 to February 2017 [5].
In Greece, the mean annual salmonellosis notification rate for the period 2008–2015 was 4·1 cases per 100 000 population, with S. enterica ser. Enteritidis being the most common serotype, dropping yearly from 63% of total isolates in 2008 to 24% in 2015 [6]. The decreasing trend of S. enterica ser. Enteritidis cases was mainly attributed to the Greek National Salmonella Control Programme implemented in poultry in accordance with the EU legislation [7]. At the same period, only small, household, outbreaks of S. enterica ser. Enteritidis were identified in the country; the last documented community outbreak was in 2011 (Hellenic Centre for Disease Control and Prevention, data not published).
On 19 June 2016, hospital authorities in Central Greece informed public health authorities of the occurrence of several cases of gastroenteritis following a christening reception at a restaurant in a popular tourist village. A 57-year-old guest with no underlying condition died after being admitted to the hospital with severe gastroenteritis symptoms. Two otherwise healthy guests, aged 26 and 60, were admitted to the intensive care unit. As a consequence, the outbreak captured wide media and public attention. An outbreak control team (OCT) was dispatched to investigate the outbreak and identify the implicated food item, the causative pathogen and factors possibly contributing to disease severity. The OCT also investigated the possible association of the outbreak with the concurrent 2016/17 European outbreak.
METHODS
Epidemiological investigation
A retrospective cohort study was performed. Restaurant management provided a list of all food items served. Telephone interviews were conducted by the fellow of the European Public Health Microbiology Training Program of ECDC (EUPHEM) after special training, using a structured questionnaire. Data were obtained on demographics, the symptoms, the need for and the length of hospitalisation, and the time taken off from work. The presence of underlying conditions and the possible contact with ill persons unrelated to the christening party were recorded. To identify potential risk factors for illness, all guests were asked whether they had consumed any of the specified food items. Information on food portions and incubation period was also collected as they were considered to be proxy measures of infectious dose. Foods doses were categorised as ‘tasting portion’, ‘one portion’ or ‘more than one portion’. For food served on a common platter for four persons, serving size corresponded to about half of a standard portion size. Incubation periods were taken as the time (in hours) from serving the course until the onset of symptoms. After reviewing descriptive data, case was defined as a christening guest who had diarrhoea (⩾3 loose stools in 24 h) within 72 h of the reception.
Statistical analysis
Categorical variables were compared using the χ 2 test. Continuous variables were expressed as median with the corresponding interquartile range, and were compared using the Mann–Whitney U test. P-values of <0·05 were considered statistically significant. The frequency and intensity of symptoms and association with outcomes were assessed. An epidemic curve was used to depict the length and case distribution of the outbreak. For each consumed food item, attack rate (AR), relative risk (RR) and 95% confidence interval (95% CI) were computed. All analyses were performed using Stata v.12.1 (StataCorp., College Station, Texas, USA).
Laboratory investigation
Stool samples were screened only for three bacterial pathogens, Salmonella spp., Campylobacter spp. and Shigella spp. The available identified Salmonella isolates were sent to the National Reference Centre for Salmonella. Serotyping was performed according to the White–Kaufmann–Le Minor Scheme [8, Reference Grimont and Weill9]. Susceptibility testing was carried out using the disk diffusion method. Antimicrobial agents appropriate for monitoring of antibiotic resistance in human Salmonella isolates, according to the relevant EU protocol [10], were tested. The European Committee on Antimicrobial Susceptibility Testing breakpoints were applied [10]. Two outbreak isolates from patients who belonged to different families and were hospitalised at different hospitals, were phage-typed by the Ward–Colindale system [Reference Ward, de Sa and Rowe11]. Pulsed-field gel electrophoresis (PFGE) was performed after the digestion of genomic DNA with XbaI macrorestriction endonuclease, according to standard operating procedure [12]. Similarity and cluster analyses were performed using the Dice coefficient and the unweighted-pair group method with the use of average linkage, using BioNumerics software v.6.0 (Applied Maths, Belgium). PFGE profiles were uploaded to the European Molecular Surveillance System (TESSy-MSS), operated by the European Centre for Disease Prevention and Control, for matching with other characterised PFGE profiles at European level [13]. The isolates were submitted for MLVA analysis, following a laboratory standard operating procedure [14], based on the five-locus MLVA method proposed by Hopkins et al. [Reference Hopkins15]. Single-nucleotide polymorphism (SNP)-based whole genome sequencing (WGS) analysis was carried out by the PHE Genome Sequencing and Development Unit [Reference Inns16]. A hierarchical, ‘SNP address’, approach was used to assess the genetic distance between our isolates and those of the concurrent European outbreak [5, Reference Inns16].
Environmental investigation
The OCT visited the restaurant where the christening event took place in order to collect information on preparation processes for the foods served. The order and delivery books of the restaurant were reviewed. Environmental swabs were taken from working benches. Food leftovers were no longer available; raw materials were collected for laboratory testing [17]. The OCT also attempted to trace raw materials back to the original suppliers. All the food handlers were interviewed regarding food handling practices and illness 1 week before and after the christening reception. They were also asked whether they had consumed foods served at the reception. Stool samples were obtained after verbal consent from all food handlers.
RESULTS
Epidemiological investigation
Of the 133 attendees, 122 (92%) individuals completed the questionnaire. Participants’ median age was 40·5 years (range 3–86); 70 (57%) participants were females. A total of 56 (46%) respondents met the case definition (median age, 43·5 years (range 3–86); 31 (55·4%) females). Of the 56 cases, 49 (87·5%) reported high body temperature (median, 39·2 °C; range 38·4–41), 37 (66%) abdominal pain and 22 (39·3%) vomiting, while 30 (54%) were hospitalised. Twenty-eight (50%) complained of intense abdominal pain. Eleven (50%) of those vomiting had at least four vomiting episodes per day (range 1–15). Half of those requiring hospitalisation stayed at the hospital for 5 days or more (range 1–10 days). Of the 42/56 (75%) working patients, 21 (50%) reported absenteeism from work, with one out of two being off work for ⩾7 days (range 1–15 days). Age and sex did not significantly differ between cases and non-cases and were not associated with any of the symptoms and outcomes. Thirty-six cases were previously healthy adults. Eight cases had an underlying condition; cardiovascular disease (n = 4); diabetes (n = 3); hypothyroidism (n = 1). One patient reported a previous upper gastrointestinal tract surgery (n = 1). Their clinical presentation and outcome did not differ from those of other cases. None reported achlorhydria or prior use of antacids or acid reducers. Affected individuals had no contact with sick people unrelated to the reception. The shape of the epidemic curve is suggestive of a common point-source outbreak (Graph 1). The median incubation period was 15 h (range 6–71). Twenty of the 56 (35·7%) patients developed diarrhoeal illness in <12 h from serving the course (Table 1). This group of affected guests passed ⩾7 loose stools in a day, had the highest proportion of fever and vomiting with more than four vomit episodes a day; and had the highest hospitalisation rate lasting >6 days. They also had symptoms of long duration (⩾8 days; n = 11, 55%). Results of the univariate analysis are displayed in Table 2. A RR of 7·8; 95% CI 3·6–16·8 was associated with having eaten cheesy penne pasta at the christening reception. Of the 63 people who ate cheesy penne pasta, 50 fell ill (AR = 79·4%). As cheesy penne pasta was served on a common platter for four persons, half of a standard portion was allocated for each guest. Most (32/50; 64%) affected guests, who had eaten cheesy penne pasta, reported that they had eaten less than half-portion. No relationship between symptoms and outcomes with consumed portion of implicated food was shown. One case was reported to have exclusively consumed cheesy penne pasta served at the christening reception.
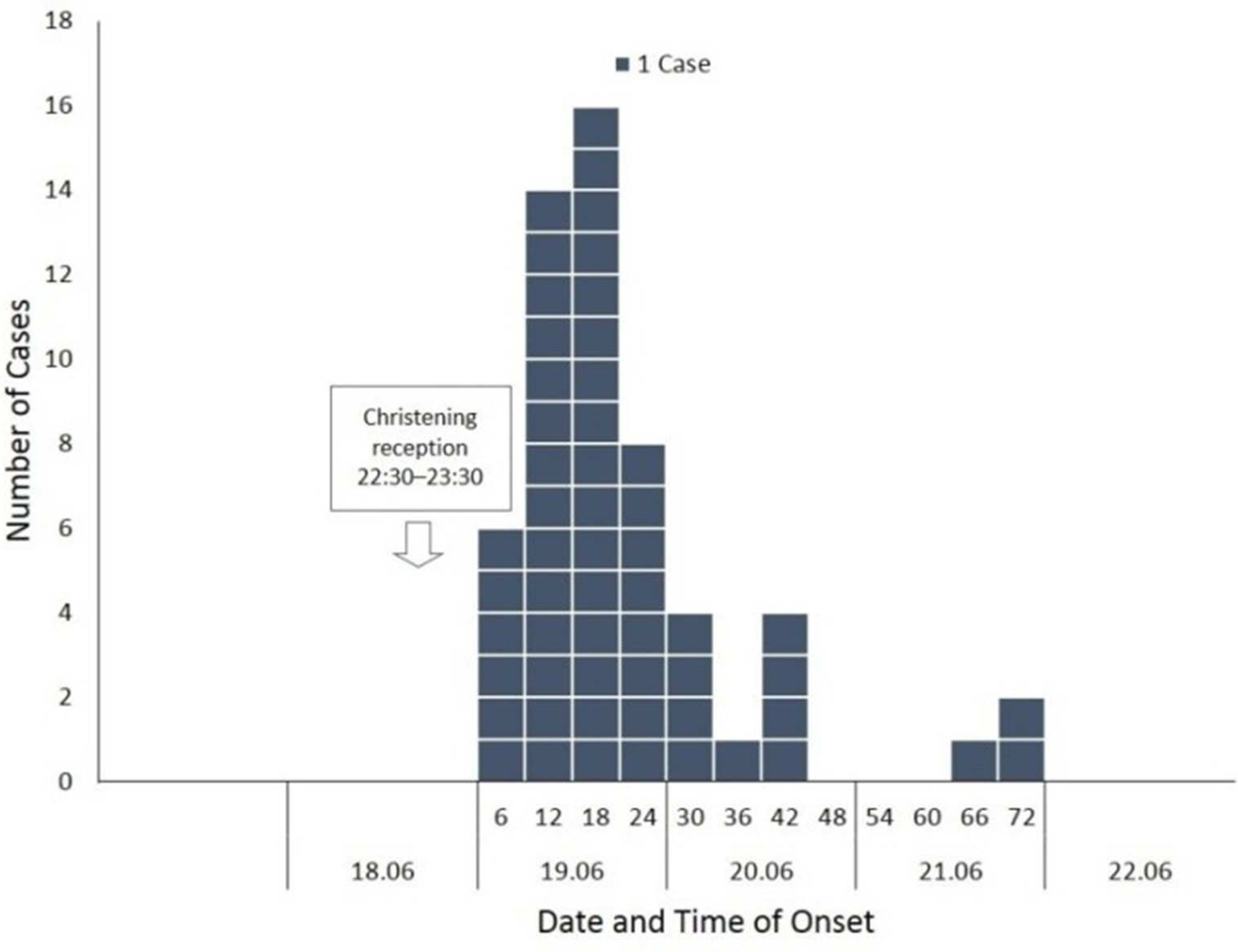
Graph 1. Interviewed guests (n = 56) who developed diarrhoea (⩾3 loose stools in 24 hours) within 72 hours following a christening reception in Central Greece, June 2016, by date and six-hour time intervals of onset of illness.
Table 1. Range of symptoms and outcomes sorted by the length of incubation period among 56 guests falling ill within 72 h after attending a christening reception in Central Greece, June 2016
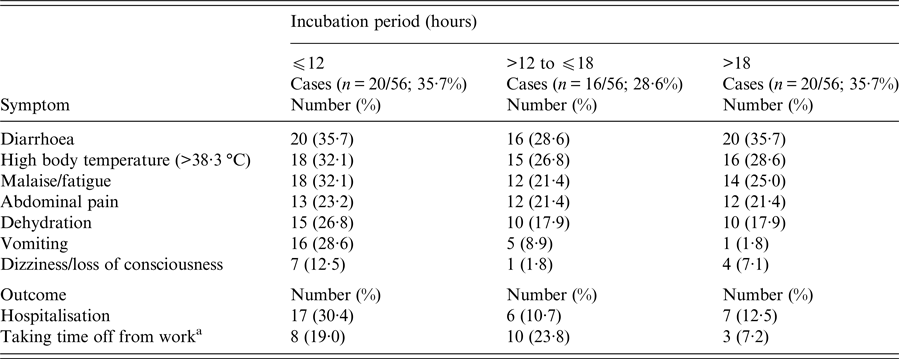
a The results refer to 42 cases of working age.
Table 2. Food-specific attack rate and relative risk of developing diarrhoea by food item among 122 persons who attended a christening reception in Central Greece, June 2016
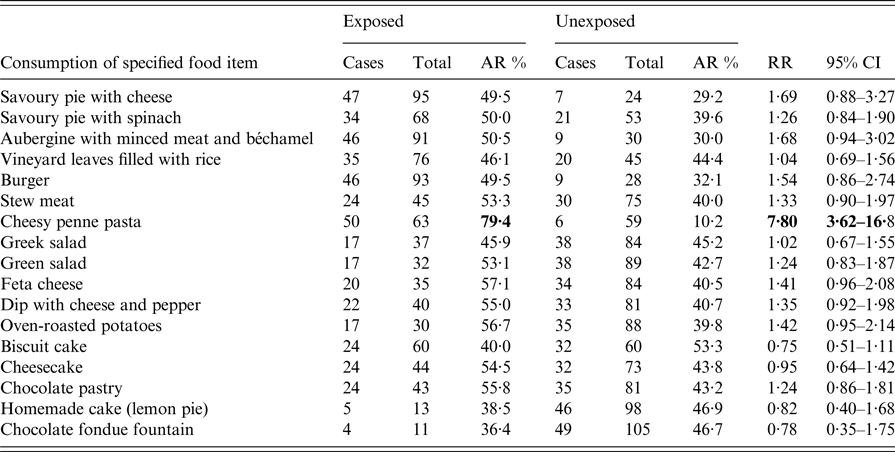
Food specific attack rates (AR), relative risks (RR) and 95% confidence intervals (95% CI) were calculated.
Laboratory investigation
Stool cultures from 17/56 (30·4%) cases were positive for Salmonella spp. Seven isolates were sent for further typing, and were identified as S. enterica ser. Enteritidis. The isolates were found to be fully susceptible to the tested antibiotics. Two isolates were typed as phage-type PT8, with PFGE profile XbaI.0024 and MLVA profile 2-9-7-3-2. The isolates shared the t5-level SNP address 1.2.3.323.323.24095628.% (with %-sign substituting any number standing for 0-SNP level) differing from each of the two European outbreak's WGS clusters by 175 (SNP address 1.2.3.175.175.175.%) and 360 (SNP address 1.2.3.18.359.360.%).
Other restaurant consumers with S. enterica ser. Enteritidis infections
The food handler that was tested positive for S. enterica ser. Enteritidis reported tasting food in the kitchen during the reception (penne pasta) and that he developed symptoms in the following days. It was also found that two persons with laboratory-confirmed S. enterica ser. Enteritidis infection had consumed cheesy penne pasta served at the reception despite not attending the event themselves (family members of one of the food handlers). Finally, stool cultures from four clients who fell ill after they had eaten at the restaurant after the reception were positive for S. enterica ser. Enteritidis. Data regarding cases other than those among the reception guests are summarised in Table 3.
Table 3. Other restaurant consumers with Salmonella enterica serovar Enteritidisin Central Greece, June 2016
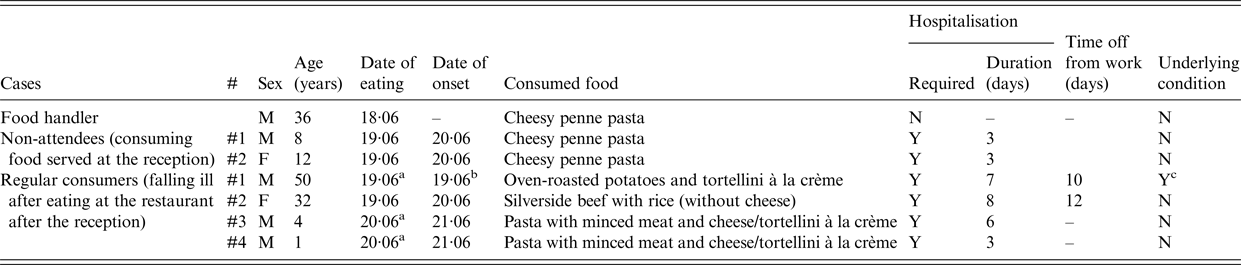
a At 12 : 00 h.
b At 23 : 00 h.
c Previous lower gastrointestinal tract surgery.
M, male; F, female; Y, yes; N, no.
Environmental investigation
The restaurant owners had voluntarily closed down their restaurant before the inspection took place. Prior to the arrival of investigators the café kitchen had been cleaned and most perishable goods had been discarded. All environmental and raw materials tested negative for Salmonella. Stool cultures from six out of seven food handlers were also negative for Salmonella. The remaining food handler, who reported diarrhoea after the event, was diagnosed with Salmonella infection. The food handler reported tasting food in the kitchen during the reception. The ingredients of the incriminated dish were penne pasta, yellow hard cheese, semi-hard cheese, cured meat, three packets of crème fraîche, six egg yolks, pepper and salt. The pasta was boiled and cheese was cut at noon. Afterwards, the ingredients were put into the refrigerator. Food was baked at some point in the afternoon. The dish was kept at room temperature for more than 2 h before being served after the completion of the first course. It has to be mentioned that the outbreak occurred during a heat wave in Greece. The temperature at the day of the reception ranged between 25·7 and 39·5 °C. Inspectors recorded the lack of a Hazard Analysis and Critical Control Point system and of standard procedures inside the kitchen for avoiding cross-contamination. Raw and cooked foods were not adequately separated. The oven's heating performance was not tested; no registry data were kept on refrigerator temperature. The origin of the eggs used to prepare the cheesy penne pasta was also investigated. An inspection of the implicated hatchery and packing station was initiated. Samples taken were negative for S. enterica spp.
Measures taken
The local public health authorities suspended the license of the restaurant for 4 months. The suspension was lifted after a thorough review of the restaurant's food safety management system and the implementation of improvements to enhance processes associated with reduction in the potential for food contamination (focus at monitoring and recording of processes and temperatures, heat treatment and preservation of food). Furthermore, all employees were re-trained on the basic concepts of Hazard Analysis and Critical Control Point (HACCP) and their compliance was further assessed by the local public health authority. The enhancement of the national Salmonella molecular surveillance was also decided; MLVA typing will be introduced in the routine work of the National Reference for salmonellosis.
DISCUSSION
A serious S. enterica ser. Enteritidis PT8, MLVA profile 2-9-7-3-2 outbreak occurred in Greece in 2016, despite a recorded decreasing trend of cases in the previous years and the absence of recorded large outbreaks due to this serotype in the country since 2011. Remarkable morbidity was associated with this outbreak, even though non-typhoidal salmonellosis is generally mild. High rates of severe symptoms and hospital admission were reported. Patients suffered from severe gastrointestinal symptoms such as profuse diarrhoea, fever and frequent vomiting episodes demanding prolonged hospitalisation and sick leave from work. One death was associated with this outbreak. Another interesting feature was that the majority of affected guests experienced serious gastrointestinal illness within <12 h of attending the reception. There has been an inconclusive debate about the infectious dose and illness severity of non-typhoidal Salmonella [Reference Glynn and Brandley18–Reference Mintz20]. In this outbreak, the small amount of consumed cheesy penne pasta did not allow us to assess the dose–response relationship. It is possible that bacteria may have survived inadequate heat processing and high-fat cheesy penne pasta may have protected Salmonella cells – encapsulated in fat – against stomach acidity [Reference Álvarez-Ordóñez21]. Another possible explanation is that leaving this perishable food out of the refrigerator on a warm summer evening allowed bacterial multiplication in the likely vehicle of infection [Reference D'Argenio, Romano and Autorino22]. The short incubation period may be attributable to very high infectious dose and/or co-infection with toxin-producing bacteria (e.g. Staphylococcus aureus), as described before [Reference Huang23]; their isolation though was not feasible in Greek local hospitals at that point of time. The severity of Salmonella-associated disease may additionally depend on host factors as well as the specific strain of Salmonella [24]. Regarding host status in the current outbreak, more than 60% of the cases were otherwise healthy adults. Over the last two decades, only a limited number of serious S. enterica ser. Enteritidis outbreaks affecting mainly non-high-risk adults have been published [Reference Rejnmark19, Reference Mintz20, Reference Cerný and Bazoutová25–Reference Mertens28]. The fact that severe S. enterica ser. Enteritidis outbreaks are infrequent is perhaps in line with the serovar's weak human invasiveness in high-income countries [Reference Feasay29]. Finally, the outbreak-associated isolates were found to belong to phage-type PT8.
Interestingly, despite lacking correlation between clonal lineage and virulence within the serovar, PT8 has been suggested to be one of the high virulent S. enterica ser. Enteritidis phage types [Reference Pang30, Reference Olsen, Tiainen and Brown31]. Our finding that the isolates of the present outbreak were identified as PT8 and produced the PFGE profile XbaI.0024 according to TESSy-MSS (corresponding to PFGE profile SENTXB.0002 according to the former PulseNet Europe network for food-borne infections in Europe) is consistent with studies showing a strong association between PT8 and SENTXB.0002 [Reference Peters32]. Phage-type PT8 has been one of the most frequently observed phage types in Eastern Europe since the 1990s [Reference Garcia-Huidobro26, Reference Cieslik33–Reference Karpiskova and Mikulaskova37].
In 2011–2015, PT8 was the predominant phage type in human cases in 13 European countries [5]; no such data were available for Greek cases. In 2016, a gastroenteritis outbreak occurred amid a S. enterica ser. Enteritidis PT8 outbreak affecting different countries in Europe [5]. The MLVA profile of our PT8 isolates was indistinguishable from the MLVA profile 2-9-7-3-2 of the multi-country outbreak [5]. SNP-based WGS analysis, however, revealed that our outbreak did not belong to either of the two WGS clusters associated with the 2016/17 European outbreak despite their temporal relatedness.
Information on the factors that contributed to the occurrence of the outbreak involved the high ambient temperature at the time of the outbreak and the lack of standard procedures inside the kitchen for avoiding cross-contamination. The fact that four people fell ill after they had eaten at the restaurant the days following the reception is supportive of the hypothesis that probably cross-contamination occurred inside the kitchen during the preparation for the reception. Even though cross-contamination after cooking is the most probable cause of the outbreak, the exact point that cross-contamination occurred remained unclear. The literature shows that this is a common limitation of the investigation of outbreaks at restaurant premises [Reference Brown38]. For this reason, environmental investigation of similar outbreaks should be more detailed, and inspection should be scheduled as soon as possible after the establishment being linked with an outbreak, ideally within a day. It is also recommended that the authorities conduct multiple establishment visits if needed to complete the environmental assessment and monitor poor handling practices. In this case due to the media attention, the restaurant owners had voluntarily closed down their restaurant before the inspection took place and investigators were restricted in their ability to hypothesise and test potential means of cross-contamination during the preparation of the incriminated meal. That most Greek local hospitals lacked the capacity to screen stools for other food-borne pathogens with very short incubation periods (at least shorter than that of Salmonella), such as S. aureus, constitutes one more limitation, since co-infection cannot be excluded. Finally, the current study is limited by the fact that no leftovers were available for microbiological testing and identification of the food vehicle.
CONCLUSION
In the aftermath of the herein described recent S. enterica ser. Enteritidis outbreak associated with high morbidity, after five consecutive years of no outbreak documentation, the occurrence of S. enterica ser. Enteritidis outbreaks remains a public health threat in Greece. Thus, despite the progress in Salmonella control, there can be no complacency; it is vitally critical to maintain and strengthen the continued implementation of the National Salmonella Control Programmes and to intensify efforts to (re)educate food handlers in the basic principles of food management and hygiene to prevent such outbreaks from occurring. A more detailed environmental investigation is needed in case of similar outbreaks so that the factors led to cross-contamination inside the kitchen be identified and the appropriate correction measures be taken. Advanced molecular data need to be incorporated into the current public health surveillance systems in Greece to help us distinguish unrelated outbreak strains and identify emerging clones.
ACKNOWLEDGEMENTS
The authors are thankful to the christening guests and the restaurant staff for their kindness in answering their questions. The authors would like to acknowledge Silvia Herrera-León, in Sección de Enterobacterias, Servicio de Bacteriología, Centro Nacional de Microbiología, Instituto de Salud Carlos III, Madrid, Spain, and Aftab Jasir, European Centre for Disease Prevention and Control, Stockholm, Sweden, for their reviewing the manuscript. The authors also thank Gitte Sørensen, Research Group for Genomic Epidemiology, National Food Institute, Technical University of Denmark, Lyngby, Denmark and Tim Dallman at Public Health England, UK for phage typing and whole genome sequencing the outbreak isolates, respectively; Saara Kotila, Scientific Officer Molecular Surveillance Epidemiological Methods, SRS, European Centre for Disease Prevention and Control, Stockholm, Sweden for all her support. The authors are also thankful to Ioannis G. Koutelekos, Faculty of Nursing, Technological Institute of Education, Athens, Greece for his entering data in worksheet cells and preparing tables in the manuscript. The authors acknowledge Alkiviadis Vatopoulos, National School of Public Health, Athens, and Kyriaki Tryfinopoulou, Central Public Health Laboratory, Vari, Attica, Greece, for their remarks. The authors also thank the Directorate of Health and Social Care, and the Hospital Authorities, Boeotia Regional Unit, Central Greece Region; and the Hellenic Food Authority for supporting OCT investigation.
DECLARATION OF INTEREST
None.






