1. Introduction
Hybodontiformes are an extinct group of euselachian sharks that ranged from the Late Devonian to the Late Cretaceous (Coates & Gess, Reference Coates and Gess2007). Based on their rich fossil record, the highest genus-level diversity was reached during the Early Cretaceous, a time interval marked by the differentiation of three palaeobiogeographic provinces (Asia, Europe–North America and Africa–South America) and the appearance of numerous non-marine forms (Cuny et al. Reference Cuny, Suteethorn, Kamha and Buffetaut2008; Cuny, Reference Cuny and Godefroit2012). In West Gondwana, the Early to mid-Cretaceous hybodont shark communities of the African–South American province were dominated by distobatids (Distobatus, Tribodus, Aegyptobatus) but also comprised taxa such as the hybodontid Priohybodus, the possible lonchidiids Bahariyodon and Diabodus and the enigmatic genus Pororhiza (Brito & Ferreira, Reference Brito and Ferreira1989; Werner, Reference Werner1989; Duffin, Reference Duffin2001; Cuny et al. Reference Cuny, Ouaja, Srarfi, Schmitz, Buffetaut and Benton2004, Reference Cuny, Suteethorn, Kamha and Buffetaut2008; Cuny, Reference Cuny and Godefroit2012; Brito et al. Reference Brito, Veiga, Dutheil and Bergqvist2025; Neves et al. Reference Neves, Medeiros, Dutheil and Brito2025). All these genera were endemic to this province except Tribodus, which also inhabited the western part of the European archipelago (Vullo & Néraudeau, Reference Vullo and Néraudeau2008; Cuny, Reference Cuny and Godefroit2012). Here we report the discovery of a new genus and species of Lonchidiidae based on isolated teeth from uppermost Albian–lower Cenomanian fluvial deposits of Algeria, and we assign to this genus some isolated teeth from Barremian–Aptian lacustrine deposits of Brazil previously described as ?Lonchidiidae (Fragoso et al. Reference Fragoso, Bittencourt, Mateus, Cozzuol and Richter2021).
The studied Algerian specimens are housed in the palaeontological collections of the Geomatics, Ecology and Environment Laboratory (LGEE) at the Mustapha Stambouli University of Mascara, Algeria, under the collection acronym LGEE-GH-SA. The studied Brazilian specimens are housed at the Instituto de Geociências of the Universidade Federal de Minas Gerais (UFGM) in Belo Horizonte, Brazil, under the collection acronym (IGC-P). The ZooBank LSID (Life Science Identifier) for this publication is: urn:lsid:zoobank.org:pub:D8890DB3-0D1F-48F1-84AA-710BE089FBE9.
2. Geological settings
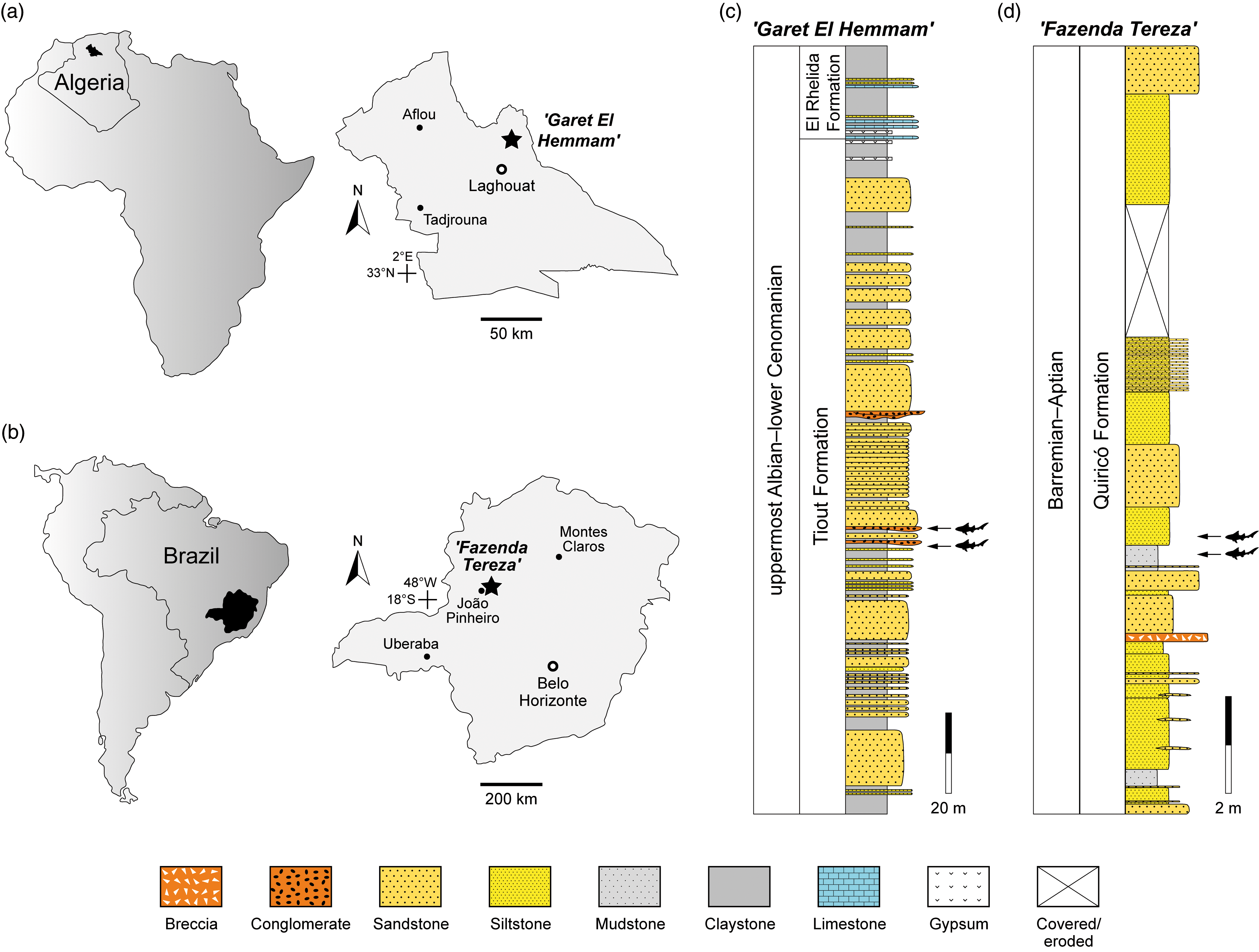
2.a. ‘Garet El Hemmam’ locality, Algeria
The Algerian material described in this study was collected from the upper, vertebrate-bearing section of the Tiout Formation in the Djebel Amour (central Saharan Atlas) (Bassoullet & Iliou, Reference Bassoullet and Iliou1967; Bassoullet, Reference Bassoullet1973), exposed at the ‘Garet El Hemmam’ locality (34° 0’ 51.39” N; 2° 57’ 20.33” E) in the northeastern part of the Laghouat Province (Figure 1a, c). This stratigraphic interval has been assigned to the uppermost Albian–lower Cenomanian based on regional biostratigraphic correlations (Benyoucef et al. Reference Benyoucef, Mebarki, Ferré, Adaci, Bulot, Desmares, Villier, Bensalah, Frau, Ifrim and Malti2017). The specimens originate from two closely spaced layers (samples Gh9 and Gh11), which are positioned about 100 m below the base of the El Rhelida Formation.

Figure 1. Maps of the Laghouat Province (N Algeria) (a) and Minas Gerais State (SE Brazil) (b) showing the location of the ‘Garet El Hemmam’ and ‘Fazenda Tereza’ sites, respectively, and stratigraphic sections (c, d) showing the position of the fossil-bearing beds that have yielded the teeth of Lonchidionoides gen. nov.
The upper part of the Tiout Formation comprises fine- to medium-grained, moderately to well-sorted sandstones (ranging from 0.20 to 50 m thick), interbedded with clay layers (0.10 to 4 m thick) and channelized, matrix-supported microconglomerates (0.10 to 0.60 m thick). Some of the sandstone beds preserve hydrodynamic sedimentary structures and invertebrate burrows and tetrapod swim tracks (Bouchemla et al. Reference Bouchemla, Benyoucef, Klein and Adaci2023). The clay intervals are predominantly red or greenish, display parallel lamination and occasionally yield fossil wood fragments, sparse smooth-shelled, low-diversity ostracod assemblages and rare fish remains (sample Gh9 comes from one of these beds). The microconglomerates are composed of sub-rounded quartz pebbles (0.1–2 cm in diameter) set in a fine- to medium-grained, poorly sorted matrix (sample Gh11 comes from one of these beds). These microconglomerates represent the primary fossiliferous units, yielding abundant vertebrate remains, including elasmobranchs (e.g. Onchopristis numida), actinopterygians (e.g. Bawitius sp.), actinistians (Mawsoniidae indet.), dipnoans (Neoceratodus africanus), testudines, crocodyliforms, non-avian dinosaurs (including the theropods Spinosaurus aegyptiacus and Carcharodontosaurus saharicus), indeterminate bones and teeth and coprolites; invertebrate remains consist of ostracods (Benyoucef et al. in prep.). In sample Gh11, fish microremains co-occurring with the new lonchidiid belong to Tribodus sp., Sclerorhynchoidei indet., Onchopristis numida, Pycnodontidae indet., Ginglymodi indet. and Amiidae indet.
The upper Tiout Formation correlates with the uppermost part of a widespread continental siliciclastic sequence across northern Africa, historically referred to as the ‘Continental Intercalaire’ (Kilian, Reference Kilian1931). In Algeria, the upper Tiout Formation is considered equivalent to the ‘Grès rouges’ Formation at the base of the Cretaceous succession in the Guir Basin (Bechar Province), near the Algerian–Moroccan border (Benyoucef et al. Reference Benyoucef, Läng, Cavin, Mebarki, Adaci and Bensalah2015), the Gara Samani Formation in the Tademait Plateau (Benyoucef et al. Reference Benyoucef, Pérez-García, Bendella, Ortega, Vullo, Bouchemla and Ferré2022) and the Djoua series along the Algerian–Libyan border (Lefranc, Reference Lefranc1983). Outside Algeria, it correlates with famous vertebrate-bearing deposits of North Africa such as the Kem Kem beds in eastern Morocco and the Bahariya Formation in western Egypt (Le Loeuff et al. Reference Le Loeuff, Läng, Cavin and Buffetaut2012).
2.b. ‘Fazenda Tereza’ locality, Brazil
The fossils from Brazil were collected at ‘Fazenda Tereza’, a traditional fossil locality (17° 37’ 32.38” S; 45° 54’ 22.48” W) in the Sanfranciscana Basin, located in João Pinheiro, in the northwestern part of the Minas Gerais State (Bittencourt et al. Reference Bittencourt, Kuchenbecker, Vasconcelos and Meyer2015, Reference Bittencourt, Fonda, Fragoso, Uhlein, Uhlein and Corecco2022) (Figure 1b, d). The local sedimentary succession comprises packages of claystone and siltstone with planar-parallel bedding (when present) and fine- to medium-grained sandstones. This succession is typical of the lacustrine strata of the Quiricó Formation (Campos & Dardenne, Reference Campos and Dardenne1997; Sgarbi et al. Reference Sgarbi, Sgarbi, Campos, Dardenne, Penha, Pinto and Martins-Neto2001) and reflects variations in the hydraulic energy of deposition, ranging from fine-grained decantation to higher-energy fluvial input of sand. Mud cracks suggest episodic drying events, although no evidence supports large-scale droughts. Both the lithofacies and the fossil record at ‘Fazenda Tereza’ indicate deposition in a freshwater environment under a warm climate.
The specimens described herein were recovered by processing claystone and siltstone samples from the lower portion of the succession (Fragoso et al. Reference Fragoso, Bittencourt, Mateus, Cozzuol and Richter2021). The fossil assemblage includes bioturbation, ostracods, spinicaudatans, actinopterygian remains (notably teeth and vertebrae of Amiidae), other hybodontiforms, abundant bone fragments of the coelacanthiform Mawsonia gigas and a single skeleton of the paramacellodid lizard Neokotus sanfranciscanus (Delicio et al. Reference Delicio, Barbosa, Coimbra and Vilella1998; Carvalho & Maisey, Reference Carvalho and Maisey2008; Leite et al. Reference Leite, Do Carmo, Ress, Pessoa, Caixeta, Denezine, Adorno and Antonietto2018; Bittencourt et al. Reference Bittencourt, Simões, Caldwell and Langer2020, Reference Bittencourt, Fonda, Fragoso, Uhlein, Uhlein and Corecco2022; Fragoso et al. Reference Fragoso, Bittencourt, Mateus, Cozzuol and Richter2021).
The age of the Quiricó Formation is generally considered Barremian–Aptian, based on ostracods and palynomorphs from other areas of the Sanfranciscana Basin (Lima, Reference Lima1979; Arai et al. Reference Arai, Dino, Milhomem and Sgarbi1995; Do Carmo et al. Reference Do Carmo, Coimbra, Whatley, Antonietto and De Paiva Citon2013; Bittencourt et al. Reference Bittencourt, Kuchenbecker, Vasconcelos and Meyer2015; Fauth et al. Reference Fauth, Strohschoen, Baecker-Fauth, Luft-Souza, dos Santos Filho, Santos, Bruno, Mescolotti, Krahl, Arai, de Oliveira Lima and Assine2024). However, the age of the strata exposed at ‘Fazenda Tereza’ remains highly controversial. Carvalho and Maisey (Reference Carvalho and Maisey2008) assigned the Mawsonia-bearing layers to the Berriasian, whereas recent research on ostracods has assigned the succession at ‘Fazenda Tereza’ to the Valanginian–Aptian interval (Leite et al. Reference Leite, Do Carmo, Ress, Pessoa, Caixeta, Denezine, Adorno and Antonietto2018, Reference Leite, Do Carmo, Gonçalves and Xi2024). In this latter interpretation, it is unclear where Mawsonia gigas occurs. In addition, Coimbra (Reference Coimbra2020, Reference Coimbra2023) has disputed the taxonomic identification of ostracods referred to Cypridea, rejecting the presence of pre-Barremian levels in the Quiricó Formation. Given the taxonomic controversy regarding the ostracods from the Quiricó Formation, and also the vertical range of Mawsonia gigas within the ostracod-based biostratigraphic framework of the ‘Fazenda Tereza’ locality, we will adopt a conservative approach and consider the Brazilian Lonchidiidae to be Barremian–Aptian in age pending further evidence.
3. Systematic Palaeontology
3.a. Lonchidionoides trifurcatum gen. et sp. nov. from Algeria
Chondrichthyes Huxley, Reference Huxley1880
Subclass Elasmobranchii Bonaparte, Reference Bonaparte1838
Cohort Euselachii Hay, Reference Hay1902
Order Hybodontiformes Patterson, Reference Patterson1966
Family Lonchidiidae Herman, Reference Herman1977
Lonchidionoides gen. nov.
ZooBank LSID. urn:lsid:zoobank.org:act:366E2C14-E128-483C-8AF6-6757F845DEA1.
Etymology. From the overall resemblance to the teeth of Lonchidion.
Type species. Lonchidionoides trifurcatum gen. et sp. nov.
Diagnosis. As for the type and the only known species.
Lonchidionoides trifurcatum gen. et sp. nov.

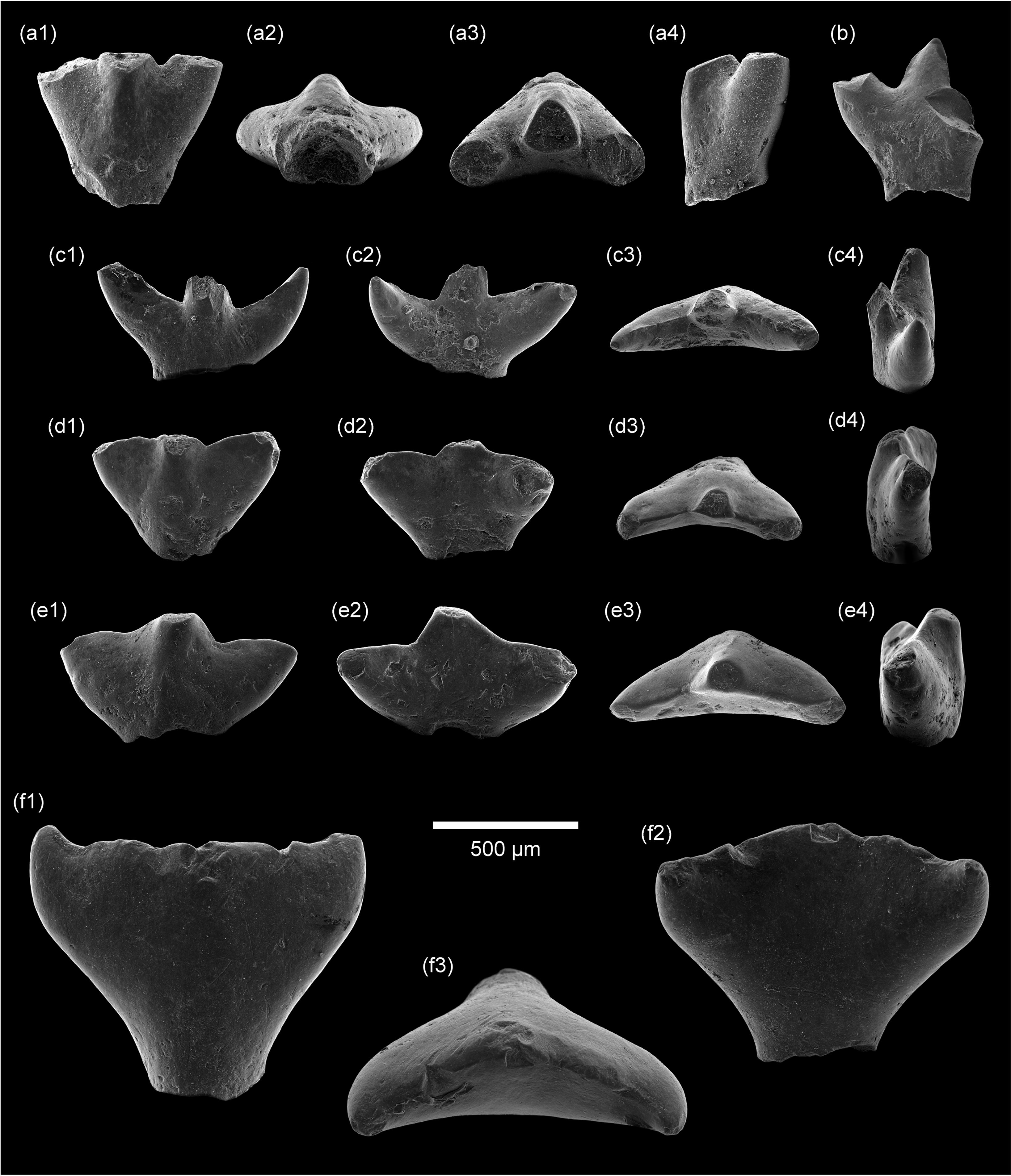
Figure 2. Teeth of the hybodont shark Lonchidionoides trifurcatum gen. et sp. nov. from the uppermost Albian–lower Cenomanian fluvial deposits of ‘Garet El Hemmam’ (Algeria). (a1–a4), symphysial tooth (LGEE-GH-SA-2) in labial, basal, occlusal and lateral views; (b) anterior tooth (LGEE-GH-SA-3) in lingual view; (c1–c4) anterior tooth (holotype; LGEE-GH-SA-1) in labial, lingual, occlusal and lateral (mesial?) views; (d1–d4) lateral tooth (LGEE-GH-SA-4) in labial, lingual, occlusal and lateral (distal?) views; (e1–e4) lateral tooth (LGEE-GH-SA-5) in labial, lingual, occlusal and lateral (mesial?) views; (f1–f3) posterior tooth (LGEE-GH-SA-6) in labial, lingual and occlusal views. Scale bar equals 500 µm.
ZooBank LSID. urn:lsid:zoobank.org:act:82B30B56-BEB0-48B2-AB0F-C16A4E77C271.
Etymology. From the trident shape of the anterior teeth.
Holotype. Specimen LGEE-GH-SA-1, an anterior tooth (Figure 2c).
Type locality and horizon. ‘Garet El Hemmam’, Djebel Amour (central Saharan Atlas), Laghouat Province, Algeria; sample Gh11, upper part of the Tiout Formation (latest Albian–early Cenomanian in age), about 100 m below the base of the El Rhelida Formation.
Referred material. Five isolated teeth (specimens LGEE-GH-SA-2 to LGEE-GH-SA-6) from the type locality (Figure 2a, b, d–f). All specimens from the type horizon (sample Gh11), except LGEE-GH-SA-6 from an underlying layer (sample Gh9, located ∼5 m above sample Gh11).
Diagnosis. Diminutive lonchidiid hybodont shark characterized by a dentition with a marked disjunct monognathic heterodonty; gracile, trident-shaped anterior teeth and low-cusped, more massively built posterior teeth; lateral teeth showing an intermediate morphology; no labial protuberance; narrow and rod-like shaped crown base; enameloid smooth and crown devoid of ornamentation.
Description. These teeth are minute, most of the specimens measuring less than 1 mm in width. All the specimens consist of well-preserved crowns with no preserved root structures. The basal face of the crown is depressed. Three main dental morphotypes can be distinguished, suggesting the existence of a disjunct monognathic heterodonty.
Tooth morphotype I (Figure 2a–c): this morphotype includes teeth (LGEE-GH-SA-1 to 3) with a markedly tricuspid (trident-shaped) crown. The erect main (central) cusp and the crescent-shaped lateral cusps are slender, especially in the holotype LGEE-GH-SA-1 (Figure 2c). The lateral cusps are slightly bent lingually. This morphotype is interpreted here as anterior teeth. One of these specimens (LGEE-GH-SA-2), showing a sub-symmetrical crown higher than wide, is interpreted as a symphysial tooth (Figure 2a). The lateral cusps are less projected laterally. In cross-section, the main cusp is subtriangular, with the crown more developed labially, whereas the lateral cusps are subcircular.
Tooth morphotype II (Figure 2d, e): this morphotype corresponds to teeth with less gracile tricuspid crown (LGEE-GH-SA-4 and LGEE-GH-SA-5). In a labiolingual view, the robust lateral cusps are triangular and project laterally. The notch between the main cusp and the distal cusp is more pronounced than on the mesial side. This morphotype is interpreted here as lateral teeth.
Tooth morphotype III (Figure 2f): this morphotype is represented by a significantly larger tooth with poorly individualized cusps (LGEE-GH-SA-6). The main cusp is broad and low, and the lateral cusps are reduced (cusplets). This morphotype is interpreted here as corresponding to posterior teeth, which include an enlarged one (interpreted here as the first posterior tooth) having a crushing function (as suggested by its functional wear).
3.b. Lonchidionoides sp. from Brazil
Lonchidionoides sp.
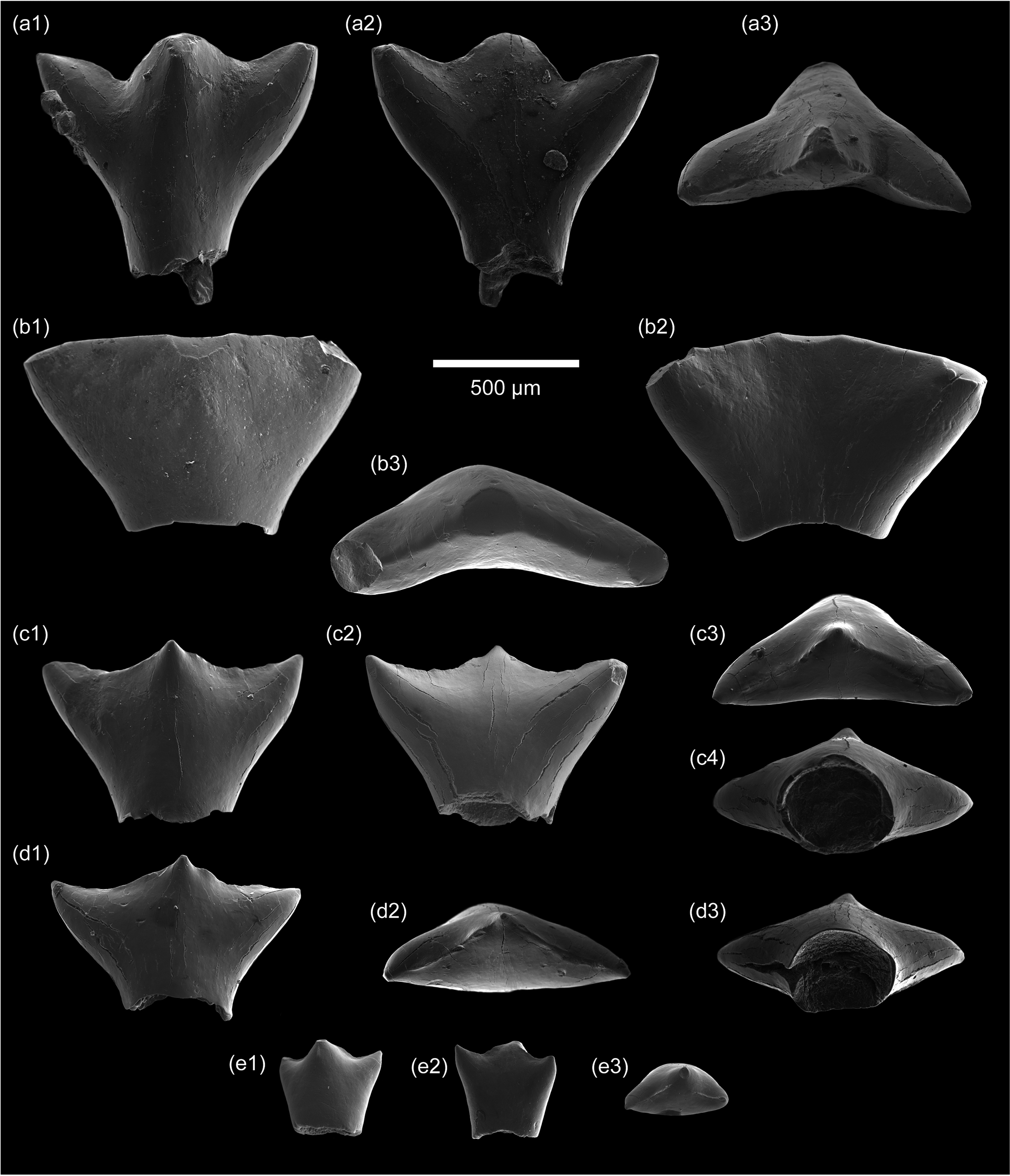
Figure 3

Figure 3. Teeth of the hybodont shark Lonchidionoides sp. from the Barremian–Aptian lacustrine deposits of ‘Fazenda Tereza’ (Brazil). (a1–a3), lateral tooth (IGC-P 0093/2) in labial, lingual and occlusal views; (b1–b3) posterior tooth (IGC-P 0093/9) in labial, lingual and occlusal views; (c1–c4) posterior tooth (IGC-P 0093/1) in labial, lingual, occlusal and basal views; (d1–d3) posterior tooth (IGC-P 0093/7) in labial, occlusal and basal views; (e1–e3) posteriormost tooth (IGC-P 0093/8) in labial, lingual and occlusal views. Scale bar equals 500 µm.

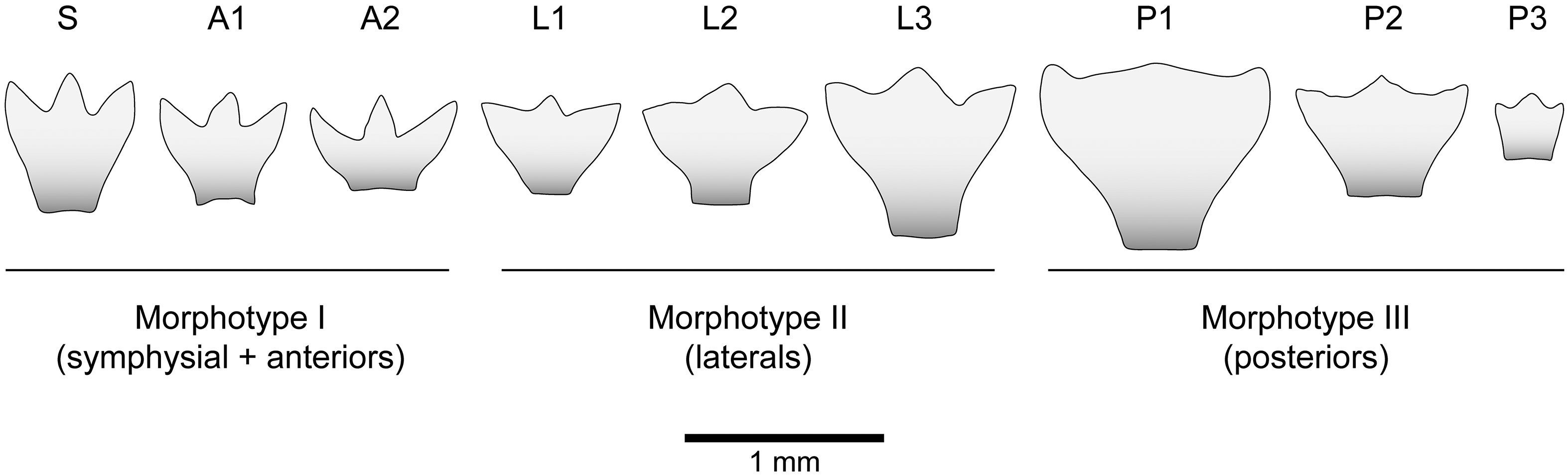
Figure 4. Tentative reconstruction of the lower dentition of Lonchidionoides trifurcatum gen. et sp. nov. from the uppermost Albian–lower Cenomanian of Algeria. Note that the material of Lonchidionoides sp. from the Barremian–Aptian of Brazil was used to supplement missing tooth positions (L3, P2, P3). Scale bar equals 1 mm.
2021 ?Lonchidiidae, Fragoso et al. Reference Fragoso, Bittencourt, Mateus, Cozzuol and Richter2021, p. 1313, fig. 3c–f.
Locality and horizon. ‘Fazenda Tereza’ in João Pinheiro, Sanfranciscana Basin, Minas Gerais, Brazil; lower portion of the Quiricó Formation (Barremian–Aptian in age).
Material. Nine isolated teeth (specimens IGC-P 0093/1 to 0093/9) (Figure 3a–e).
Description. Some of the specimens were described and figured by Fragoso et al. (Reference Fragoso, Bittencourt, Mateus, Cozzuol and Richter2021). The material available includes the tooth morphotypes II and III described above for the Algerian material. The morphotype I, characterized by a gracile trident-shaped crown, has not been recovered so far among the Brazilian specimens. The root is not preserved in all specimens.
Tooth morphotype II (Figure 3a): this morphotype is represented by one complete crown (IGC-P 0093/2), which is similar in shape and size to the Algerian specimen LGEE-GH-SA-5 (Figure 2e). In a labiolingual view, the distal cusp projects more laterally than the mesial cusp, and the notch that separates it from the main cusp is more marked. The basal part of the crown shows a well-developed rod-like portion.
Tooth morphotype III (Figure 3b–e): this morphotype includes a significantly larger crown (IGC-P 0093/9) (Figure 3b), which is similar to the Algerian specimen LGEE-GH-SA-6 (Figure 2f) (interpreted here as the first posterior tooth). The cusps are abraded. The main cusp is broad and low, and the lateral cusps are reduced (cusplets). In addition, some smaller crowns (IGC-P 0093/1 and IGC-P 0093/7) are also assigned to this morphotype and interpreted here as more posterior teeth (Figure 3c, d). In a labiolingual view, the main cusp and lateral cusplets are low and pointed, and the occlusal crest is concave between these cusps. In the occlusal view, the main cusp and lateral cusplets slightly project labially and lingually, respectively. A minute crown (IGC-P 0093/8), showing similar morphological features, is interpreted as a posteriormost commissural tooth (Figure 3e). It mainly differs from the other teeth of this morphotype by the lateral cusplets that are closer to the main cusp, the occlusal crest that does not reach the main cusp apex and the base of the crown that is less constricted basally.
4. Discussion
4.a. Comparisons
The material of Lonchidionoides trifurcatum gen. et sp. nov. from Algeria includes three main dental morphotypes, which are interpreted as representing distinct tooth positions (i.e. morphotype I: symphysial and anterior teeth; morphotype II: lateral teeth; morphotype III: posterior teeth). The material from Brazil includes some teeth that are almost identical in shape and size to some Algerian specimens (lateral and posterior tooth positions), thus strongly supporting its assignment to the new genus Lonchidionoides. The absence of trident-shaped anterior teeth (morphotype I) in the Brazilian material and of posteriormost teeth in the Algerian material is likely due to the small size of the available samples. However, the Brazilian specimens are left in open nomenclature (as Lonchidionoides sp.) pending the discovery of additional material, which would help to determine whether the North African and South American teeth represent a single or two species. The tooth root is unknown in both Lonchidionoides trifurcatum and Lonchidionoides sp., as in most hybodontiform species based on isolated teeth (Böttcher, Reference Böttcher2024).
Lonchidionoides is included here in the family Lonchidiidae based on its general morphological resemblance to Lonchidion. Although the anterior teeth of Lonchidionoides show a unique trident-shaped crown, the morphology of the lateral and posterior teeth is roughly similar to that of Lonchidion teeth (e.g. Rees & Underwood, Reference Rees and Underwood2002). Both taxa share some features (i.e. crown mesiodistally wider than high, crown shape subtriangular in occlusal view, base of the crown constricted, low to poorly defined cusps), but the latter has a more or less developed labial protuberance (= labial peg of some authors) that is absent in the former. Similarly, the teeth of the Triassic genus Diplolonchidion mainly differ from those of Lonchidionoides by the presence of two labial protuberances (Heckert, Reference Heckert2004). Parvodus, Jiaodontus and Pristrisodus have teeth with a labial protuberance, vertical folds and generally two or three pairs of lateral cusplets (Rees & Underwood, Reference Rees and Underwood2002; Klug et al. Reference Klug, Tütken, Wings, Pfretzschner and Martin2010; Bhat et al. Reference Bhat, Ray and Datta2018). Luopingselache has large teeth (up to 15 mm in mesiodistal width) showing a labial protuberance, a strong ornamentation and up to six pairs of lateral cusplets (Wen et al. Reference Wen, Zhang, Kriwet, Hu, Zhou, Huang, Cui, Min and Benton2023). These features clearly distinguish Parvodus, Jiaodontus, Pristrisodus and Luopingselache from Lonchidionoides. Vectiselachos differs from Lonchidionoides by its larger, bulky teeth that are devoid of lateral cusplets and ornamented with folds and granulae (Rees & Underwood, Reference Rees and Underwood2002).
Several other hybodont genera (i.e. Bahariyodon, Diabodus, Hylaeobatis, Isanodus, Lissodus) have been tentatively referred to or excluded from the Lonchidiidae by various authors (see Rees & Underwood, Reference Rees and Underwood2002; Cuny et al. Reference Cuny, Ouaja, Srarfi, Schmitz, Buffetaut and Benton2004, Reference Cuny, Suteethorn, Kamha, Buffetaut and Philippe2006, Reference Cuny, Laojumpon, Cheychiw and Lauprasert2010; Fischer, Reference Fischer2008; Rees, Reference Rees2008; Cappetta, Reference Cappetta2012; Bhat et al. Reference Bhat, Ray and Datta2018; Wen et al. Reference Wen, Kriwet, Zhang, Benton, Duffin, Huang, Zhou, Hu and Ma2022, Reference Wen, Zhang, Kriwet, Hu, Zhou, Huang, Cui, Min and Benton2023). Regardless of the systematic position of these genera, their relatively large and massively built tooth crowns are easily distinguishable from those of Lonchidionoides. In addition, most of the species included in these genera have ornamented teeth.
Within the African–South American Tribodus–Priohybodus palaeobiogeographic province defined by Cuny (Reference Cuny and Godefroit2012), Lonchidionoides is characterized by teeth that are clearly distinct from those of other unambiguous Early Cretaceous lonchidiids, which are represented only by Lonchidion marocensis from the ?Berriasian of Morocco (Duffin & Sigogneau-Russell, Reference Duffin and Sigogneau-Russell1993; Rees & Underwood, Reference Rees and Underwood2002) and Parvodus sp. from the ?Early Cretaceous of Brazil (Cupello et al. Reference Cupello, Bermúdez-Rochas, Martill and Brito2012). As noted above, the latter two genera share the presence of a labial protuberance, which is lacking in Lonchidionoides.
4.b. Dentition reconstruction
Articulated dentitions of hybodontiforms indicate that enlarged lateral or posterior teeth with a crushing function were present in the monognathic heterodont dentitions of various taxa with a wide range of sizes, such as Acrodus anningiae (Day, Reference Day1864: pl. 3), Acrodus georgii (Rieppel, Reference Rieppel1981: fig. 2), Lissodus africanus (Rees & Underwood, Reference Rees and Underwood2002: fig. 1), Luopingselache striata (Wen et al. Reference Wen, Zhang, Kriwet, Hu, Zhou, Huang, Cui, Min and Benton2023: fig. 2) and Palaeobates polaris (Romano & Brinkmann, Reference Romano and Brinkmann2010). Based on material consisting of isolated teeth, a similar type of dentition was also inferred for Jiaodontus vedenemus (Klug et al. Reference Klug, Tütken, Wings, Pfretzschner and Martin2010), Lissodus nodosus (Duffin, Reference Duffin1985: text-fig. 12), Lissodus sp. (Duncan, Reference Duncan2004: fig. 7), Reticulodus synergus (Voris & Heckert, Reference Voris and Heckert2017) and Vectiselachos ornatus (Rees & Underwood, Reference Rees and Underwood2002), among others. The different morphotypes recognized in Lonchidionoides suggest that this kind of monognathic heterodonty was also present in this genus, and a tentative reconstruction of the dentition of Lonchidionoides can thus be proposed. From the symphysis to the commissure, it is suggested that the lower jaw bears a symphysial tooth, two anterior, three lateral and three posterior teeth (Figure 4). According to this reconstruction, the total number of tooth files present in the lower jaw would be estimated at 17. This is consistent with the tooth file number (lower jaw) known for various hybodont taxa, ranging from 15 to 21: one symphysial file and seven files on each side in Palaeobates polaris (Romano & Brinkmann, Reference Romano and Brinkmann2010); one symphysial file and eight files on each side in Luopingselache striata (Wen et al. Reference Wen, Zhang, Kriwet, Hu, Zhou, Huang, Cui, Min and Benton2023); one symphysial file and nine files on each side in Acrodus anningiae (Day, Reference Day1864), Asteracanthus ornatissimus (Stumpf et al. Reference Stumpf, López-Romero, Kindlimann, Lacombat, Pohl and Kriwet2021), ‘Polyacrodus’ brevicostatus (Patterson, Reference Patterson1966) and possibly Lissodus africanus (Rees & Underwood, Reference Rees and Underwood2002); and one symphysial file and ten files on each side in Acrodus georgii (Rieppel, Reference Rieppel1981) and Egertonodus basanus (Maisey, Reference Maisey1983).
4.c. Dental features and diet inferences
The dentition of Lonchidionoides shows a strong disjunct monognathic heterodonty, well distinct from the moderate disjunct or gradient monognathic heterodonties observed in the above-mentioned hybodont genera. Moreover, the high degree of heterodonty of Lonchidionoides seems to be unique among hybodont taxa with dentitions of the clutching–crushing type. Lonchidionoides evolved a specialized dentition that clearly separates it from the European and North American lonchidiid genera (Lonchidion, Parvodus, Vectiselachos), which show a conservative dental morphology (Rees & Underwood, Reference Rees and Underwood2002; Cuny, Reference Cuny and Godefroit2012).
Some resemblances resulting from parallel evolution can be noted. Among non-euselachian elasmobranchs, trident-shaped teeth roughly similar to the anterior teeth of Lonchidionoides are known in some Palaeozoic phoebodonts (e.g. Isacrodus, Jalodus) and xenacanths (Bransonella) (Ginter, Reference Ginter2001; Ginter et al. Reference Ginter, Hairapetian and Klug2002; Hampe & Ivanov, Reference Hampe and Ivanov2007; Ivanov et al. Reference Ivanov, Duffin and Richter2021). However, these early sharks had homodont or weakly heterodont dentitions, with no crushing posterior teeth. Among neoselachians, the reconstructed dentition of Lonchidionoides is somewhat similar (although much smaller) to that of young individuals of modern bullhead sharks (e.g. Heterodontus portusjacksoni; Reif, Reference Reif1976: fig. 24c).
Non-marine hybodont sharks underwent a major diversification during the Early Cretaceous Period, resulting in a wide array of dental morphologies and trophic habits (Cuny et al. Reference Cuny, Suteethorn, Kamha and Buffetaut2008, Reference Cuny, Cavin and Suteethorn2009; Cuny, Reference Cuny and Godefroit2012). The specialized dental morphology of Lonchidionoides suggests that these dwarf sharks seized minute, elusive prey items with their tricuspid anterior teeth and crushed them with their more massive, low-cusped posterior teeth. Hence, it is likely that microcrustaceans such as ostracods formed a large part of the diet of Lonchidionoides. These small shelled organisms are common to abundant in the beds that have yielded the shark teeth here described (Leite et al. Reference Leite, Do Carmo, Ress, Pessoa, Caixeta, Denezine, Adorno and Antonietto2018; M.B. pers. obs.). In modern ecosystems, freshwater ostracods are predated by small teleost fishes that can have similar tricuspid teeth (e.g. the cyprinodontiform Cyprinodon brontotheroides; Hernandez et al. Reference Hernandez, Adriaens, Martin, Wainwright, Masschaele and Dierick2018).
4.d. Palaeobiogeographic significance
The discovery of a new Cretaceous genus of non-marine hybodont sharks occurring in both North Africa and South America further documents the biogeographic history of fishes related to the Gondwana break-up and South Atlantic opening (Maisey, Reference Maisey2000; Capobianco & Friedman, Reference Capobianco and Friedman2019; Parméra et al. Reference Parméra, Gallo, Silva and Figueiredo2019). The original distribution area of Lonchidionoides may have been divided into two separate areas, possibly with subsequent vicariance (assuming that the Brazilian and Algerian teeth represent two distinct species). This aligns with the spatial distribution of other non-marine cartilaginous and bony fish taxa such as the distobatid sharks Tribodus, Distobatus and Aegyptobatus (Vullo & Néraudeau, Reference Vullo and Néraudeau2008; Brito et al. Reference Brito, Veiga, Dutheil and Bergqvist2025; Neves et al. Reference Neves, Medeiros, Dutheil and Brito2025), the holosteans Calamopleurus and Obaichthys (Forey & Grande, Reference Forey and Grande1998; Grande, Reference Grande2010) and the coelacanths Mawsonia and Axelrodichthys (Cavin, Reference Cavin2008). This faunal link is also supported by the occurrence of loricarioid catfishes in the Cenomanian of North Africa (Brito et al. Reference Brito, Dutheil, Gueriau, Keith, Carnevale, Britto, Meunier, Khalloufi, King, de Amorim and Costa2024). Furthermore, Lonchidionoides adds to the list of non-marine hybodont genera that appeared and diversified during the Early Cretaceous Period, and it represents a new component of the peculiar hybodont fauna characterizing the African–South American Tribodus–Priohybodus province (Cuny et al. Reference Cuny, Suteethorn, Kamha and Buffetaut2008; Cuny, Reference Cuny and Godefroit2012).
5. Conclusion
A new genus of hybodontiform sharks, Lonchidionoides (Lonchidiidae), is described here based on isolated teeth from the uppermost Albian–lower Cenomanian of Algeria and the Barremian–Aptian of Brazil. The distribution of this newly recognized lonchidiid provides additional evidence of the biogeographic connection that existed between the non-marine ichthyofaunas of Africa and South America during the Late Jurassic–mid-Cretaceous interval. In the Early to mid-Cretaceous ecosystems of West Gondwana, ecological niche partitioning appears to have occurred among synchronic and sympatric hybodonts. Medium-sized distobatids with crushing or grinding dentitions coexisted with the small-sized genus Lonchidionoides, which may have occupied a specialized trophic niche as suggested by its unique, delicate clutching–crushing dentition.
Acknowledgements
We thank L. Joanny (CMEBA, Rennes) for SEM images of the specimens from Algeria. We also thank J. Kriwet for his helpful comments that improved the manuscript. This research received funding from the Tellus–INTERRVIE programme of INSU–CNRS (R.V., project ‘Vegace’), the FAPEMIG (J.S.B., grant APQ-00474-23), the CNPq (J.S.B., grant 313565/2021-0), the FAPESP (J.S.B., grant 2020/07997-4) and the Spanish Ministerio de Ciencia, Innovación y Universidades (A.P.-G., grant PID2023-148083NB-I00).
Competing interests
The authors declare none.