Introduction
Beringia is the region spanning eastern Asia to northwestern North America. It has long been recognised as both a migration corridor and a biotic refugium due to its ice-free conditions during Pleistocene glaciations (e.g., Elias et al. Reference Elias, Short, Nelson and Birks1996; DeChaine Reference DeChaine2008). This ice-free landscape was home to the mammoth steppe ecosystem, which supported a mosaic of grasslands and tundra that bridged Eurasia and North America across the Bering Strait (Guthrie Reference Guthrie2001). Dominated by herbaceous vegetation and having a cold and arid climate, the steppe–tundra ecosystem supported a diverse megafauna, of which most are now extinct (e.g., woolly mammoth, steppe bison, and Yukon horse). The remains of large charismatic megafauna have been extensively studied (e.g., Fox-Dobbs et al. Reference Fox-Dobbs, Leonard and Koch2008), but with the exception of beetles (Coleoptera) (e.g., Elias et al. Reference Elias, Berman and Alfimov2000; Berman et al. Reference Berman, Alfimov and Kuzmina2011), considerably fewer records of Pleistocene Beringian invertebrates have been found. Fleas (Siphonaptera) and mites (Parasitiformes and Acariformes) are poorly represented from Pleistocene deposits in eastern Beringia. To date, grasshoppers (Orthoptera) and thrips (Thysanoptera) have not been reported from Quaternary deposits in any part of Beringia. Most entomologists studying Quaternary fauna have focused on beetles, relying on their abundance, diversity, variation in ecological requirements, and relative ease of identification to reconstruct past climates and environments (e.g., Coope Reference Coope1979; Ashworth Reference Ashworth2003). Because most groups of invertebrates lack both the very broad habitat associations and strong preservation potential of beetles, most invertebrates are underrepresented in Quaternary sedimentary records. This is particularly true for taxa with strong host associations, such as fleas and parasitic mites, and for taxa with weakly sclerotised exoskeletons (Matthews and Telka Reference Matthews, Telka, Danks and Downes1997).
Middens (nests and food caches) of the Arctic ground squirrel, Urocitellus parryii Richardson (Rodentia: Sciuridae), preserved in Pleistocene-aged, ice-rich permafrost deposits provide detailed records of the rodents’ foraging behaviour and of local vegetation within the steppe–tundra ecosystems (Zazula et al. Reference Zazula, Froese, Westgate, La Farge and Mathewes2005, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2007, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2011; Froese et al. Reference Froese, Zazula, Westgate, Preece, Sanborn, Reyes and Pearce2009; Langeveld et al. Reference Langeveld, Mol, Zazula, Gravendeel, Eurlings and McMichael2018). In addition to records of past vegetation, these unique archives also contain remains of Arctic ground squirrels, other small mammals (Cocker et al. Reference Cocker, Zazula, Hall, Jass, Storer and Froese2024), and invertebrates (Zazula et al. Reference Zazula, Froese, Westgate, La Farge and Mathewes2005, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2007, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2011).
Here, we report a unique record of mites, fleas, thrips, and grasshoppers from six permafrost-preserved Arctic ground squirrel middens spanning approximately 80 000–13 500 years BP from the Klondike goldfields in central Yukon Territory, Canada. All four taxa are vastly underrepresented in Quaternary entomological literature; importantly, this study provides the first records of grasshoppers and thrips from Quaternary deposits in Beringia.
The Orthoptera of Yukon Territory
Almost 30 000 species of Orthoptera exist, covering all continents except Antarctica (Cigliano et al. Reference Cigliano, Braun, Eades and Otte2024). The order includes two suborders: Ensifera (crickets, katydids, and relatives) and Caelifera (grasshoppers and relatives). Within the Caelifera, three extant families are found in North America (Acrididae, Tetrigidae, and Tridactylidae), with 139 species in Canada (Miskelly and Paiero Reference Miskelly, Paiero, Langor and Sheffield2019). The modern fauna of grasshoppers in Yukon Territory is represented by 20 species from two families: the short-horned grasshoppers (Acrididae) and the dwarf grasshoppers (Tetrigidae), of which the majority inhabit grasslands (Miskelly et al. Reference Miskelly, Cárcamo, Giberson, Cárcamo and Giberson2014). Within Acrididae, three subfamilies are reported from Yukon Territory: the slant-faced grasshoppers (Gomphocerinae), the spur-throated grasshoppers (Melanoplinae), and the band-winged grasshoppers (Oedipodinae). Tetrigidae are represented in Yukon Territory by one subfamily, Tetriginae.
Fossil record of grasshoppers
Orthopteroid insects have been reported as far back as the Late Carboniferous and in younger deposits preserved in amber (Skejo et al. Reference Skejo, Kasalo, Thomas and Heads2024). Nonadult life stages are rarely preserved, with few exceptions. For example, Lee et al. (Reference Lee, Famoso and Lin2024) reported an Oligocene-aged grasshopper egg mass from the Turtle Cove Member (31.45–26.6 Ma) in the John Day Basin of central and eastern Oregon, United States of America. Such reports are uncommon but provide glimpses into the ontogenetic stage of orthopteran evolution.
Despite a widespread distribution in modern environments and sturdily sclerotised exoskeletons, Orthoptera are notably rare as fossils in Quaternary deposits globally and, until now, were unreported from Beringia. Coope (Reference Coope1970) and Matthews and Telka (Reference Matthews, Telka, Danks and Downes1997) briefly discuss this omission, and Coope (Reference Coope1970, Reference Coope1979) references the preservation of subfossil Orthoptera at a late Pleistocene woolly rhinoceros site in Starunia, Poland (Zeuner Reference Zeuner1934). Late Pleistocene records from the Rancho La Brea tar pits in Los Angeles, California, United States of America, include individuals of both Stenopelmatus Burmeister (Orthoptera: Stenopelmatidae) and undetermined Acrididae (Stock Reference Stock1992), but no records of specimens are reported in the peer-reviewed literature. Beyond these few studies, few publications of global scale in Quaternary entomology even discuss the occurrence of Orthoptera.
Modern fleas and their relationship with ground squirrels
Adult fleas (Siphonaptera) are ectoparasites that have morphologically and physiologically evolved to spend most of their lives either on the bodies of mammals and birds or in their nests. Adults are wingless and laterally flattened, with varying levels of sclerotisation of their exoskeletons. Although they feed exclusively on blood, most fleas are considered to be temporary ectoparasites that spend considerable time in the nests and middens of their hosts. Galloway (Reference Galloway, Foottit and Adler2018) attributes this behaviour to survival by limiting the attention of hosts to groom in response to irritation. Most species that are darkly pigmented and heavily sclerotised may spend more time on their hosts, whereas those that are pale yellow and weakly sclerotised usually are considered to be nest fleas (Galloway Reference Galloway, Foottit and Adler2018).
Because of the prevalence of zoonotic diseases in ground squirrel populations, a focus on the relationship between these hosts and their fleas is important to understand the risk of flea-borne pathogens to wildlife and humans and the impact of ectoparasites on host health (Raveh et al. Reference Raveh, Neuhaus and Dobson2015). Compared to their southern counterparts, Arctic ground squirrels have a considerably less diverse flea fauna. Although many flea species have been recorded as incidental occurrences, only two extant species of primary parasites of Arctic ground squirrels are known: Oropsylla alaskensis (Baker) and O. idahoensis (Baker) (Ceratophyllidae) (Nadler and Hoffman Reference Nadler and Hoffmann1977; Holland Reference Holland1985; Lewis and Eckerlin Reference Lewis and Eckerlin2024).
Fossil records of fleas
Siphonaptera are represented by approximately 2500 described species (Zhu et al. Reference Zhu, Hastriter, Whiting and Dittmar2015; Durden and Hinkle Reference Durden, Hinkle, Mullen and Durden2018), and they are hypothesised to have evolved from Cretaceous-aged mecopterids (scorpionflies), although the relationship remains a subject of ongoing research (e.g., Zhao et al. Reference Zhao, Wang, Bashkuev, Aria, Zhang and Zhang2020). Flea-like arthropods have been described from Cretaceous deposits, but collections of true “modern” fleas have been primarily from Baltic and Dominican amber (Beaucournu and Wunderlich Reference Beaucournu and Wunderlich2001; Poinar Reference Poinar2015).
Palaeoentomological studies of fleas have largely focused on their presence at archaeological sites and their role as vectors for pathogenic micro-organisms. The transmission of bubonic plague, Yersinia pestis (Lehmann and Neumann) van Loghem (Yersiniaceae), from the Oriental rat flea, Xenopsylla cheopis Rothschild (Siphonaptera: Pulicidae), is likely the best-known example of flea-based zoonosis from archaeological records (Panagiotakopulu Reference Panagiotakopulu2004). The biogeography of this flea is tied largely to major port cities and trade routes that facilitated the movement of its main host, the black rat, Rattus Linnaeus (Rodentia: Muridae). This flea, although now a lesser vector of bubonic plague, is still a vector of murine typhus, Rickettsia typhi (Wolbach and Todd) Philip (Rickettsiales: Rickettsiaceae), from natural reservoirs in rat populations, Rattus spp. (Christou et al. Reference Christou, Psaroulaki, Antoniou, Toumazos, Ioannou and Mazeris2010). Similarly, the origin and introduction of the human flea, Pulex irritans Linnaeus (Siphonaptera: Pulicidae), has been a focus of research linking the early development of synanthropic environments to the spread of disease and guiding interpretations of past sanitary conditions (Buckland and Sadler Reference Buckland and Sadler1989; Bain Reference Bain2004; Panagiotakopulu and Buckland Reference Panagiotakopulu and Buckland2017). Panagiotakopulu and Buckland (Reference Panagiotakopulu and Buckland2017) summarize comprehensive overview of European pests, including the human flea.
Records of fleas from Quaternary localities in east Beringia are almost completely absent. Matthews and Telka (Reference Matthews, Telka, Danks and Downes1997) note that no Siphonaptera remains had been collected from Quaternary sediments from the Yukon and adjacent regions of Alaska at the time of publication. Subsequently, Zazula et al. (Reference Zazula, Froese, Elias, Kuzmina and Mathewes2007) recorded one individual, identified only as Siphonaptera, from an Arctic ground squirrel midden preserved in permafrost at Quartz Creek, central Yukon Territory. A similar absence of flea collections from west Beringian studies exists. The insect’s small size and its restriction to host bodies and nests are likely the limiting factors contributing to the rarity of their collection from palaeontological deposits.
Modern records of thrips
Of the approximately 6000 known extant species of Thysanoptera, most feed on living plants, others feed on fungal hyphae, and about 100 species are predators of other arthropods (Grimaldi et al. Reference Grimaldi, Shmakov and Fraser2004; Mound Reference Mound2005; Wang et al. Reference Wang, Mound, Hussain, Arthurs and Mao2022). Of the 147 species of thrips reported in Canada, 33 are introduced, and five of these are found only indoors (Foottit and Maw Reference Foottit and Maw2019). Among recent publications on thrips in Canada, most studies have focused on the economic impact of thrips as agricultural pests (e.g., Nystrom and Syme Reference Nystrom and Syme1994; Opit et al. Reference Opit, Peterson, Gillespie and Costello1997; Nakahara and Foottit Reference Nakahara and Foottit2007). Records of Thysanoptera are limited from Yukon Territory. Turney et al. (Reference Turney, Altshuler, Whyte and Buddle2018) report thrips from the forest–tundra ecotone of subarctic Yukon but identify specimens only as “Thysanoptera”.
Fossil records of thrips
Thrips (Thysanoptera) constitute an order of small-bodied insects that can have fully developed, reduced, or absent wings (Mound Reference Mound2005). The earliest known thrips are reported from late Triassic deposits in Virginia and Kazakhstan. By the early Cretaceous, thrips become considerably more abundant in the fossil record (Grimaldi et al. Reference Grimaldi, Shmakov and Fraser2004).
The modern fauna is dominated by taxa that derived from ancestors that are 40–50 million years old (Grimaldi et al. Reference Grimaldi, Shmakov and Fraser2004). Fossils of pre-Quaternary thrips have been reported from Miocene-aged Dominican amber, which include the earliest known examples of the currently most species-rich family, Thripidae, and members of the family Phlaeothripidae. No records of thrips from Quaternary deposits, globally, have been published, and both Swain (Reference Swain1965) and Matthews and Telka (Reference Matthews, Telka, Danks and Downes1997) note this lack of material.
Modern records of mites from Urocitellus spp. ground squirrels
Hilton and Mahrt (Reference Hilton and Mahrt1971) provide the most complete record of mites associated with congeners of the Arctic ground squirrel, Columbian and Richardson’s ground squirrels, in Alberta, Canada. In that study, four true rodent parasites were identified in their assemblages, and the free-living faunas that make use of ground squirrel burrow complexes were also described. In total, four species of mites have been recorded in association with Columbian ground squirrels, Urocitellus columbianus Ord (Rodentia: Sciuridae) (Strandtmann Reference Strandtmann1949; Hilton and Mahrt Reference Hilton and Mahrt1971), and 15 mite species have been reported to be associated with the Richardson’s ground squirrel, U. richardsonii Sabine (Rodentia: Sciuridae) (McLeod Reference McLeod1933; Burgess Reference Burgess1955; Hilton and Mahrt Reference Hilton and Mahrt1971). Androlaelaps fahrenholzi Berlese (Mesostigmata: Laelapidae) is the only mite previously recorded in association with Arctic ground squirrels (Strandtmann and Wharton Reference Strandtmann and Wharton1958).
Fossil records of mites
Mites are a diverse group of arachnids traditionally placed in Subclass Acari, but they are now frequently classified into two superorders, Acariformes and Parasitiformes, that may not be each other’s closest relatives (e.g., Dabert et al. Reference Dabert, Witalinski, Kazmierski, Olszanowski and Dabert2010). Mites have palaeontological records spanning the last 410 Ma. The earliest known mites are recorded from Scotland’s Lower Devonian Rhynie Chert (Hirst Reference Hirst1923; Dunlop and Garwood Reference Dunlop and Garwood2018). Comprehensive pre-Quaternary palaeontological records of mites can be found in Sidorchuk (Reference Sidorchuk2018) and Dunlop et al. (Reference Dunlop, Penney and Jekel2020). Published Quaternary records are dominated by mites in the order Oribatida. Also known as moss mites or beetle mites, oribatid mites range from 0.15 to 2.0 mm (Behan-Pelletier and Lindo Reference Behan-Pelletier and Lindo2023), and their application in Quaternary palaeoenvironmental studies has relied on their diverse niche habitat requirements and well-sclerotised exoskeletons (Solhøy and Solhøy Reference Solhøy and Solhøy2000; Solhøy Reference Solhøy, Smol, Birks and Last2001).
Records of mites associated with Beringian rodents are limited to those from five narrow-skulled vole mummies collected in 1968 from permafrost in the basin of the headwaters of the Indigirka River, Yakutia, Russia, by the Zoological Institute of the Russian Academy of Sciences. From these specimens, Sosnina (Reference Sosnina1972) recorded Hirstionyssus Fonseca and Hyperlaelaps Zakhvatkin (Parasitiformes: Mesostigmata: Laelapidae), Myocoptes Claparède (Acariformes: Astigmata: Myocoptidae), and nine individuals of Myobiidae (Acariformes: Prostigmata). Of particular interest was the collection of the New World myobiid, Radfordia hylandi Fain and Lukoschus, which, because of its absence in the Old World, aided in the morphology-driven revision of the taxonomy of mummified narrow-skulled voles from Microtus gregalis egorovi Fejgin (Rodentia: Cricetidae) to a subspecies of a Nearctic species, Mynomes miurus egorovi (Fejgin) (Rodentia: Cricetidae) (Dubinina and Bochkov Reference Dubinina and Bochkov1996; Golenishchev Reference Golenishchev2008).
Materials and methods
The Arctic ground squirrel middens investigated in this study were collected during summer field seasons spanning 2009–2022 in the Klondike goldfields of central Yukon Territory, Canada. Samples were collected on placer gold mining property, with permission from placer mine owners and the Tr’ondëk Hwëch’in First Nation Territory whose traditional territory includes the study region. Six middens from four sites were analysed for this study (Fig. 1). The Arctic ground squirrel middens and invertebrate specimens presented in this study have been assigned Yukon Government accession numbers (Tables 1 and 2). All specimens and middens will be returned to the Yukon Palaeontology collection in Whitehorse, Yukon Territory.
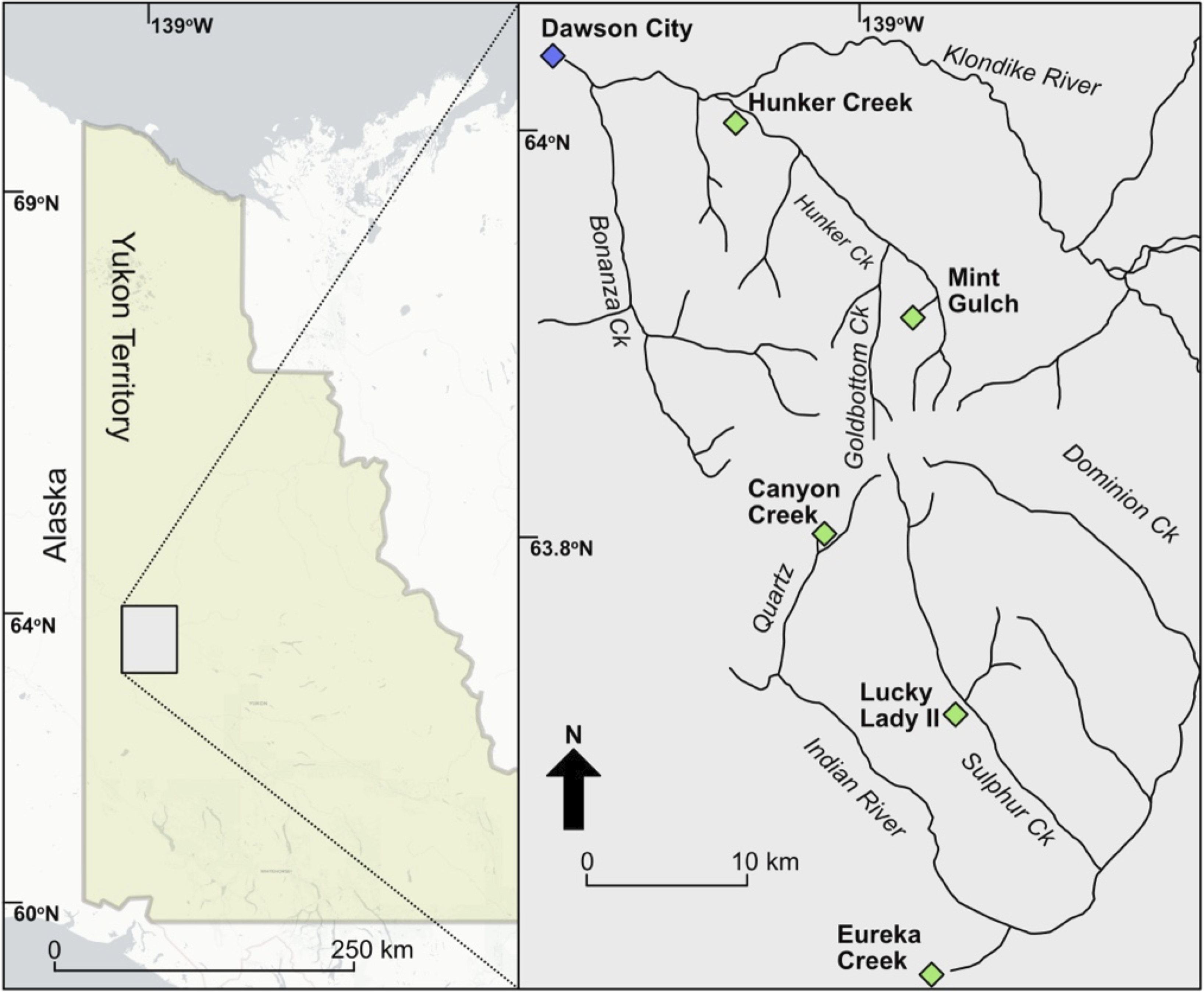
Figure 1. Map showing location of the Hunker Creek, Mint Gulch, Canyon Creek, Lucky Lady II, and Eureka Creek sites in Yukon Territory, Canada.
Table 1. Chronology for Arctic ground squirrel middens analysed in study. Radiocarbon dates (14C) were calibrated using OxCal, version 4.4 (Bronk Ramsey Reference Bronk Ramsey2009), and the Intcal20 radiocarbon calibration curve (Reimer Reference Reimer2020). All calibrated ages are presented at 2σ uncertainty and rounded to the nearest five years
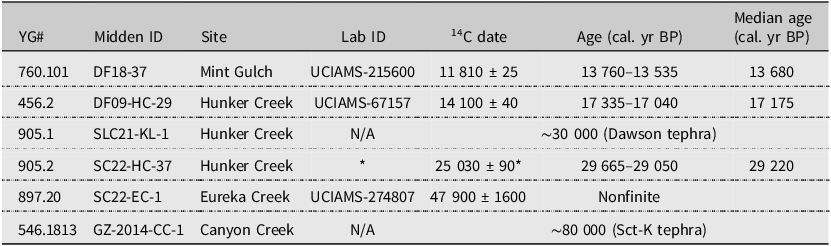
YG#, Yukon Government accession number, cal. yr BP, calibrated years before present.
*14C age for adjacent sample, SC22-HC-38 (UCIAMS-274808).
Table 2. Subfossil invertebrate data from the studied midden sites in Yukon Territory, Canada
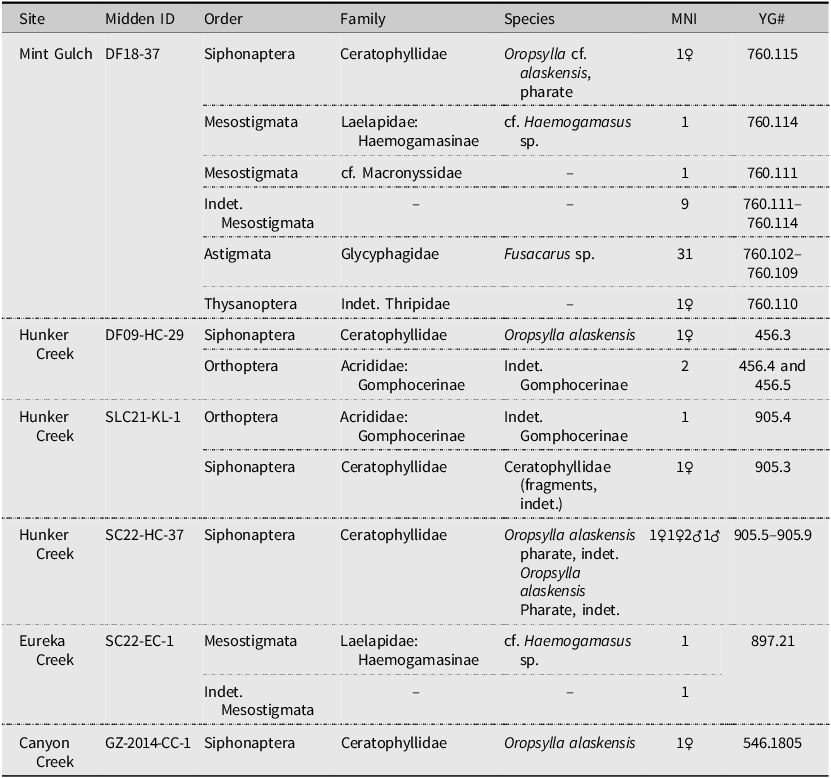
Abbreviations: Indet., indeterminate; MNI, minimum number of individuals; YG#, Yukon Government accession number.
Methods
We collected middens from permafrost exposures at Hunker Creek in 2009, 2021, and 2022, at Mint Gulch in 2018, at Canyon Creek in 2018, and at Eureka Creek in 2022. The middens were kept frozen at the University of Alberta, Edmonton, Alberta, until analysis.
In the laboratory, we thawed half of each sample, washed it through 500-μm and 150-μm sieves, and analysed all the fractions individually. The remaining sample was then stored at –20 °C in the Permafrost Archive Science Laboratory at the University of Alberta. We dried the residues at 40 °C and dispersed them among Petri dishes for sorting under a dissecting microscope. The invertebrate remains were sorted by S.L. Cocker and were prepared and identified by T. Galloway (Siphonaptera), H. Proctor (Acariformes and Thysanoptera), and J. Miskelly (Orthoptera). We present our specimens as minimum number of individuals.
Mites and thrips were cleared in lactic acid for 24 hours, slide-mounted in a polyvinyl alcohol mounting medium (BioQuip Products, Rancho Dominguez, California) and cured for four days at approximately 40 °C on a slide warmer. The morphology of slide-mounted individuals was compared to illustrations and diagnoses in the following taxonomic resources: for Mesostigmata, Baker (Reference Baker1999) and Lindquist et al. (Reference Lindquist, Krantz, Walter, Krantz and Walter2009); for Astigmata, Zakhvatkin (Reference Zakhvatkin1941) and OConnor (Reference OConnor, Krantz and Walter2009).
We initially were concerned that the fleas might disarticulate if standard processing techniques were used. One specimen was processed using methods described by Richards (Reference Richards1964), a technique originally developed for aphids but now used successfully for a wide variety of invertebrates, including fleas. The first specimen was placed directly into 10% potassium hydroxide (KOH) solution and maintained at room temperature overnight. The specimen was cleared successfully, and the remaining steps described by Richards (Reference Richards1964) were followed. This was followed by further clearing in lactophenol, secondary passage through 10% KOH, and then dehydration in acid–oil and oil–acid before the specimen was slide-mounted in Canada balsam, as described by Richards (Reference Richards1964). Subsequent specimens were processed, even when fragmented, using standard procedures, with only occasional disarticulation occurring. We cured the slides in a drying oven at 45°C for 4–6 weeks. Fleas were identified using keys and descriptions in Holland (Reference Holland1985) and Lewis and Eckerlin (Reference Lewis and Eckerlin2024). Additionally, specimens were compared with voucher slide-mounted specimens deposited in the J.B. Wallis/R.E. Roughley Museum of Entomology in the Department of Entomology, University of Manitoba, Winnipeg, Manitoba, Canada.
Orthopteran remains required no additional processing. Subfossil legs were identified using keys and illustrations in Otte (Reference Otte1981), Vickery and Kevan (Reference Vickery and Kevan1985), and Haberski et al. (Reference Haberski, Woller and Sikes2021) and by comparison with specimens deposited in the Royal British Columbia Museum, Victoria, British Columbia, Canada.
Chronology
The ages associated with our samples are presented in Table 1. Radiocarbon pre-treatment was completed at the University of Alberta, following a standard acid–base–acid methodology (e.g., Reyes et al. Reference Reyes, Froese and Jensen2010). The samples were then frozen, freeze dried overnight, and then stored in airtight sterilised vials. Carbon dioxide production, graphitisation, and measurement of radiocarbon abundance of all samples were completed at the Keck Carbon Cycle AMS facility (UCIAMS), University of California, Irvine (Irvine, California, United States of America).
Where samples were beyond the limit of radiocarbon dating, we made use of the tephrostratigraphic framework available in Yukon Territory (e.g., Davies et al. Reference Davies, Jensen, Froese and Wallace2016). Samples SLC21-KL-1 and SC22-EC-1 are associated with the Dawson tephra, which is dated to 25 300 14C years BP, approximately 30 000 cal. yr BP (29 055–29 470 cal. yr BP; Froese et al. Reference Froese, Westgate, Preece and Storer2002, Reference Froese, Zazula and Reyes2006; Demuro et al. Reference Demuro, Roberts, Froese, Arnold, Brock and Ramsey2008; Davies et al. Reference Davies, Jensen, Froese and Wallace2016). Additionally, sample GZ-2014-CC-1 was collected approximately 1 m below tephra sample AVR-14-CC-1, which has been identified as Sheep Creek Klondike tephra (SCt-K). This tephra has been dated to late MIS 5a or early MIS 4 using single-grain optically stimulated luminescence to obtain an age of approximately 80 000 years BP (Westgate et al. Reference Westgate, Preece, Froese, Pearce, Roberts and Demuro2008; Demuro et al. Reference Demuro, Arnold, Froese and Roberts2013).
Results
We present the remains of four underrepresented invertebrate groups from Quaternary deposits in Beringia. Data in Table 2 are presented as minimum number of individuals (noted as “MNI” below) and are separated by site and by individual midden.
Siphonaptera
The Canyon Creek midden (GZ-2014-CC-1) is the oldest in this study, at approximately 80 000 years BP, and the Mint Gulch midden (DF18-37) is the youngest at 13 680 cal. yr BP.
All identifiable flea specimens presented in this study are Oropsylla alaskensis (Ceratophyllidae). Of the nine fleas collected, five are females and four are males. Most specimens were heavily sclerotised (Fig. 2A and C), with the exception of fleas from SC22-HC-37 (Fig. 2D), four of which (3♂, 1♀) are pharate (Fig. 2B), and around which there was particulate materials, perhaps associated with their cocoons. Two pharate males could be identified as O. alaskensis. One female from this site was well sclerotised and identified as O. alaskensis. The male from SLC21-KL-1 was badly fragmented, with only head and thoracic segments present in pieces; it could be identified only as Ceratophyllidae. Specimen DF18-37 is a pharate female Oropsylla cf. alaskensis. The hilla of the spermatheca in this specimen is partially collapsed, and measurements of the hilla could not be made. Although the abdomen of DF09-HC-29 is slightly retracted, the hilla and posterior margin of STVII are sufficiently intact to identify this specimen as O. alaskensis. Specimen GZ-2014-CC-1 is the oldest specimen in the series (∼80 000 years old) and is a fully sclerotised female. It is in remarkably good condition despite being separated into two pieces at the posterior abdomen (Fig. 2A). The spermatheca (cracked) and STVII are sufficiently intact and characteristic of O. alaskensis (Fig. 3B).
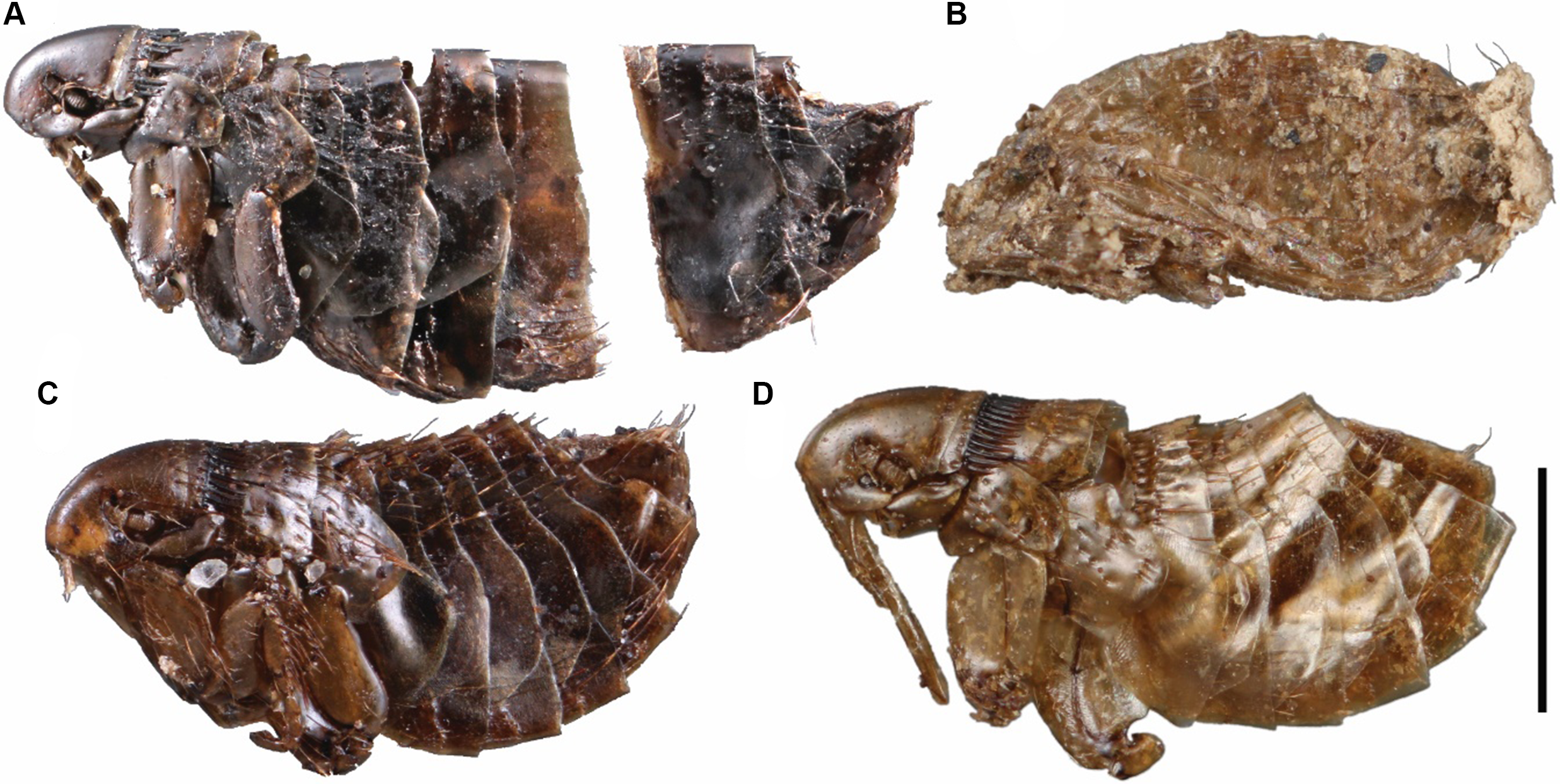
Figure 2. Oropsylla alaskensis flea specimens collected from middens of Urocitellus parryii in the Klondike goldfields, Yukon Territory, Canada: A, female, GZ-2014-CC-1; B, pharate male, SC22-HC-37; C, female, DF09-HC-29; and D, female, SC22-HC-37. Scale bar is 1 mm.

Figure 3. Thrips and flea specimens: A, indeterminate female Thysanoptera (ovipositor is apparent at the terminus of the abdomen) Thripidae (DF18-37); B, abdomen of a female Oropsylla alaskensis showing the characteristic spermatheca and margin of STVII.
Orthoptera
We identified three grasshopper legs (Fig. 4) from middens DF09-HC-29 (MNI = 2) and SLC21-KL-1 (MNI = 1) as belonging to short-horned grasshoppers (Acrididae) and, more specifically, slant-faced grasshoppers (Gomphocerinae). Only five known species of this subfamily are known to occur in Yukon Territory, but the absence of diagnostic features required for species identification meant the three specimens could be identified only to indeterminate subfamily Gomphocerinae.

Figure 4. Grasshopper (Acrididae: Gomphocerinae) specimens collected from middens of Urocitellus parryii in the Klondike goldfields, Yukon Territory, Canada: A and B, DF09-HC-29; and C, SLC21-KL-1. Scale bars are 1 mm.
Thysanoptera
One Thysanoptera specimen was collected from midden DF18-37 from Mint Gulch (Fig. 3A). The specimen preserves features characteristic of thrips (a rounded head with small, rounded eyes and a short tarsus without claws) but lacks features to enable identification further than indeterminate Thripidae (Laurence Mound, Commonwealth Science and Industrial Research Organization, Canberra, Australia, personal communication).
Mites: Mesostigmata and Astigmata
In all, 44 mites were collected from two middens, SC22-EC-1 (MNI = 2) and DF18-37 (MNI = 42) and represent members of Astigmata and Mesostigmata in various life stages. Identification to finer taxonomic levels using keys was problematic because of many missing or obscured features. Midden SC22-EC-1 from Eureka Creek contained two individuals of Mesostigmata, of which one lacks characters to identify it further than indeterminate Mesostigmata (male). The other was identified as cf. Haemogamasus sp. (Laelapidae: Haemogamasinae) (male or a nymph), based in part on the dense setae covering the idiosoma (= hypertrichy; Fig. 5A). Midden DF18-37 from Mint Gulch contained individuals of both Mesostigmata (MNI = 11) and Astigmata (MNI = 31). Individuals of Mesostigmata are represented by cf. Haemogamasus sp. (MNI = 1; possible female), cf. Macronyssidae (MNI = 1; female), and indeterminate Mesostigmata (MNI = 9; two nymphs, one possible male, one possible female, and five unclear as to sex or life stage). Astigmata are represented among the specimens solely by the genus Fusacarus sp. (Glycyphagidae), of which three specimens are males, seven are females, and 21 are indeterminate as to sex or life stage (Fig. 5B–D). This genus is unusual among Glycyphagidae in being strongly sclerotised rather than having a soft cuticle (OConnor Reference OConnor, Krantz and Walter2009). Female Fusacarus spp. have a very distinctive oviporus (egg opening) region with strongly sclerotised shields (Fig. 5C; Halliday and Walter Reference Halliday and Walter2006). Like Fusacarus laminipes Michael, Reference Michael1903, F. volantis Volgin, 1971, and an undescribed species of Fusacarus from Algeria (Grandjean Reference Grandjean1953), both sexes of our specimens have well-developed flanges on the ventral sides of the femora and genua of first and second pairs of legs (Fig. 6).
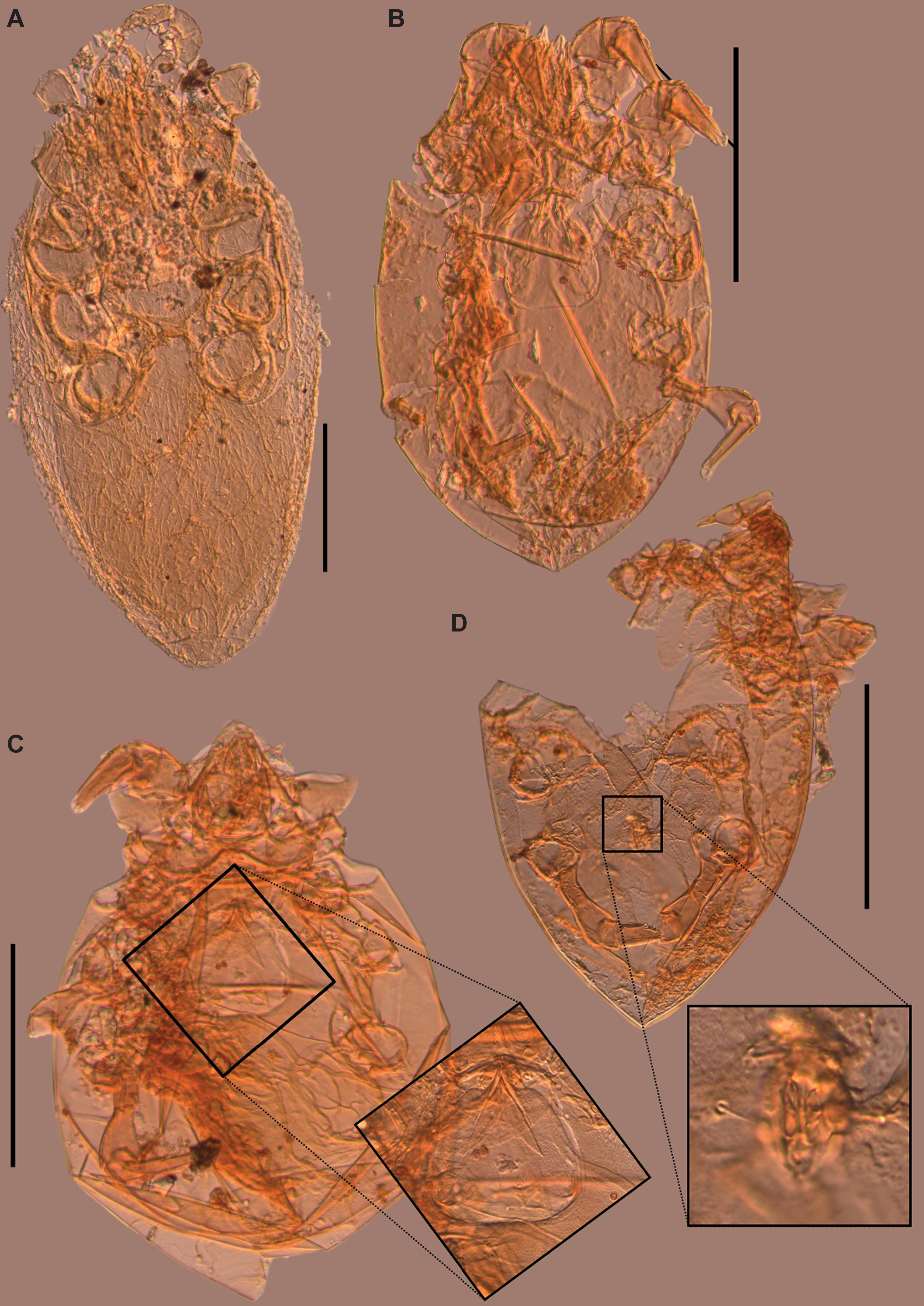
Figure 5. Mites collected from middens of Urocitellus parryii in the Klondike goldfields, Yukon Territory, Canada: A, cf. Haemogamasus sp. (male or a nymph), SC22-EC-1; B, Fusacarus sp. (female), DF18-37; C, Fusacarus sp. (female; inset showing distinctive genital region), DF18-37; and D, Fusacarus sp. (male; inset shows genital region), DF18-37. Scale bars are 200 µm.
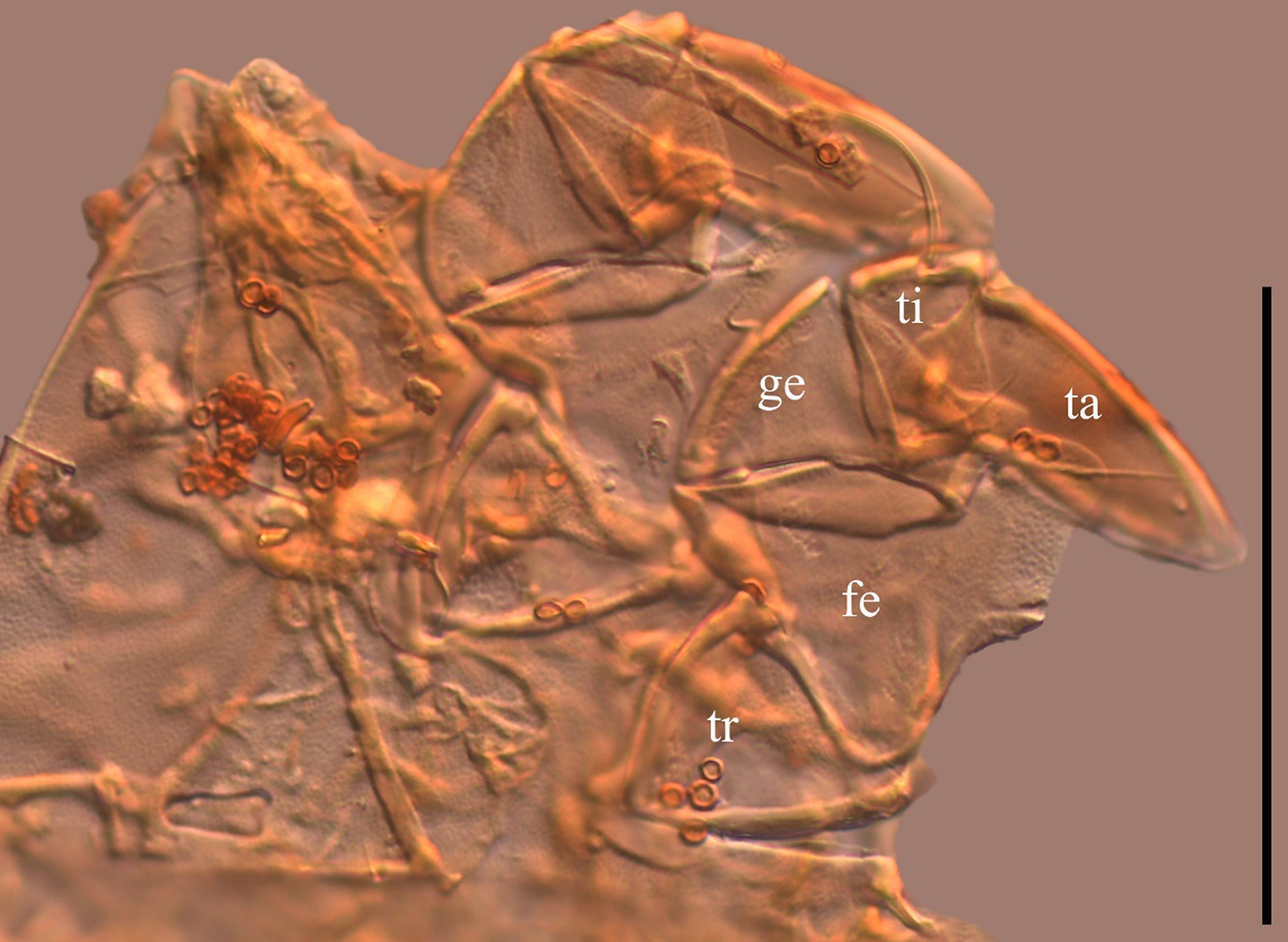
Figure 6. Anterior two legs of a male Fusacarus sp. specimen (stacked image). Scale bar is 100 µm. Abbreviations: tr, trochanter; fe, femur; ge, genu; ti, tibia; ta tarsus.
Discussion
Palaeoecology and taxonomic remarks
We present the records of four rarely reported invertebrate taxa from deposits in east Beringia and the first record of grasshoppers and thrips from Pleistocene Beringia.
Fleas
Due to taxonomically distinctive features of the preserved fleas, we are confident in their identification as the ceratophyllid, Oropsylla alaskensis. This is therefore evidence of an at least 80 000-year relationship between this taxon and Arctic ground squirrels. Oropsylla alaskensis is an amphi-Beringian species that is a specific parasite of Arctic populations of U. parryii (Holland Reference Holland1963, Reference Holland1985; Hopla Reference Hopla1965). Females of O. alaskensis can be difficult to distinguish from those of Oropsylla idahoensis Baker (Siphonaptera: Ceratpohyllidae), especially in the absence of associated males. However, the spermatheca in the latter is relatively small (Lewis and Eckerlin Reference Lewis and Eckerlin2024). In a series of 15 females of O. idahoensis from Malheur National Forest (Oregon), Fish Lake (Washington state, United States of America), and southwest British Columbia (Canada), all from Cascade golden-mantled ground squirrels, Callospermophilus saturatus Rhoads (Rodentia: Sciuridae), and associated with clearly identifiable males, the mean length and width of the spermathecae are 0.129 × 0.056 mm (ranges, 0.125–0.145 × 0.055–0.065 mm, respectively). One female collected from U. parryii at Toolik Lake (Alaska, 4 August 1989) has spermathecal dimensions of 0.19 × 0.075 mm. Spermathecae from three females from Yukon goldfield deposits in the present study had spermathecal dimensions ranging from 0.165 × 0.19 to 0.065 × 0.09 mm, similar to the Toolik Lake female. There are also biogeographic considerations, especially because O. idahoensis is known today to infest subarctic populations of U. parryii (Holland Reference Holland1985; Lewis and Eckerlin Reference Lewis and Eckerlin2024). In fact, Hopla (Reference Hopla1965) presented records where both O. idahoensis and O. alaskensis infested the same individuals of U. parryii. However, this occurs in subarctic regions where forest had been cleared and where Arctic ground squirrels subsequently had invaded (Hopla Reference Hopla1965). It is apparent today that O. idahoensis does not occur in truly Arctic habitats and is unlikely to have occurred in the region’s steppe–tundra at the times being considered in the present study. Holland (Reference Holland1963) believed that the range of Arctic ground squirrels once extended much further south than it does today, perhaps even overlapping with the ranges of species of Rocky Mountain ground squirrels. Lewis and Eckerlin (Reference Lewis and Eckerlin2024) note that O. idahoensis has the least-specific host range of Oropsylla spp., infesting four species of prairie dogs, Cynomys Rafinesque (Rodentia: Sciuridae), and 14 species of ground squirrels.
Mites
Additional parasitism is suggested by the presence of the mite cf. Haemogamasus sp. from midden SC22-EC-1 (Eureka Creek, ∼30 000 cal yr BP), because Haemogamasus spp. are commonly associated with rodent populations (e.g., Whitaker and Wilson Reference Whitaker and Wilson1974; Whitaker et al. Reference Whitaker, Walters, Castor, Ritzi and Wilson2007; Herrin and Sage Reference Herrin and Sage2012). Most species of the astigmatan family Glycyphagidae have deutonymphs that are highly modified for phoresy on mammals (OConnor 2009). Stages other than the deutonymph live off the phoretic host, usually in the host’s nest. Diet of the free-living stages is not known for most species (OConnor 2009). Fusacarus spp. have been recorded in nests of birds (Fain and Philips Reference Fain and Philips1977), moles (Michael Reference Michael1903), flying squirrels (Volgin Reference Volgin1971a, Reference Volgin1971b) and in leaf litter and tree holes that had not obviously been recently occupied by a mammal (Grandjean Reference Grandjean1953; Halliday and Walter Reference Halliday and Walter2006).
Grasshoppers
Diagnostic features required for definitive species identification were absent in the orthopterid remains collected in this study. However, the family Tetrigidae was excluded based on the size of the legs. The shape of the femora is consistent with the subfamily Gomphocerinae (Acrididae). The femora are slender and evenly tapering, whereas the femora of potential species of Melanoplinae and Oedipodinae are proportionally wider with a more curving outline. The size and proportions of the femora were compared to specimens of Gomphocerine species known to occur in Yukon Territory and Alaska, and all species were broadly similar when sexual dimorphism was considered. Definitive species identification of Gomphocerinae requires examination of head structure (especially foveolae), antennae, fore-tibiae, tibial spurs, and tegmina (Vickery and Kevan Reference Vickery and Kevan1985). None of these features are available for the subfossil legs. Using dichotomous keys, Pseudochorthippus curtipennis (Harris), Aeropedellus arcticus Hebard (Orthoptera: Acrididae), and A. clavatus (Thomas) (Orthoptera: Acrididae) are the only three taxa that use features of the hind legs to distinguish them (Haberski et al. Reference Haberski, Woller and Sikes2021). Pseudochorthippus curtipennis has a black femur tip, whereas A. arcticus and A. clavatus are distinguished by their lack of black colouration on the femur tip. Neither Chloealtis abdominalis (Thomas) (Orthoptera: Acrididae) nor Bruneria yukonensis Vickery (Orthoptera: Acrididae) are included in this part of the key, but reference to images and specimens suggest that neither species has a particularly dark femur tip.
Of the five members of Gomphocerinae potentially present in Yukon Territory, only B. yukonensis is recognised as a species endemic to Yukon. This taxon is known primarily from southern Yukon, with the holotype recorded from the shores of Lake Laberge. Although initially appearing inconsistent with environments of the mammoth steppe, an additional label on this specimen notes that it was recovered from a “deep creek, no trees, grassy slope burned out by sun” by collector D. Marsh (Vickery Reference Vickery, Danks and Downes1997). Specimens collected since 1997 have likewise been associated with native grassland on south-facing slopes (specimens in Royal British Columbia Museum). These Yukon grasslands are home to relict invertebrate species of steppe–tundra environments such as the weevil, Connatichela artemisiae Anderson (Coleoptera: Curculionidae), another Beringian endemic (Anderson Reference Anderson1984). As with C. artemisiae, B. yukonensis may have been more widely distributed during the Pleistocene, but due to the lack of fossil material, this cannot be confirmed.
The presence of grasshoppers in Arctic ground squirrell middens DF09-HC-29 and SLC21-KL-1 suggests palaeoenvironmental conditions at Hunker Creek. The following is a summary of habitat preferences for four Gomphocerinae (slant-faced grasshoppers) species found in Yukon today: Aeropedellus arcticus (Arctic club-horned grasshopper) has been recorded from turfy limestone tundra with distribution across Alaska, Yukon, and Northwest Territories, Canada (Catling Reference Catling2008). Aeropedellus clavatus (club-horned grasshopper) is primarily found in dry, grassy, and often sandy areas from prairies to alpine tundra (Catling Reference Catling2008). Bruneria yukonensis (Yukon grasshopper) is a Yukon endemic found on open grassy slopes, with a known distribution restricted to Yukon Territory (Catling Reference Catling2008). Chloealtis abdominalis (cow grasshopper) has a large geographic range from the Great Lakes to Alaska and can be found in parklands and dry forests with grassy clearings (Catling Reference Catling2008). Pseudochorthippus curtipennis (marsh meadow grasshopper) is widespread across North America, with habitats spanning heath bogs, fens, marshes, dry prairies, and tundra (Vickery and Kevan Reference Vickery and Kevan1985; Catling Reference Catling2008).
All gomphocerine grasshoppers have habitat preferences that could align with conditions found in ice-free Yukon Territory during the Pleistocene. The environment would not have been a homogenous ecotone because the mammoth steppe encompasses a range of environments, from tundra-like conditions to more temperate grasslands, and would have featured not only open grassy plains but also localised shrublands, wetlands, and riparian systems (Guthrie Reference Guthrie, Hopkins, Mathews, Schweger and Young1982).
Considering the factors above, we speculate that the specimens identified here (Fig. 4) may represent individuals of P. curtipennis for two main reasons: (1) their current Beringian distribution, which is consistent with other Beringian taxa (Shafer et al. Reference Shafer, Cullingham, Côté and Coltman2010) and (2) the dark colouration at the tip of the femur (Catling Reference Catling2008; Haberski et al. Reference Haberski, Woller and Sikes2021). It has been noted that this pigmentation can fade over time on modern pinned specimens and so variation in femur tip colouration may occur (Adam Haberski, Mississippi State University, Starkville, United States of America, personal communication). Specimens of P. curtipennis often have only the knees showing pigmentation, similar to the subfossil specimens presented here. Even though Catling (Reference Catling2008) does not consider P. curtipennis a Beringian species, it occurs in a diversity of wet, grassy habitats, including bogs, fens, marshes, grassland, and tundra. Although confidence cannot be applied to this identification, P. curtipennis appears to be the most likely candidate for the subfossil specimens. We could consider the application of ancient DNA to help answer this question. However, the main barrier to this approach is the availability of appropriate reference genomes on databases, such as GenBank NCBI (National Center of Biotechnology Information). Currently, none of the potential species of subfamily Gomphocerinae have this information, although recent museum specimens are available for all species. Without sequencing mitogenomes of modern Gomphocerinae, the lack of reference data will prevent molecular-level species identification and will limit assignment to higher taxonomic classifications. Smith et al. (Reference Smith, Kamiński, Kanda, Sweet, Betancourt and Holmgren2021) addressed a similar issue for Pleistocene-aged tenebrionid beetles, which provides an important basis for the increased application of ancient DNA to subfossil insect populations.
Thrips
Thrips are a primarily phytophagous group, but some species are fungivores, and others are invertebrate predators (Mound Reference Mound2005). Without increased taxonomic resolution, the presence of only one thrips specimen is generally uninformative for palaeoecological reconstructions. However, this specimen still represents the first record of thrips in Beringian records and, apparently, in Quaternary records globally.
Unique taphonomic setting
Permafrost has been shown to provide excellent conditions for the preservation of ancient life (Fisher et al. Reference Fisher, Tikhonov, Kosintsev, Rountrey, Buigues and van der Plicht2012; Murchie et al. Reference Murchie, Monteath, Mahony, Long, Cocker and Sadoway2021; Monteath et al. Reference Monteath, Kuzmina, Mahony, Calmels, Porter and Mathewes2023). The results presented here show that middens of Arctic ground squirrels continue to preserve unique palaeoecological records (Zazula et al. Reference Zazula, Froese, Elias, Kuzmina and Mathewes2007, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2011; Gaglioti et al. Reference Gaglioti, Barnes, Zazula, Beaudoin and Wooller2011; Langeveld et al. Reference Langeveld, Mol, Zazula, Gravendeel, Eurlings and McMichael2018). These hibernacula are built to ensure successful thermoregulation during a prolonged winter (Chappell Reference Chappell1981). This association is so crucial for Arctic ground squirrel survival that the animals do not occur in areas of Yukon Territory where permafrost tables are too high, preventing burrowing depths to at least one metre (Buck and Barnes Reference Buck and Barnes1999).
The ecological impact of ground squirrel burrow complexes to their local ecosystem should not be underestimated (Hansell Reference Hansell1993). Arctic ground squirrels are not the only inhabitants of their burrow complexes. Cocker et al. (Reference Cocker, Zazula, Hall, Jass, Storer and Froese2024) previously reported Pleistocene records of arvicoline rodents, providing the first chronologically constrained record of these rodents from the region. Invertebrate records, dominated by Coleoptera, have been reported from several middens both in the Klondike (Zazula et al. Reference Zazula, Froese, Elias, Kuzmina and Mathewes2007, Reference Zazula, Froese, Elias, Kuzmina and Mathewes2011) and from Siberia (Zanina et al. Reference Zanina, Gubin, Kuzmina, Maximovich and Lopatina2011). These records are mostly the result of co- or reinhabitation by arvicolines or may be due to scavenging and cannibalistic behaviours exhibited in modern Arctic ground squirrel populations (Musacchia Reference Musacchia1954; McLean Reference McLean1983; Gillis et al. Reference Gillis, Morrison, Zazula and Hik2005; Zazula et al. Reference Zazula, Mathewes and Harestad2006). Although cached material is a direct result of Arctic ground squirrel behaviour, a large portion of material collected from these middens will be of incidental origin. An emphasis should be placed on the attractiveness of warm, below-ground hibernacula filled with dried plants, a seed cache, faecal pellets, and possibly carrion or a host to mites, fleas, grasshoppers, and other invertebrates. Once frozen, these middens become time capsules of information for less common taxa that might otherwise be lost in a rapidly accumulating loess landscape.
Direct comparisons can and should be made between the records presented here and the invertebrate records obtained from packrat middens of North and Central America (Hall et al. Reference Hall, Van Devender and Olson1988; Elias Reference Elias1990; Elias et al. Reference Elias, Mead and Agenbroad1992; Clark and Sankey Reference Clark and Sankey1999; Smith et al. Reference Smith, Kamiński, Kanda, Sweet, Betancourt and Holmgren2021). More than 3000 packrat middens have been studied, but the rarity of invertebrate assemblages relative to the abundance of plant macrofossils has meant fewer reports of invertebrate remains have been published. Elias (Reference Elias1990) discussed the taphonomic conditions that lead to the preservation of invertebrate remains in packrat middens by comparing subfossil insect faunas to the modern regional fauna. That author concluded that most specimens collected are inquiline in nature, co-inhabitants of rock-shelters where packrat middens are typically found, or both. Parallels can be drawn to the midden complexes of Arctic ground squirrels, but research on the modern invertebrate fauna associated with Arctic ground squirrel middens has yet to be conducted.
Quaternary entomology in an ancient DNA world
In the present study, we present several specimens that were challenging to identify due to fragmented preservation and the absence of diagnostic features. In Quaternary palaeoecology, ancient DNA has been used to identify remains, mainly of vertebrates, where traditional morphology-based identifications have not been possible. However, species identification is limited by the availability of reference genomes on databases such as GenBank NCBI, an issue noted in regards to our grasshopper specimens (see grasshopper section, above). Although projects have been undertaken to tackle this lack of data (Global Invertebrate Genomics Alliance Community of Scientists 2014), the diversity of invertebrate taxa proves to be a consistent barrier to the accumulation of these data. In lieu of genetic data, assignments are made to high taxonomic classifications that are not solely informative of the community structure and diversity.
To date, a few studies have attempted to extract DNA from ancient subfossil invertebrates. An important contribution by Thomsen et al. (Reference Thomsen, Elias, Gilbert, Haile, Munch and Kuzmina2009) established a nondestructive DNA-extraction method from invertebrates of various ages. In that study, the authors indicated success from museum specimens of historical origins (back to 1820 AD) but struggled to apply the method to Pleistocene-aged specimens. Despite these difficulties, subsequent advances in ancient DNA-extraction methods over the past 15 years should be considered and attempts to replicate this study should be made, particularly where a nondestructive method must be used. In situations where specimens are plentiful and destructive extraction methods can be used, Smith et al. (Reference Smith, Kamiński, Kanda, Sweet, Betancourt and Holmgren2021) highlight the successful extraction of endogenous DNA from samples as old as approximately 34 355 years old. Smith et al. (Reference Smith, Kamiński, Kanda, Sweet, Betancourt and Holmgren2021) provide important evidence to highlight the potential for palaeogenetic studies of past invertebrate populations. However, given the lack of genetic reference material, ancient DNA analysis of subfossil invertebrates remains in the early stages of development.
Alternative sources of subfossil material
We discuss the occurrence of taxa with strong host associations, namely fleas and parasitic and nest-associated mites. The context of their presence cannot be limited just to Arctic ground squirrels because previous studies have highlighted the occupation of ground squirrel burrow complexes by other rodents (Cocker et al. Reference Cocker, Zazula, Hall, Jass, Storer and Froese2024). Skeletal and faecal remains of lemmings and voles from Pleistocene Arctic ground squirrel middens show that arvicoline rodents at least temporarily inhabited these hibernacula either to pilfer seed caches or to make their homes in abandoned burrows. Given that all the identifiable flea specimens are the ceratophyllid, O. alaskensis, a known parasite of Arctic ground squirrels, this occurrence seems appropriate. However, the records of the parasitic mite cf. Haemogamasus presented here might represent an overlap in rodent habitation and may be from arvicoline rodents rather than from Arctic ground squirrels. In any interpretation of the data, the source of these invertebrates does not detract from the importance of rodent middens as valuable sources of underrepresented Pleistocene invertebrate data.
Biogeographic implications and challenges
Quaternary glaciations in North America have played a considerable role in the structure of present-day invertebrate communities (Matthews Reference Matthews and Danks1979). Of particular significance are regions, such as central Yukon Territory, that remained ice-free and would have been home to invertebrates for prolonged periods of refugium (e.g., Morgan et al. Reference Morgan, Morgan, Ashworth, Matthews, Porter and Porter1983).
Of the four invertebrate groups we report in the present study, grasshoppers are the most represented in biogeographic studies. These mainly analyse the current distributions of grasshoppers (e.g., Vickery Reference Vickery1989) or use phylogenetic analysis to identify the timing of divergence and dispersal events (e.g., Contreras and Chapco Reference Contreras and Chapco2006; Shafer et al. Reference Shafer, Cullingham, Côté and Coltman2010). One limiting factor for understanding more recent biogeographic histories has been the preservation of subfossil specimens. Only five species of slant-faced grasshopper legs (subfamily Gomphocerinae) are known from present-day Yukon Territory, and so the preservation of subfossil specimens in middens DF09-HC-29 (MNI = 2) and SLC21-KL-1 (MNI = 1) presents the first opportunity to establish biogeographic histories of this subfamily from the region using direct subfossil evidence. However, as previously discussed, the omission of taxonomically identifiable features in these specimens resulted in a lack of species-level identification. If identified, these remains could provide direct evidence for species endemicity in the region.
With the exception of Oribatida (e.g., Behan-Pelletier Reference Behan-Pelletier, Danks and Downes1997), records of mites from northern ecosystems are poor. This is particularly true when considering taxa with specific habitat associations, such as those living in mammal burrows. As a result, few opportunities exist to study the biogeographic histories of northern mite populations. The issue is further exacerbated by poor preservation – for example, our subfossil mite specimens lacked features to allow identification to genus, except for Fusacarus. To date, only two specimens of Fusacarus have been reported from Canada. OConnor (Reference OConnor1981) identified a male and female from the nest of a lemming on Banks Island, Northwest Territories, collected in 1968 by W.R. Mason. Few records for North America are known to occur, in general. The subfossil specimens reported in the present study represent a first occurrence in Yukon Territory and are the earliest known examples of Fusacarus globally.
Commensal and parasitic relationships in palaeoecology
Palaeoecological records of commensal relationships and ectoparasites and their hosts are often lost during the process of taphonomy (Tapanila Reference Tapanila2008), and some entomologists argue that no commensal relationships cannot be identified from the fossil record (Zapalski Reference Zapalski2011). We will not discuss the merits and issues of describing palaeoecological relationships as commensal.
Extant species of both Fusacarus and Haemogamasus have close relationships with rodents. Members of Glycyphagidae, including Fusacarus spp., are typically found in the nests of mammals and birds (e.g., Volgin Reference Volgin1971a, Reference Volgin1971b; Fain and Philips Reference Fain and Philips1977). For example, a survey of troglobitic species from Crystal Lake Cave, Iowa, United States of America, recorded a Fusacarus sp. from a mouse nest (Peck and Christiansen Reference Peck and Christiansen1990). However, Halliday and Walter (Reference Halliday and Walter2006) described a species of Fusacarus from Australia, F. australis Halliday and Walter, that may not be associated with vertebrates. Although identification to species was not possible for our specimens of Fusacarus, the occurrence of this genus in the midden of an Arctic ground squirrel strongly implies that these specimens represent a commensal species (e.g., F. laminipes Michael or F. volantis Volgin) associated with mammal burrows rather than a free-living individual such as F. australis. Of additional geographical relevance is the description of F. volantis from the nest of a flying squirrel, Pteromys volans Linnaeus (Rodentia: Sciuridae), near Yuzhno-Sakhalinsk in far eastern Russia (Volgin Reference Volgin1971a, Reference Volgin1971b).
Identification of the mite, cf. Haemogamasus, and of the flea, O. alaskensis, provide more direct evidence of parasitism. As previously discussed, species of Haemogamasus are blood-feeding ectoparasites regularly associated with rodents (e.g., Whitaker and Wilson Reference Whitaker and Wilson1974; Whitaker et al. Reference Whitaker, Walters, Castor, Ritzi and Wilson2007; Herrin and Sage Reference Herrin and Sage2012), and O. alaskensis is one of two known flea species found on present-day Arctic ground squirrels (Nadler and Hoffman Reference Nadler and Hoffmann1977). These specimens provide an opportunity to study long-term host–parasite relations directly and to document the uncommon preservation of ectoparasites, in comparison to their endoparasite counterparts, from the fossil record. Quaternary records of endoparasites are most regularly recorded from coprolites (e.g., Beltrame et al. Reference Beltrame, De Souza, Araújo and Sardella2014; Tietze et al. Reference Tietze, Urquiza and Beltrame2021), whereas the record of ectoparasites often relies on damage to host integument (e.g., Moura et al. Reference Moura, Nascimento, de Barros, Robbi and Fernandes2021; Zonneveld et al. Reference Zonneveld, Wilson and Holroyd2024) or, as we present here, the preservation of rodents and their burrow or midden complexes (see also Sosnina Reference Sosnina1972). These records can serve to improve understanding of host–parasite relations through time, to establish the origin and evolution of these parasites, and to document the occurrence of parasite populations both temporally and spatially (Qvarnström et al. Reference Qvarnström, Niedźwiedzki and Žigaitė2016).
Conclusions
The middens of Arctic ground squirrels are a rich natural archive of life in Pleistocene Beringia’s mammoth steppe ecosystem. In addition to being a valuable source of botanical remains, these middens provide a unique opportunity to study previously underrepresented invertebrate communities. The current study presents the rare preservation of fleas and mites and the first records of thrips and grasshoppers from Quaternary Beringian deposits as a direct result of the favourable taphonomic conditions of middens. We subsequently establish the oldest known individuals of all the taxa presented.
We confirm that a strong host association between Arctic ground squirrels and the ceratophyllid flea, O. alaskensis, has existed for at least the last 80 000 years and provide evidence of a 30 000-year-old association between parasitic mites (cf. Haemogamasus sp.) and rodents. This study proves the use of midden analysis for understanding the origin and evolution of parasite–host relations and that middens are an important resource for palaeoparasitological studies.
We present the first account of the glycyphagid mite, Fusacarus sp., dating to 13 680 cal yr BP. The collection of legs from three slant-faced grasshoppers (subfamily Gomphocerinae) improves our understanding of the biogeographic history of grasshoppers in Beringia. These specimens should be the subject of an ancient DNA study to confirm the species-level identification. Genomic data from the specimens could also facilitate comparison to Yukon endemic species, such as the prairie-sage weevil, C. artemisiae, which is now less widely distributed than during the Pleistocene, thereby expanding our understanding of northern biodiversity during the Pleistocene. We recommend that the collection and analysis of Pleistocene Arctic ground squirrel middens continue to be a focus of Beringian palaeoecological studies to provide a holistic view of the mammoth steppe ecosystem.
Acknowledgements
The authors acknowledge and thank the Tr’ondëk Hwëch’in First Nation for allowing us to conduct this research on their traditional territories and to the placer gold mining community for granting access to their mines. The authors also thank A. Haberski, S. Cannings, and D. Johnson for their discussions surrounding grasshoppers, E. Francis and H. Stormer for their aid in picking and processing of invertebrate remains from the fossil middens, and L. Mound for aid in identifying the thrips specimen. The authors also thank Jordan Bannerman, Thilina Hettiarachchi, and Jillian Detwiler, University of Manitoba, for their aid in imaging our flea specimens. We thank Bruce Halliday, Australian National Insect Collection (Canberra), for sharing PDF copies of Volgin (Reference Volgin1971a) and its English translation Volgin (Reference Volgin1971b). The authors thank Fred Beaulieu and Wayne Knee, Canadian National Collection of Insects, Arachnids and Nematodes (Ottawa, Ontario), for gathering information on the Fusacarus specimens from the Northwest Territories. Thanks also to Michael Hastriter, Monte L. Bean Life Science Museum (Brigham Young University, Provo, Utah, United States of America), for early discussion on processing the fleas. We also thank Grant Zazula and Elizabeth Hall, Yukon Palaeontology Programme (Yukon Territorial Government, Whitehorse, Yukon Territory), for facilitating the analysis of the middens and for creating accession numbers. Funding for this project was provided by an NSERC Discovery Grant to H.C.P. and by University of Alberta Northern Research Awards to S.L.C. T.D.G. acknowledges the continued support of the Department of Entomology, Faculty of Agricultural and Food Sciences, University of Manitoba. The authors thank two anonymous reviewers for their comments and suggestions that improved the manuscript.
Competing interests
The authors declare that they have no competing interests.










