Introduction
Avian conservation efforts increasingly involve reintroduction programmes where birds are most often sourced from the wild or captive bred (Snyder et al. Reference Snyder, Koenig, Koschmann, Snyder and Johnson1994, Oelher et al. Reference Oelher, Boodoo, Plair, Kuchinski, Campbell, Lutchmedial, Ramsubage, Maruska and Malowski2001, Collazo et al. Reference Collazo, White, Vilella and Guerrero2003, Brightsmith et al. Reference Brightsmith, Hilburn, del Campo, Boyd, Frisius, Frisius, Janik and Guillen2005, Oritz-Catedral et al. Reference Ortiz-Catedral, Kearvell, Hauber and Brunton2009), but also from facilities that care for confiscated or rehabilitated birds (Sanz and Grajal Reference Sanz and Grajal1998, Metz and Zimmerman Reference Metz and Zimmerman2010). Understanding factors contributing to the success or failure of such releases will improve their effectiveness as conservation measures.
With regard to previous studies involving psittacids, predation (Snyder et al. Reference Snyder, Koenig, Koschmann, Snyder and Johnson1994, White et al. Reference White, Collar, Moorhouse, Sanz, Stolen and Brightsmith2012) and deficits in foraging and socialisation skills (Snyder et al. Reference Snyder, Koenig, Koschmann, Snyder and Johnson1994) have contributed to poor survival of released birds. Post-release provisioning, particularly for captive-reared psittacids, and pre-release training to facilitate recognition of appropriate food items have been associated with increased success (Brightsmith et al. Reference Brightsmith, Hilburn, del Campo, Boyd, Frisius, Frisius, Janik and Guillen2005, White et al. Reference White, Collar, Moorhouse, Sanz, Stolen and Brightsmith2012), as has selecting sites with an existing resident or establishing population (Sanz and Grajal Reference Sanz and Grajal1998, Collazo et al. Reference Collazo, White, Vilella and Guerrero2003, Brightsmith et al. Reference Brightsmith, Hilburn, del Campo, Boyd, Frisius, Frisius, Janik and Guillen2005). Larger releases have been more successful than smaller ones (Snyder et al. Reference Snyder, Koenig, Koschmann, Snyder and Johnson1994, Brightsmith et al. Reference Brightsmith, Hilburn, del Campo, Boyd, Frisius, Frisius, Janik and Guillen2005). The key factors vary between species, source of birds and habitats.
Carnaby’s Cockatoo Zanda latirostris is an ‘Endangered’ species (BirdLife International 2016) that is declining in both population size and range (Saunders Reference Saunders1990). Birds in the western and eastern portions of its range are genetically distinct, likely as a result of the extensive clearing of land for agriculture between the two portions (White et al. Reference White, Bunce, Mawson, Dawson, Saunders and Allendorf2014). Knowledge of how to release and reintegrate birds successfully into wild flocks may become an important conservation action in these areas.
Increasing numbers of Carnaby’s Cockatoos are entering care through anthropogenically related injury and illness (such as that caused by vehicle strike or illegal shooting), particularly in the suburbs and rural areas surrounding the city of Perth (Le Souëf Reference Le Souëf2012, Groom et al. Reference Groom, Mawson, Roberts and Mitchell2014a, Le Souëf et al. 2015). There are no conservation benefits in directing rehabilitated birds into aviculture, as captive breeding of Carnaby’s Cockatoos has proven difficult (Saunders et al. Reference Saunders, Rowley, Smith, Keast, Recher, Ford and Saunders1985), and it is also not sustainable to retain in perpetuity the number of birds entering care. Release of rehabilitated birds is therefore the preferred outcome. These rehabilitated birds provide the opportunity to optimise release protocols with birds that would otherwise be lost from the population without intervention.
Carnaby’s Cockatoos are social birds that forage during the day in large flocks and roost communally at night. They breed in late winter and spring and by late December have migrated to their non-breeding range (Saunders Reference Saunders1977, Reference Saunders1980) which includes the suburbs of Perth (31°57’S, 115° 51’E), the capital city of Western Australia, where this study was undertaken. Carnaby’s Cockatoos are long lived with females breeding until at least 30 years old (Saunders et al. Reference Saunders, Dawson and Mawson2011, Reference Saunders, Mawson and Dawson2014) and young of those Zanda spp. and closely related Calyptorhynchus spp. studied so far accompany their parents for at least a year (Saunders Reference Saunders1974a, McInnes and Carne Reference McInnes and Carne1978, Saunders Reference Saunders1982). During this first year young cockatoos learn foraging behaviour from their parents and flock mates (McInnes and Carne Reference McInnes and Carne1978, Saunders Reference Saunders1982). Their long life-span and low reproductive output mean long term population viability may be improved by any measures to increase adult survival, such as the release of rehabilitated individuals. To assess the survival and reintegration of rehabilitated Carnaby’s Cockatoos into wild flocks, we studied the movements and behaviour of 23 rehabilitated birds, fitted with satellite tracking devices and released in 2012 and 2013. To assess longer-term survival of rehabilitated birds we collated records of leg-banded birds re-admitted to care or found dead after release, between 2005 and 2013.
Methods
Study birds and release strategy
All study birds were rescued from the wild following debilitation, most commonly due to traumatic injury. The majority of rescued cockatoos were hospitalised at Perth Zoo Veterinary Department for assessment and treatment, and then transferred to rehabilitation centres until considered fit for release. Prior to release, birds were observed for natural behaviours and were specifically tested for manoeuvrability, landing and walking. All assessments were made by Rick Dawson (Senior Investigator, Nature Protection Branch, Department of Parks and Wildlife) who has extensive field experience with the species.
In preparation for release, cockatoos were housed in flight aviaries (e.g. 64 x 6m at Kaarakin Black Cockatoo Conservation Centre, 30 x 10m at Native Animal Rescue) and fed mixed seed and native browse (e.g. Corymbia, Banksia) ad libitum. Rehabilitated birds were housed together to form an ‘aviary flock’ (Le Souëf Reference Le Souëf2012). ‘Aviary flocks’ consisted of individuals that were rescued from broadly the same area, and were usually released within 40 km of their original rescue locations. Release locations were places where flocks of more than 100 cockatoos were known to congregate regularly to forage or roost, and releases were timed to occur when wild birds were present (seasonally and time of day).
To enable individual identification, all birds in rehabilitation were implanted with a Trovan® microchip. Since 2005 all released birds were fitted with stainless steel size 21 or 32 Australian Bird and Bat Banding Scheme leg bands and in 2012 and 2013 they also had an individual colour and letter combination marked on the tail feathers (Groom et al. Reference Groom, Mawson, Warren, Roberts and Page2013).
Data on the health history and length of time in captivity were collated for each study bird. Age was estimated from plumage characteristics, physical changes (e.g. beak turning black and a pink eye ring developing in adult males around four years of age) and behaviour (e.g. juvenile begging behaviour). Pre-release haematology and serum biochemistry values of all 23 birds fitted with tracking devices and released in this study were interpreted using species-specific reference values established by Le Souëf et al. (Reference Le Souëf, Holyoake, Vitali and Warren2013a). Results were within normal ranges indicating that the birds were healthy. Faecal samples were collected from the aviaries to screen for endoparasite infections. From June 2013, on veterinary advice following the detection of gastrointestinal parasites, all birds were wormed prior to release with Worm Out Gel® (Vetafarm, Wagga Wagga, NSW, Australia).
Satellite tracking and flock follows
Satellite tracking devices (Telonics TAV 2617), weighing 17g (less than 4% of body weight; adult cockatoos weigh between 520 and 790 g) were attached to the two central tail feathers of study birds in 2012 and 2013 (Groom et al. Reference Groom, Warren, Le Souëf and Dawson2014b). Argos satellite tracking devices were chosen as they ensured that location fixes could be obtained irrespective of dispersal distance and without the need to recapture or gain close proximity to birds to download data.
Tracking devices were programmed to maximise battery life. Releases in 2012 determined short-term survival and dispersal during the first two weeks post-release with tracking devices programmed to switch on between 06h00 and 18h00 (with a 2-hour break around midday when no satellite passes occurred). After two weeks, devices were switched on for five hours each morning every fifth or tenth day to obtain longer-term survival and dispersal data. Releases in 2013 enabled night roost locations to be determined by switching on for up to four hours each night, and allowed close observation of social interactions and behaviour of study birds through flock follows by switching on between 03h00 and 11h00 on two mornings and 13h00 and 20h00 for two afternoons each week. Due to limitations of battery life, this study focused on the non-breeding season, and due to the requirement to follow flocks closely and observe study birds, this study focused on the urban landscape of Perth which provided suitable vehicle access.
In 2013 an Argos Locator AL-1 (Communications Specialists) was used to locate and follow flocks containing study birds. Flock follows were undertaken by vehicle using the road network of the urban landscape. Location fixes obtained via satellite provided a vicinity to start tracking, which was particularly useful for tracking study birds from night roosts. Flocks were usually followed as they left a night roost in the morning or else were located whilst foraging and followed to the roosting site in the evening.
Following Drake and Dingle (Reference Drake and Dingle2007), the movements of cockatoos were assigned to one of three categories: 1) foraging and commuting, 2) exploratory ranging movements and 3) migratory movements. Study birds often travelled regularly between a subset of spatially proximate roosts and these sets of roosts, and the foraging movements around them, were used to define 10 transitory foraging ranges that were named according to the broad geographic characteristics of the study area. Birds shifted between sets of roosts and these moves were scored as a shift in transitory foraging range. The transitory foraging ranges enabled the movements of study birds to be summarized in both space and time. In order to define loose boundaries for each foraging range minimum convex polygons were constructed around the location fixes for birds originating from roosts allocated to a particular transitory foraging range. Distances between roosts within the foraging ranges were typically no greater than twice the average maximum distance birds foraged from a roost. Sometimes arbitrary decisions were needed to allocate particular study birds to specific transitory foraging range for periods of time when there was overlap in sets of roosts used. If study birds travelled between roosts that were normally not used interchangeably and the distance between night roosts was greater than average, then this was considered a ranging movement. If the ranging movements continued in a consistent direction for more than two consecutive nights, then this was considered a migratory movement.
Assessment of rehabilitation success
Survival and reintegration of birds rehabilitated into the wild was assessed by collating band returns, opportunistic field observations, targeted observations through flock follows and data on location and movement from tracking devices. Band returns are defined as when a bird was found dead, debilitated or returned to care, and does not include sightings of banded birds in the field. We determined whether rehabilitated birds were joining flocks, interacting with flock mates and attempting to form pair bonds (allo-preening, courtship displays), and assessed their ability to find and manipulate appropriate foods and any evidence of imprinting or habituation to humans. Releases were considered successful if all of the following were met: a) birds survived the first month; b) did not return to care due to starvation; c) demonstrated a capacity to interact with wild flocks; and d) if mature, demonstrated basic pair bonding or courtship behaviours.
Annual survival rate was estimated using the Kaplan-Meier staggered entry model (Pollock et al. Reference Pollock, Winterstein, Bunck and Curtis1989). This method enables an estimate of survival to be calculated from tracking data and allows for animals to be lost from the study (e.g. through failure of tracking devices) and, for new animals to be added. Longer-term survival was assessed by collating records of leg-banded birds re-admitted to care or found dead after release, between 2005 and 2013. To determine if tracking devices were affecting survival we used a two-tailed Fisher’s exact test to compare the band returns of birds released with and without tracking devices.
Results
Study birds and release strategy
Seven hundred and sixty Carnaby’s Cockatoos were admitted to Perth Zoo Veterinary Department between 2005 and 2013. Of these, 310 (40.8%) were euthanized, 126 (16.6%) were dead on arrival or died whilst in care, while 324 (42.6%) survived and were passed on to long-term rehabilitation facilities. Of the rehabilitated birds, 145 (19.1%) have been released back into the wild. Most birds admitted for care had injuries consistent with vehicle collisions (fractures, bruises, wounds and/or feather loss), but often incidents were not directly observed, so the cause of many injuries could not be definitively attributed.
Of 36 (17 males and 19 females) Carnaby’s Cockatoos released in the greater Perth region (the central portion of the Swan coastal plain between Lancelin and Waroona) during 2012–2013, time in rehabilitation varied from 103 days to almost 3.5 years (mean 337 days, median 268 days). Birds with wing fractures spent the longest time in captivity (800 days ± 475 n = 4). Approximately equal numbers of males and females were released from rehabilitation between 2005 and 2013 (63 males, 68 females with 14 birds unsexed). A total of 23 birds were fitted with satellite tracking devices and released (11 in 2012 and 12 in 2013). At least 14 of 23 satellite-tracked study birds were less than four years old, including four birds likely to have been less than one year old when they entered rehabilitation.
Group size of all released birds (leg banded and those carrying tracking devices) varied from one to 28 individuals. Releases at Perry Lakes and Collier Park were timed to occur when wild birds were congregating at night roosts. They occurred between 0 and 77 minutes before sunset. On release a group would typically land in nearby trees for a period until wild Carnaby’s Cockatoos were heard. The released birds would then call to the wild birds and fly in their direction. On their first night, seven out of 10 study birds, where the roost could be determined, roosted at their release roost site. The birds that did not roost at their release roost were in the group released 77 minutes before sunset. This group joined a passing flock on their way to another, nearby roost.
Released birds did not remain together after release although some individuals were subsequently found in the same foraging flock or communal night roost. Of 857 occasions where it was known where a study bird roosted for the night, there were 13 (4.5%) occasions where at least three study birds roosted together, 81 (18.9%) where two study birds roosted together and 656 (76.5%) occasions only one study bird was recorded at the roost.
Flock follows and field observations
Tracking devices monitored movements of the 23 study birds for between one and 289 days during two consecutive non-breeding seasons. A total of 173 flock follows were undertaken ranging from seven minutes to 10 hours and 28 minutes (average 3 hours 30 minutes) duration, and resulting in over 540 hours following flocks and sighting study birds. Most flocks followed (160 of 173) contained one or more study birds.
Physical fitness
Study birds in 2013 were located at least once a week whilst their tracking devices were active and half (six birds) were found alone on one or more occasion suggesting they may not have been able to keep up with the flock or were having difficulty reintegrating socially (Table 1). However, there was no indication of deterioration of health in those birds, and all were subsequently observed with a flock or at a communal roost.
Table 1. Social and behavioural indicators of successful reintegration of rehabilitated Carnaby’s Cockatoos released into the wild in 2013. Colours and letters refer to markings applied to the white panels of tail feathers to identify individual study birds in the field.
Study birds varied greatly in their movement patterns after release (Figure 1). One bird was sedentary and used a single roosting area the entire 60 days it was monitored. In contrast, another study bird moved 224 km from its release site in less than two months. This bird spent the first three days close to the release site at Yanchep National Park or in the nearby pine plantation, then spent a week 40 km to the north before travelling approximately 180 km further north, where it spent two months before returning approximately 75 km south for one to two weeks, then moving 140 km south-east where it remained for at least three weeks until the battery failed in its tracking device (Figure 1 and Figure 5 of Groom et al. Reference Groom, Warren, Le Souëf and Dawson2014b).
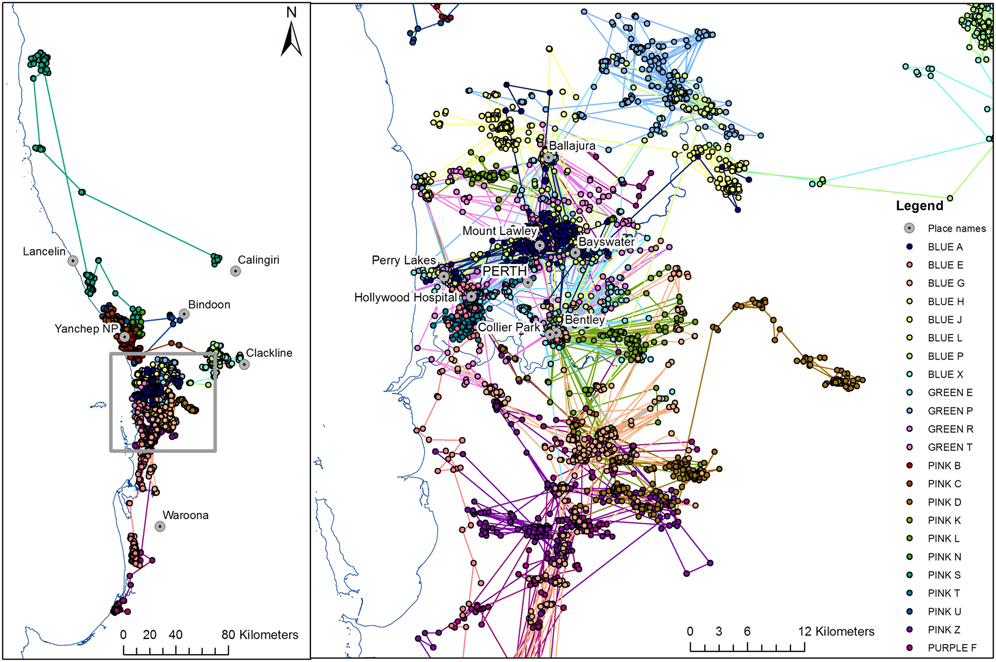
Figure 1. Movements of 23 rehabilitated Carnaby’s Cockatoos released with satellite tracking devices in 2012 and 2013.
Three other study birds made long-distance movements immediately or soon after release, indicating physical fitness and possible prior spatial knowledge of areas they once frequented (Figure 1). One travelled south for 25 days reaching an area 110–125 km from the release site where it remained for at least 135 days. This bird had a lengthy rehabilitation (963 days) recovering from a wing fracture. The other two travelled east together for two days following release, then stayed about 55 km east-north-east from their release site for at least 107 days. Five birds made distinct changes in their movement pattern and moved inland at the usual time migration to breeding areas occurs in this species. Most study birds (16 of 23) moved between roosts within the greater Perth region and did not disperse further than 50 km from their release sites whilst being monitored (Figure 1 and 2).
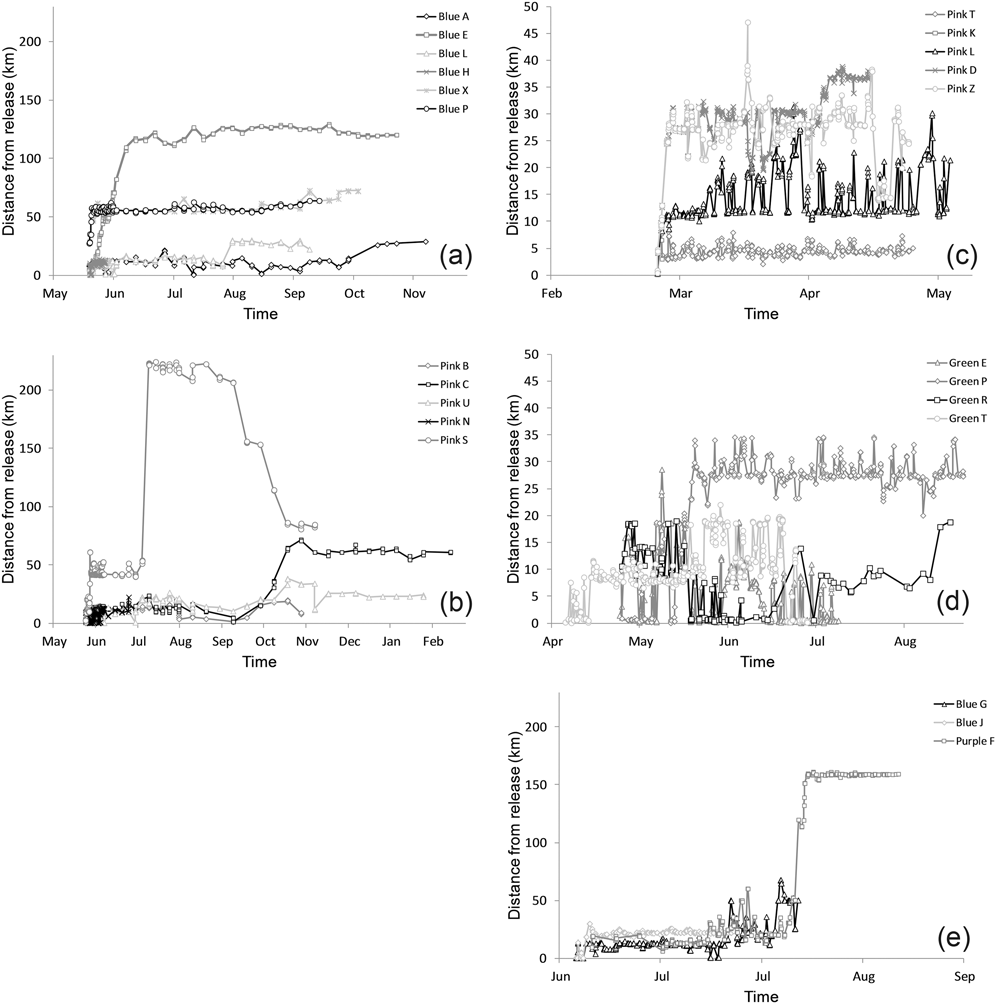
Figure 2. Distance from release site over time for rehabilitated Carnaby’s Cockatoos released with satellite tracking devices on (a) 18 May 2012 at Perry Lakes, (b) 24 May 2012 at Yanchep National Park, (c) 25 February 2013 at Perry Lakes, (d) 5 and 24 April 2013 at Collier Park and (e) 5 June 2013 at Collier Park.
Three study birds fitted with tracking devices died. One was illegally shot, one died following a suspected collision with a vehicle, and the cause of death of the other is unknown as the remains were not recovered until many months after death. Time of death was assumed on the basis of fixes being clustered. Necropsy within hours of the death of the bird that was shot showed that its body condition was very good, it had food in its crop, and that it weighed 34 g less than its release weight of 602g.
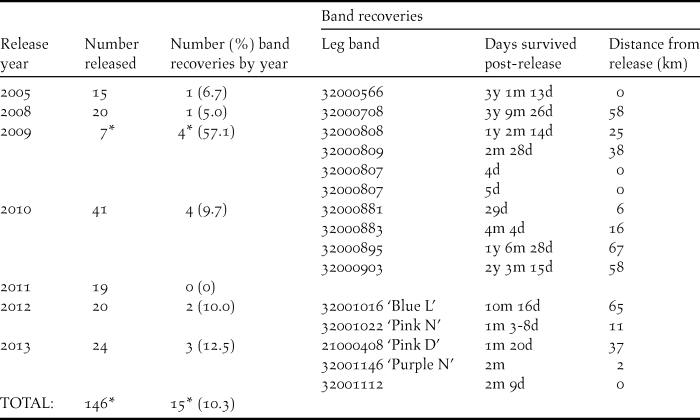
For satellite-tracked study birds released in 2012 and 2013 the daily survival rate was estimated at 0.9991 with an estimated annual survival rate of 0.73. Leg band returns provided evidence of long-term survival with the longest survival record after release being three years and nine months (Table 2). There have been 15 band returns from 14 birds (one was recovered twice) of 145 banded birds released between 2005 and 2013 (10.3%) (Table 2). The band recovery rate during the two years of this study was 13.0% for birds fitted with tracking devices (n = 23) and 9.5% for all other cockatoos released without tracking devices (n = 21) which was not significantly different (P = 1). Two leg-banded birds were only recovered because they were also fitted with tracking devices.
Table 2. Releases and band returns from rehabilitated Carnaby’s Cockatoos between 2005 and 2013. *Includes one bird released and recovered twice.
Social and behavioural fitness
Study birds were observed feeding with, and interacting with wild flocks. All were competent at handling foods and none were returned to care due to poor body condition or starvation. No satellite-tracked rehabilitated birds returned to rehabilitation centres, sought human company or behaved unusually when in close proximity to humans. One banded female (not fitted with a tracking device) returned voluntarily twice to the rehabilitation centre where it had previously bonded with a male in the facility. Return visits stopped when both birds were released together.
On several occasions study birds used a set of roosts interchangeably before transitioning to another set of roosts, often with a period of overlap in roosting habits. For example, for the period April to June 2013 up to six study birds interchangeably used roosts at Bentley, Hollywood Hospital, Perry Lakes and Ballajura and it was common for more than one study bird to forage in the same flock despite roosting in different locations. The birds congregated primarily to feed on nut trees (macadamia, pecan and almond) and liquid amber Liquidambar styraciflua on private properties in the vicinity of Bayswater and Mount Lawley (part of the Central Perth transitory foraging range illustrated in Figure 3). Each study bird varied considerably in the amount of time spent foraging within a transitory foraging range and the number of times they shifted to different areas (Figure 3). Several study birds that were released at different times used the same transitory foraging ranges indicating that the transitory foraging ranges reflect patterns of spatial use of the landscape by wild flocks. The Central Perth transitory foraging range was used most extensively.
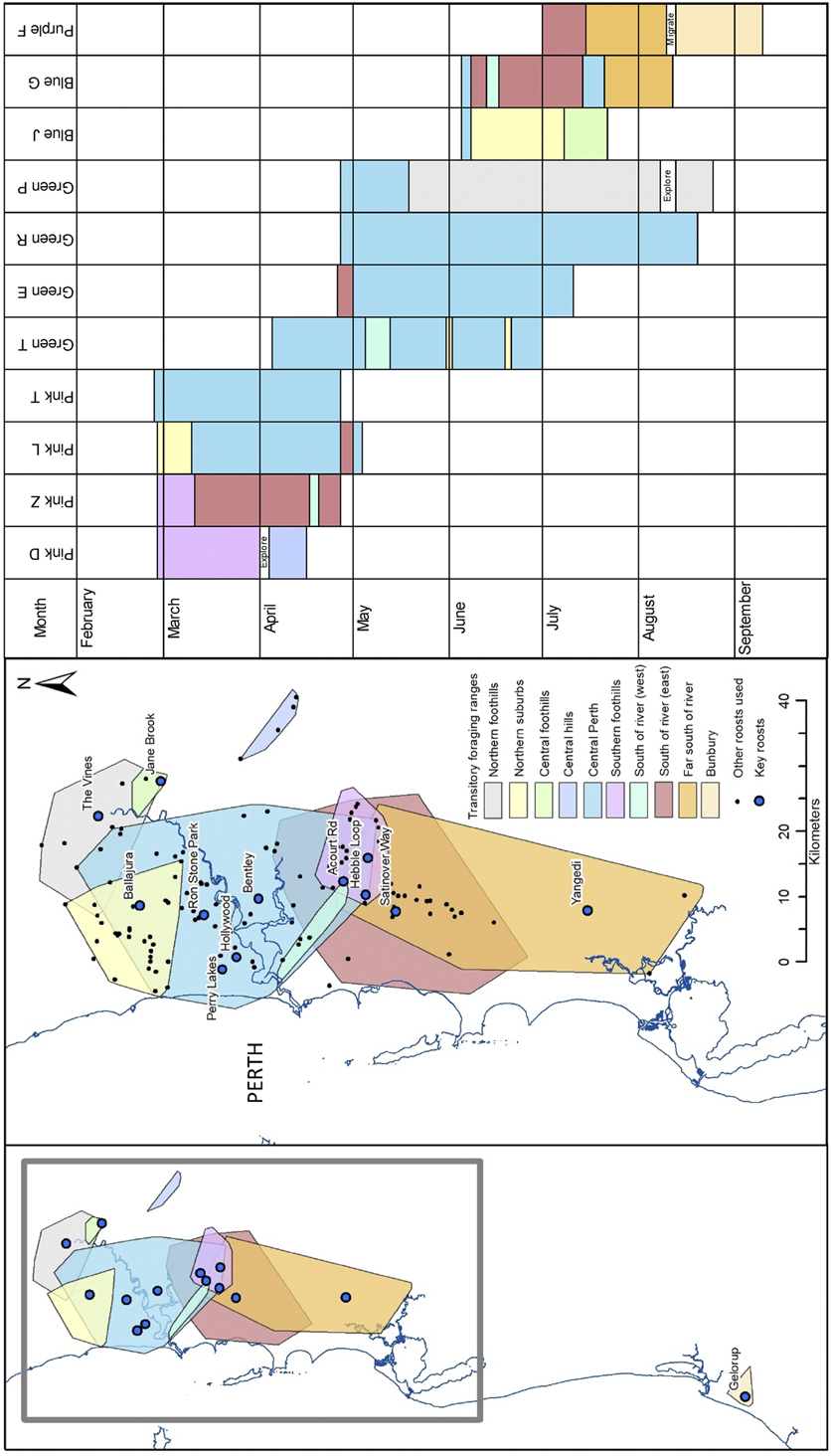
Figure 3. Spatial extent of transitory foraging ranges and temporal use by study birds based on data from Argos tracking devices attached to released rehabilitated Carnaby’s Cockatoos in 2013. Key roosts were used on ten or more nights by one or more study birds
Of 160 follows of flocks containing at least one study bird, 147 (91.9%) involved direct observation of a study bird(s) foraging with a flock and roosting communally. Three study birds were found alone more than once (Table 1). The night roosts and feeding locations used by lone study birds were often ones previously visited with a flock, potentially indicating a capacity for spatial learning and memory.
Evidence of study birds showing breeding behaviour included observation of five of seven study birds for which tracking devices were active during or after September, making distinct changes in their movement patterns coinciding with when migration to breeding sites normally occurs. These birds moved to areas including Bindoon, Calingiri and Clackline where breeding is known or suspected to occur. Further, five of 11 study birds intensively followed were observed allo-preening and three were involved in male courtship displays indicative of bonding (Saunders Reference Saunders1974a) (Table 1).
Wedge-tailed Eagles Aquila audax are a predator of Carnaby’s Cockatoos (Saunders Reference Saunders1982, Reference Saunders1988) and one incident of an unsuccessful attack on a flock containing a study bird was observed. The 37 birds subject to the attack flew high in a tight group and circled before moving away from the area after the eagle landed in the Banksia woodland where they had been feeding. The study bird behaved in the same way as the rest of the flock members.
Discussion
Assessment of survival, physical fitness and the rehabilitation strategy
The data presented here indicate the rehabilitation protocols that were followed resulted in rehabilitated Carnaby’s Cockatoos that were capable of surviving, integrating with local flocks and moving considerable distances. Their estimated annual survival rate of 73% was similar to adult survival of 61% for females and 69% for males reported for wild Carnaby’s Cockatoos (Saunders Reference Saunders1982). Actual survival of wild cockatoos is likely to be higher given that the patagial tags used in the earlier study increased mortality through predation by Wedge-tailed Eagles (Saunders Reference Saunders1982, Reference Saunders1988). The survival rate in our study indicates that the rehabilitation process and assessment of release candidates was successful in identifying individuals ready for release that could survive in the wild.
White et al. (Reference White, Collar, Moorhouse, Sanz, Stolen and Brightsmith2012) have defined reintroduction success for psittacines as: 1) ≥ 50% of released individuals surviving the first year: and, 2) released birds breeding with conspecifics. Neither of those criteria could be adequately assessed in the time period of this study due to the limited battery life of the tracking devices. However, survival rates were high, allo-preening and male courtship displays were observed, an established pair bond was maintained and movements towards known breeding areas were detected. Taken together, these observations suggest successful rehabilitation, release, and likely reintegration back into the wild of study birds. Future studies should aim to confirm success against the criteria of White et al. (Reference White, Collar, Moorhouse, Sanz, Stolen and Brightsmith2012).
Over half the 23 satellite-tracked study birds were estimated to be less than four years old, but post-release survival was high despite their inexperience. The success of rehabilitating juveniles (and adults) in this study is likely due to several factors. Juvenile birds were often housed with ‘nanny’ birds (usually experienced wild-born adult birds whose injuries prevented their release) who helped to re-socialise them and demonstrated food handling skills (Le Souëf Reference Le Souëf2012). These ‘nanny’ birds were often adult males which is consistent with the increased role that males play in feeding and caring for newly fledged cockatoos (Saunders Reference Saunders1982). The release strategy also involved housing birds together to form an aviary flock of familiar individuals to increase learning opportunities and possibly provide an easier social transition to the wild. In other avian studies, larger release group sizes (Snyder et al. Reference Snyder, Koenig, Koschmann, Snyder and Johnson1994; Brightsmith et al. Reference Brightsmith, Hilburn, del Campo, Boyd, Frisius, Frisius, Janik and Guillen2005) and selecting release sites where there is an existing resident or establishing population (Sanz and Grajal Reference Sanz and Grajal1998, Collazo et al. Reference Collazo, White, Vilella and Guerrero2003, Brightsmith et al. Reference Brightsmith, Hilburn, del Campo, Boyd, Frisius, Frisius, Janik and Guillen2005) have been associated with greater success of reintroductions. Rehabilitated Carnaby’s Cockatoos were released at occupied night roosts or popular congregation areas to help ensure they would come into contact with wild birds that had current local knowledge of the spatial distribution of food resources and roosts. The satellite-tracked birds all interacted with wild flocks and none returned to the rehabilitation centres, indicating that they had successfully disassociated themselves from humans and food provided in captivity.
Social and behavioural responses to rehabilitation and release
Study birds were observed to forage and roost interchangeably with different flocks and move between different areas of the Swan coastal plain (Figure 3) which provides evidence that wild cockatoos do not form stable groups, and instead form flocks with a loose interchangeable association of individuals, pairs and pairs with young. Saunders (Reference Saunders1980) observed membership of flocks changing through sightings of individuals identified by patagial tags, and we also observed different combinations of wild individuals with distinctive natural tail markings (Usher et al. Reference Usher, Groom and Saunders2015) in foraging flocks originating from different roosts over time. Variable numbers of birds counted at roost sites on consecutive nights (Shah Reference Shah2006, Berry Reference Berry2008) provides further evidence for the composition of flocks changing over time.
Despite our aviary flocks consisting of individuals housed together for varying periods, most did not stay together post-release. Sanz and Grajal (Reference Sanz and Grajal1998) also found aviary groups of Yellow-shouldered Parrots Amazona barbadensis did not stay together post-release, but they did join wild groups with integration occurring five days to nine months after release. Younger parrots were slower to reintegrate. Similarly, older Carnaby’s Cockatoos (4+ years) appeared to reintegrate more rapidly with wild flocks. This was inferred based on the frequency with which they were found with a flock and made more decisive movement patterns. This suggests birds retain spatial memory and social flocking skills. Younger birds had spent proportionally more time in captivity than the wild and may not have developed an understanding of social flocking cues. A study of released, captive-reared Thick-billed Parrots Rhynchopsitta pachyrhyncha showed an inadequate tendency to flock and this was attributed to the young birds lacking the inducement of their calling parents to maintain contact with the flock (Wallace Reference Wallace1994). Released parrots can lose condition if they cannot keep up with the flock moving from one feeding area to another, and also became more vulnerable to predation without the predator awareness and warnings provided by flock members (Wallace Reference Wallace1994). Similarly, Collazo et al. (Reference Collazo, White, Vilella and Guerrero2003) observed that captive-reared Hispaniolan Parrots A. ventralis had difficulty keeping up with wild birds, but this improved if birds were subjected to a more rigorous exercise routine prior to release. The conditions birds are kept in prior to release appear to greatly affect survival and reintegration back into the wild, and efforts should be made to prepare birds both physically and socially for release.
Behavioural plasticity to learn and adapt to change may be important for long-lived and wide-ranging species such as Carnaby’s Cockatoos, and should be advantageous when rehabilitated birds are released back into the wild. For example, Salinas-Melgoza et al. (Reference Salinas-Melgoza, Salinas-Melgoza and Wright2013) showed that translocated wild Yellow-naped Amazon Parrots A. auropalliata demonstrated flexibility in ranging movements and communal night roosting behaviours by matching the behaviours of resident birds at release sites. Behavioural plasticity in psittacines can be used in conservation planning to improve the recovery of species (Ortiz-Catedral et al. Reference Ortiz-Catedral, Kearvell, Hauber and Brunton2009). Behaviourally adaptive species learn and adapt to new surroundings and social groups, which is helpful when planning where and how to release individuals for greatest conservation benefit. We know little about the social structure of flocks of Carnaby’s Cockatoos or of the population as a whole, which makes it difficult to gauge the best way to reintegrate rehabilitated birds back into the wild. However, their apparent capacity to learn and adjust behaviours helps ensure the cockatoos survive long enough to develop necessary foraging skills and spatial knowledge to reintegrate over time.
Our study birds varied greatly in movement patterns after release with individuals demonstrating movement patterns consistent with sedentary, nomadic and migratory behaviours (Figure 1). This is partly due to the relatively short monitoring period, but also the time of year during which each bird was observed. Some birds appeared to find all the resources they needed in a small area, while others moved their foraging areas over time (Figure 3), and some were released later in the year and moved long distances coinciding with expected movements to breeding areas. These varied movement patterns are consistent with Carnaby’s Cockatoos being highly mobile and responding to changes in spatial availability of resources (Johnston et al. Reference Johnston, Stock and Mawson2016).
Assessment of the value of rehabilitation and conservation implications
The value of rehabilitation to conservation is controversial, with some practitioners arguing that the number of individuals released is too small to have a beneficial effect on wild populations, or that rehabilitation works against processes of natural selection and evolutionary fitness (Aitken Reference Aitken1997). Most of the cockatoos were in need of rehabilitation as a result of human threats, which are evolutionarily novel, and therefore they cannot be argued to be surviving against natural selection. Additionally, when threatened species are long-lived and where the survivorship of adults is critical to the viability of the population, it may be more beneficial to ensure adult survival than to try to improve reproductive success (Grier Reference Grier1980). Consequently, as Carnaby’s Cockatoos are a long-lived species with low annual reproductive output, survival of adults is fundamental to the long-term survival of the species. Hence, reintegration of rehabilitated birds back into the wild, and their subsequent breeding, should improve population viability.
Release of rehabilitated birds may be helpful to augment genetically isolated parts of the population. A study of the population genetics of Carnaby’s Cockatoos has revealed evidence of population structuring such that birds in the western and eastern portions of the distribution of the species are genetically distinct (White et al. Reference White, Bunce, Mawson, Dawson, Saunders and Allendorf2014). This was likely caused by extensive clearing of habitat for agriculture between the two edges of their current distribution (White et al. Reference White, Bunce, Mawson, Dawson, Saunders and Allendorf2014). Through the strategic release of rehabilitated individuals it may be possible to increase the genetic diversity of the eastern and western portions of the species range and to ensure gene flow.
In addition to contributing valuable adults to the population, and potentially genetically augmenting populations, rehabilitated individuals also provide the opportunity to mark and release individuals for study. Capture of wild Black Cockatoos, including Carnaby’s Cockatoos, for marking is problematic, with mist netting having limited success (Kurucz Reference Kurucz2000, Murdoch Reference Murdoch2012). Capture at the nest is suitable for banding individuals but less suitable for attaching tracking devices because the preferred method of attachment involves the tail feathers (Le Souëf et al. Reference Le Souëf, Stojanovic, Burbidge, Vitali, Heinsohn, Dawson and Warren2013b, Groom et al. Reference Groom, Warren, Le Souëf and Dawson2014b) and a major moult occurs in the months following breeding (Saunders Reference Saunders1974b) which would limit the time period individuals could be followed. Rehabilitated cockatoos therefore provide an opportunity to attach tracking devices to individuals that can then be followed after release. The normal behaviours and high survival demonstrated by rehabilitated birds in this study indicate that they provide the first opportunity to estimate spatial ecology parameters (foraging distance, roost site fidelity etc.) for this species that may be refined by future study of wild-caught individuals.
Acknowledgements
The efforts of staff and volunteers at Perth Zoo, Kaarakin Black Cockatoo Conservation Centre, Native Animal Rescue and Jamarri Black Cockatoo Sanctuary to rescue, treat and rehabilitate Black Cockatoos are gratefully acknowledged. The authors thank Department of Parks and Wildlife, particularly Rick Dawson, for their support and assistance. Thank you to the many people who assisted following flocks; in particular Mark Blythman, Rebecca Kay and Abby Thomas. Matt Williams performed the statistical analysis of survival. J. Dale Roberts, Nicki Mitchell, Simone Vitali, Denis Saunders and an anonymous reviewer provided constructive comments on earlier versions. This project was supported with funds received as part of an offset package approved by the Australian Government Department of the Environment. The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals. This research was approved by the University of Western Australia, Murdoch University and Department of Parks and Wildlife Animal Ethics Committees (RA/3/100/1100; R2485/12; DEC AEC 2011/30) and carried out under the Australian Bird and Bat Banding Authority No. 1862 issued to PRM, and under licence SF008648 from Department of Parks and Wildlife, Western Australia.






