Introduction
Seeds of almost all Australian Acacia species have physical dormancy (PY) due to a water-impermeable epidermal layer of palisade cells in the testa (Burrows et al., Reference Burrows, Virgona and Heady2009, Reference Burrows, Alden and Robinson2018). Acacia seeds can remain viable and with PY unbroken after many decades in the soil and after storage in seed stores (Cavanagh, Reference Cavanagh1980). Following various natural events (e.g. fire) or nursery treatments (e.g. hot or boiling water), a very small part of the palisade layer (the lens) ‘pops’ almost instantaneously, thus breaking PY and allowing the seed to imbibe and germinate when moisture becomes available.
Rate of imbibition depends on water availability, seed structure and composition, water permeability of seed coat layers and temperature (Obroucheva, Reference Obroucheva1999). Imbibition is usually slower at lower temperatures, which is usually attributed to the increased viscosity of water at lower temperatures and to the effect of temperature on plant membranes (Booth and Bai, Reference Booth and Bai1999). The effect of temperature on imbibition of Acacia seeds has rarely been studied.
While there have been numerous studies of germination in species of the Mimosoideae and Caesalpinioideae, few have specifically investigated the initial imbibition phase of the process (e.g. Clemens et al., Reference Clemens, Jones and Gilbert1977; Wilson and Witkowski, Reference Wilson and Witkowski1998; Funes and Venier, Reference Funes and Venier2006; Commander et al., Reference Commander, Merritt, Rokich and Dixon2009; Venier et al., Reference Venier, Funes and García2012; Pound et al., Reference Pound, Ainsley and Facelli2014; Matos et al., Reference Matos, Ataíde and Borges2015; Jaganathan et al., Reference Jaganathan, Wu, Han and Liu2017, Reference Jaganathan, Yule and Biddick2018; Suleiman et al., Reference Suleiman, Dixon, Commander, Nevill, Bhat, Islam, Jacob and Thomas2018). Most of these studies have been of control and mechanically scarified seeds over relatively short time frames (e.g. 1–4 days). Longer term studies of imbibition in seeds with popped lenses, which is closer to what happens in nature and in most production nurseries, have rarely been reported. In addition, many of these studies used replicates but weighed all the seeds of a replicate together, rather than seeds individually. Thus, it is not known how imbibition proceeds at an individual seed level.
Many germination studies of acacias provide only a final germination percentage at a certain time point. Whether the germination rate was constant or variable over the assessment period was often not stated. In some studies, a time course for cumulative germination after different treatments was given (e.g. Doran and Gunn, Reference Doran, Gunn and Turnbull1987; Burrows, Reference Burrows1991; Burrows et al., Reference Burrows, Virgona and Heady2009). These studies have usually shown that after manual scarification (e.g. nicking, sandpapering) almost all viable seeds germinated after a few days as there were large and/or numerous water entry points. The most effective hot water (HW) or boiling water (BW) treatments can have similarly high final germination percentages. In some species the HW- or BW-treated seeds germinated almost as soon as, and at much the same rate as, the nicked seeds. In other species it took several weeks to reach this percentage (e.g. see Doran and Gunn, Reference Doran, Gunn and Turnbull1987; fig. 3). Burrows et al. (Reference Burrows, Virgona and Heady2009) considered that the difference in germination rate between the two treatment types (scarification versus HW/BW) was related to the size of the disruption in the palisade layer. For example, in A. melanoxylon seeds a small nick (just to cotyledon level at the distal end) produced an area of damage of about 1.3 mm2, while the internal diameter of a fully popped lens was only about 0.004 mm2 (about 300 times smaller) (Burrows et al., Reference Burrows, Virgona and Heady2009). Other authors have also discussed the popped lens as a possible constriction to water entering a seed when compared with imbibition after nicking or strong acid scarification (e.g. Tran, Reference Tran1979; Cavanagh, Reference Cavanagh1980). If the lenses of almost all Acacia seeds ‘pop’ immediately after a brief exposure to HW (Burrows et al., Reference Burrows, Alden and Robinson2018), why don't all these seeds germinate at much the same time, perhaps a few days after the bulk of the nicked seeds have germinated?
Burrows et al. (Reference Burrows, Alden and Robinson2018) identified two ways Acacia seeds might be non-dormant (‘soft seeded’) without a specific dormancy-breaking treatment. Firstly, the seeds of very few species are genuinely soft seeded (non-functional lens and very short or non-existent palisade cells, e.g. A. cambagei and A. harpophylla); secondly, species where the lens of at least some seeds can pop without a pronounced heat treatment (e.g. fire or boiling water). The imbibition of these soft-seeded species has not been previously studied.
We studied seed imbibition at the individual seed level for a wide range of Australian Acacia species to investigate why a staggered germination can occur when PY has clearly been broken at the lens. Specifically, we studied the following questions:
(i) Do non-treated seeds maintain their PY when exposed to warm and moist conditions for several weeks?
(ii) Do soft- and hard-seeded Acacia species have different imbibition patterns?
(iii) Does temperature influence the imbibition of Acacia seeds?
(iv) Can investigation of imbibition at the individual seed level help explain the staggered germination of HW- or BW-treated Acacia seeds?
(iv) If staggered germination is related to staggered imbibition, could this have ecological implications for establishment of Acacia seedlings?
Materials and methods
Seed samples
Seeds of 47 Australian species of Acacia sensu stricto were sourced mostly from a commercial supplier. The seeds had a median collection date of 2010, with experimental work carried out during in 2016–2018. Australian acacias are currently classified into seven sections (Table 1). Six to nine species were obtained from all sections except for the Lycopodiifoliae (smallest section, about 20 species) where two species were available from the supplier. Within each section, except the Lycopodiifoliae, a wide range of average seed mass was sampled. The wide taxonomic (all seven sections), geographic (all Australian states and territories) and seed mass variation (species average seed mass 3–245 mg) was associated with a wide variation in habitat (central Australian desert to coastal rainforest) and habit (shrubs to trees) [see Burrows et al. (Reference Burrows, Alden and Robinson2018), table 2, for additional detail]. Species were chosen on the basis of commercial availability, taxonomic distribution and seed mass. In addition, three species (A. cambagei, A. harpophylla, A. oswaldii) were selected as they had been noted to produce a large proportion of non-dormant seeds. The seed samples used in the study have been lodged with the Charles Sturt University Herbarium, Thurgoona Campus.
Table 1. Various parameters associated with the imbibition of 47 Australian Acacia species.

Species are divided into their taxonomic sections. Seed mass* = seed plus aril where present. C % increase mass* - excluding the small percentage of imbibed seeds. HW % increase mass – only fully imbibed seeds before germination. ** these values for A. cambagei and A. harpophylla (soft-seeded species) are the control imbibition t 50 values. C, control; HW, hot water treated. na, not applicable.
Imbibition of 47 Acacia species, non-treated and HW-treated seeds
To investigate the rate of imbibition two treatments were used, a control and a HW treatment. Seeds with obvious insect holes or cracks in the testa were not used. For the control treatment, 30 seeds of each species were selected at random and then divided into 10 seed replicates. For the HW treatment, 30 seeds per species were placed in a 175 ml container, which then had 75 ml of water at 95°C poured over them. The seeds were left in the water for 1 min before it was poured off and the seeds spread to cool and dry. The water had cooled to about 90°C at the end of the 1 min treatment. The control and HW-treated seeds were placed on moistened paper towel in 90 mm diameter Petri dishes. The paper towel was folded so that there were seven layers of paper below the seed and one above. This arrangement was used as some HW-treated seeds without an aril and with a water-repellent cuticle appeared to have a low initial rate of moisture uptake through the popped lens if the seed only sat on top of the paper. Seeds were individually weighed (to 0.0001 g) on a near daily basis, until all or almost all HW-treated seeds had imbibed. The seeds were considered imbibed when their mass had increased by at least 100%. The seeds of the soft-seeded species A. cambagei and A. harpophylla were weighed hourly during the first 6 h and then daily after that. Seeds were maintained at 21–22°C in the dark. Imbibition t 50 values (i.e. time for 50% of seeds in a replicate to imbibe) were calculated for each replicate. Any seeds that had fully imbibed in the first 24 h were inspected under a dissecting microscope for signs of damage (e.g. seed coat fractures, holes in the seed coat from insect damage). Any seeds that had not imbibed by the end of the experiment had their viability assessed with tetrazolium chloride (TTC). Most seeds had arils attached and, if present, these were left on the seeds as in some species the arils were very firmly attached and forcibly removing them could have fractured the hilum and the area around it, thus possibly breaking the palisade layer. For this paper, unless specified, ‘seed’ is used in the sense of ‘seed plus aril’. The results of this experiment are presented in Table 1 with no formal statistical analysis.
Table 2. ANOVA results to compare differences between imbibition t 50 at three different incubation temperatures in 11 Acacia species

Cells with no results are where the response did not vary between temperature treatments. Values in bold are where significantly different responses were recorded between the three temperatures (P < 0.05).
Statistical analysis of possible correlation between imbibition t50 and seed structure
We tested for significant correlation between average HW imbibition t 50 and (i) average seed plus aril mass, (ii) average popped lens area, (iii) average popped lens morphology rating [0, unpopped; 1, mound; 2, mound with cracks; 3, tube; 4, complete detachment; for more detail see Burrows et al. (Reference Burrows, Alden and Robinson2018), fig. 4], (iv) average testa palisade thickness and (v) average total testa thickness, across the 47 species. We used Spearman's rank correlation statistic as there was no reason to expect that any relationship would be linear. Seed plus aril mass was based on the 60 seeds per species from the present study. The other four parameters could not be based on the seeds used in the present study (would have either destroyed seeds or disturbed the area around the hilum and lens) so data from Burrows et al. (Reference Burrows, Alden and Robinson2018) was used as this study used the same seed batches. Rank correlation was also investigated of average HW imbibition t 50 with average annual rainfall for 29 species for which a specific collection locality and average annual rainfall were known.
Imbibition of two provenances of Acacia melanoxylon
In a previous study, Burrows et al. (Reference Burrows, Virgona and Heady2009) investigated the germination of three Tasmanian provenances of A. melanoxylon. The experiments reported in Burrows et al. (Reference Burrows, Virgona and Heady2009) were carried out during 2003–2005, with date of seed collection unknown. After a short exposure to BW, seeds of provenance L08 had a slow and gradual germination (germination t 50 about 25 days), while those of provenance L21 germinated much more rapidly (germination t 50 about 15 days) [Burrows et al. (Reference Burrows, Virgona and Heady2009), fig. 4]. For both provenances final (40 days) germination percentages were very similar for both short BW and nicking treatments (84–91%). The seed lots used in this experiment were still available (stored at a near constant 20°C) and were processed as above to see if imbibition patterns correlated with previously recorded germination patterns. A nicking treatment was also performed and percentage germination was recorded. A Student's t-test was used to determine whether the average imbibition t 50 for HW-treated seeds of L08 differed significantly from that of L21.
Influence of temperature on seed imbibition of 11 Acacia species
To determine if incubation temperature influenced imbibition (and facilitate additional replication of the main experiment), non-treated and HW-treated seeds of 11 Acacia species were incubated at 15, 20 and 25°C. The species (A. aphylla, A. adoxa, A. browniana, A. cardiophylla, A. coolgardiensis, A. falcata, A. gracillima, A. leptoloba, A. mearnsii, A. patagiata, A. truncata) were selected from the main experiment to have a wide range of HW imbibition t 50 values, seed mass and popped lens morphology. Imbibed seeds are easily distinguished from non-imbibed seeds as the average mass increase of about 150% results in much larger seeds that are usually lighter in colour. Thus, this experiment was prepared as above (3 × 10 seeds per replicate) except that imbibition of the individual seeds was assessed visually rather than by weighing. We performed a single-factor ANOVA for each species to assess whether t 50 imbibition of HW-treated seeds was different between the three incubation temperatures. Assumptions of normality and homoscedasticity were assessed by inspection of the residuals (Zar, Reference Zar2010). Results for ANOVAs for species that showed no variation in the response were ignored, and all other results were tabulated for interpretation. When there was a significant overall effect, we used pairwise comparison of averages and the least significant difference test to determine which temperature treatments had significantly different average imbibition t 50.
To compare the overall effects of HW and temperature on final percentage imbibition (control and HW-treated seeds) for the 11 select species, we performed a two-factor generalized linear model with a binomial response (imbibed or not) comparing between temperatures (15, 20 or 25°C) and treatment (control or HW). To further investigate the effects of temperature, we performed a single-factor generalized linear model with a binomial response comparing per cent imbibition after 1 day (HW-treated) and per cent germination (HW-treated) between seeds treated at the three temperatures. The models used maximum likelihood and were assessed for goodness of fit by considering the convergence status of the likelihood algorithm and sensibility of standard error estimates (small enough to keep confidence intervals interpretable). Significant effects were investigated using pairwise comparisons of the least parameter estimates and their Wald confidence intervals.
Relative contribution of seed and aril imbibition to total mass increase
The main experiment showed that non-treated seeds had a relatively wide variation in percentage increase in mass from being placed on a moist substrate. We investigated the relative contributions of the seed and the aril to this increase. Nine species (A. adoxa, A. blayana, A. cochlearis, A. elata, A. falcata, A. genistifolia, A. gilbertii, A. grasbyi, A. restiacea) had the arils separated from 30 seeds, split into 3 × 10 seed and aril replicates, placed on moist paper towel, weighed after 24 and 48 h and percentage increase in mass calculated.
Seed moisture content
Seed moisture content has been considered a factor in PY. For the 11 species used in the incubation temperature experiment, seeds were separated into 3 × 10 seed replicates and each seed was cut in half with a scalpel. The three replicates of seed halves were then weighed, dried at 100°C for 48 h, weighed again and percentage moisture content was calculated. A Student's t-test was used to determine if the average seed moisture content was significantly different between species with HW imbibition t 50 between 15 and 63 h and those with HW imbibition t 50 between 186 and 358 h.
Results
Imbibition of 47 Acacia species
Non-treated seeds
On average, 20.7% (median 13.3%) of control seeds imbibed, with six species having 0% imbibition and the two soft-seeded species having 100% imbibition (A. harpophylla and A. cambagei) (Table 1). Excluding these two species, average control imbibition was 17.2% (median 10.0%). On average, the non-imbibed seeds had a 17% increase in mass (Table 1), most of which occurred in the first 24 h. There were varying contributions between the seed and the aril to this increased mass. In species with glossy seeds (e.g. A. gilbertii) there was almost no increase in seed mass, while seeds with a matt finish (e.g. A. cochlearis) had up to 17% increase in mass. Arils made up varying percentages of the seed plus aril mass (Burrows et al., Reference Burrows, Alden and Robinson2018) and the arils absorbed different percentages of water (e.g. A. gilbertii 83%, A. adoxa 179%). Most imbibition of control seeds in the first 24 h was due to water entry through bruchid exit holes, often minute (as small as 150 µm diameter, 20,000 μm2 area, Fig. 1), in the seed coat, with physical damage such as cracks in the testa also observed. This was a very small percentage of all control seeds (average 5%, median 3%). Burrows et al. (Reference Burrows, Alden and Robinson2018) recorded several species with a relatively high proportion of pre-popped lenses. These species had a relatively high percentage of control seeds that had imbibed by the end of the experiment.

Fig. 1. Two seeds of Acacia glaucoptera with insect exit holes. The hole on the right is smaller in area than many popped lenses but would allow full seed imbibition in less than 24 h. Scale bar, 500 µm.
HW-treated seeds
An average of 95% (median 97%) of the HW-treated seeds imbibed (Table 1). The lowest percentage was for A. dunnii (75%), which was the species with the largest seeds and thickest testa. On average, imbibed seeds increased in mass by 154% (Table 1). Some seeds were observed when partially imbibed and a distinct wetting front was usually present (Fig. 2). Average t 50 imbibition time for HW-treated seeds was 170 h (about 7 days) (median 168 h) (Table 1). Twelve species had a t 50 imbibition of less than 30 h, while two species took around 600 h (25 days) to reach 50% imbibition. For almost all species with a t 50 imbibition of greater than 30 h, a graph of average cumulative per cent imbibition over time revealed a progressive, near-linear increase (e.g. Fig. 3). The curve of this graph gives the impression that imbibition was a gradual and consistent process. However, examination at the level of the individual seeds within a replicate showed that seeds remained unimbibed for varying lengths of time and then became fully imbibed in less than 24 h (Fig. 4). Within each replicate of 10 seeds, for all species with >30 h HW imbibition t 50, this same imbibition pattern was recorded (i.e. a seed might not increase in mass for several weeks, then be fully imbibed in less than 24 h). The only partial exception was for some of the larger seeds, e.g. A. dunnii (mass 245 mg), which took up to 48 h to be fully imbibed. In most species with longer imbibition t 50 times, at least some seeds would imbibe after 1 or 2 days of being placed on a moist substrate (Fig. 4). In A. leptoloba (and to a lesser extent A. glaucoptera, A. deanei, A. gilbertii and A. doratoxylon), almost no seeds imbibed during the first 8 days, after which percentage imbibition increased relatively rapidly (Fig. 5).

Fig. 2. Two seeds of Acacia cardiophylla that have partially imbibed. Note there is a very distinct wetting front and that the seeds have imbibed from the hilar end. Scale bar, 1000 µm.

Fig. 3. Cumulative percentage imbibition of seeds over time of Acacia falcata that had been hot water-treated. Bars denote standard error of the mean.

Fig. 4. Percentage increase in mass over time for 10 seeds for the three replicates making up the data in Fig. 3. The seeds had been hot water-treated and physical dormancy had been broken. Note that the seeds remained unimbibed for varying lengths of time (20–420 h) but then imbibed rapidly.
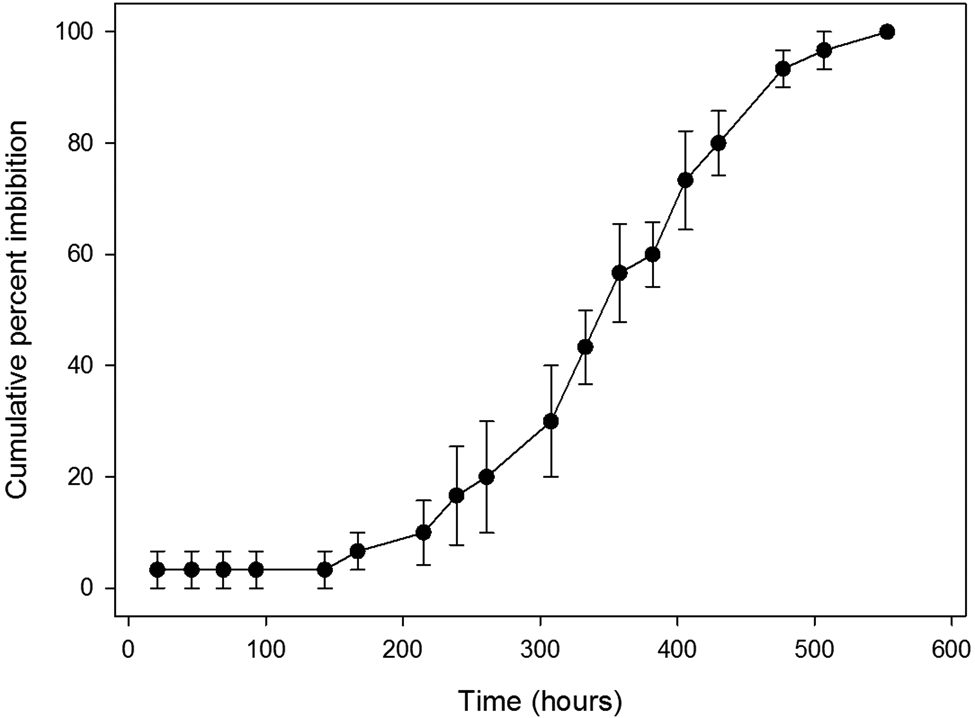
Fig. 5. Cumulative per cent imbibition of hot water-treated Acacia leptoloba seeds. Note that no imbibition occurred in the first 7 days. Bars denote standard error of the mean.
For the 47 species there was a significant negative correlation between t 50 imbibition and popped lens area (i.e. the larger the average popped lens area, the shorter the average t 50 imbibition) (r s = –0.433, n = 46, P < 0.005) and positive relationships with palisade thickness (r s = 0.36, n = 46, P < 0.02) and total testa thickness (r s = 0.36, n = 48, P < 0.02). Species from locations with higher average annual rainfall tended to have a higher t 50 (r s = 0.45, n = 29, P < 0.02). No significant correlation of imbibition t 50 with average seed plus aril mass (r s = –0.01, n = 48, P = 0.95) or average popped lens morphology rating (r s = 0.21, n = 46, P = 0.15) was found.
Non-dormant species (A. cambagei, A. harpophylla, A. oswaldii)
Acacia harpophylla responded differently to the two treatments compared with almost all other species. All control seeds quickly imbibed (37% mass increase in 1 h, 139% after 24 h), with seeds beginning to germinate between 23 and 28 h and 97% germination after 48 h. The HW-treated seeds failed to fully imbibe (104% increase in mass after 23 h) and none germinated. A TTC test indicated that the HW-treated seeds were non-viable (embryo presumably killed by the relatively short exposure to HW). For A. cambagei, only a control treatment was conducted as there was an insufficient number of seeds for a HW treatment. As in A. harpophylla, there was 100% imbibition and average 153% increase in mass after 23 h, with 67% germination after 71 h.
Unlike the two species above, A. oswaldii had a hard seed coat, well-developed palisade cells and a functional lens (Burrows et al., Reference Burrows, Alden and Robinson2018). Twenty-three per cent of the control seeds had imbibed after 25 h and additional control seeds imbibed over the next 8 days, with a final average imbibition of about 68% after 8 days. The HW-treated seeds imbibed more rapidly in a shorter time (83% were imbibed in 3 days) (t 50 imbibition control 62 h, t 50 imbibition HW 30 h). A previous structural study (Burrows et al., Reference Burrows, Alden and Robinson2018) indicated that about 33% of control seeds had pre-popped lenses. A batch of 42 non-treated seeds were sorted into pre-popped (14 seeds) and non-popped seeds (28 seeds). Without a HW treatment, 92% of pre-popped seeds had imbibed after 43 h. Of the non-popped seeds, 50% had imbibed after 43 h, rising to 75% after 138 h. Examination of these seeds after 138 h showed that in over 68% of the seeds the lens had popped without a HW treatment.
Imbibition of two provenances of Acacia melanoxylon
Both provenances had an average of >80% germination for both HW and nicking treatments, indicating that seed viability was still high after another 12–14 years storage. The germination curves for L08 and L21 [Burrows et al. (Reference Burrows, Virgona and Heady2009), fig. 4] and the imbibition curves of the present study (Fig. 6) were very similar, especially the more gradual imbibition and germination of HW-treated L08 seeds (t 50 imbibition averages of 15.5 and 8.0 days for L08 and L21, respectively, which were significantly different; t = 6.4; d.f. = 2, 2; P < 0.005). Both sets of curves (Fig. 6) suggest that the HW-treated seeds gradually imbibed, but again investigation at an individual seed level (Fig. 7) showed that seeds remained unimbibed for differing lengths of time before becoming fully imbibed in less than 24 h. Once imbibed, almost all seeds, both nicked and HW treated, germinated within 2–3 days.

Fig. 6. Cumulative per cent imbibition of two seed provenances of Acacia melanoxylon (L08 and L21). The nicking and the control curves are very similar, but after the hot water (HW) treatment L21 imbibed more rapidly than L08. Bars for standard error of the mean are supplied only for the hot water treatments.

Fig. 7. Percentage increase in mass over time for the 10 seeds in the three replicates making up the data in Fig. 6 for Acacia melanoxylon provenance L08 (seeds hot water-treated). Note that the seeds remained unimbibed for varying lengths of time (5–22 d) but then imbibed rapidly.
Influence of temperature on seed imbibition of 11 Acacia species
Again, all non-treated seeds had low imbibition (average 5%), e.g. no A. leptoloba seeds imbibed during 40 days at 25°C surrounded by moist surfaces (Fig. 8a). HW-treated seeds of seven species reached 100% imbibition at all temperatures, with 94% imbibition being the lowest recorded (A. mearnsii, 15°C) (Fig. 8a). The HW-treated seeds of the 11 species tested at 15, 20 and 25°C produced the same pattern of imbibition responses as in the main experiment. For example, for A. adoxa and A. coolgardiensis, over 70% of seeds imbibed at all three temperatures in less than 24 h (i.e. t 50 < 24 h) (Fig. 8b). Only A. aphylla showed a significant difference between temperature treatments in the proportion of imbibed seeds after 24 h (χ2 = 7.1, d.f. = 2, P < 0.05) (Fig. 8b). Almost all species reached t 50 imbibition sooner at higher temperatures (Fig. 8c). Acacia aphylla, A. cardiophylla, A. leptoloba and A. mearnsii had no significant difference in average imbibition t 50 between temperatures, and A. adoxa and A. coolgardiensis were invariant and did not require analyses. The other five species had a significant temperature influence on t 50 where seeds treated at 15°C always had a significantly greater t 50 than those at 25°C, with 20°C in between (Fig. 8c). Final germination percentages were not significantly influenced by temperature, except where inhibited at higher temperatures (Fig. 8d). The proportion of seeds that germinated was significantly different between temperatures for A. browniana (χ2 = 71.4, d.f. = 2, P = < 0.0001), A. truncata (χ2 = 65.8, d.f. = 2, P = <0.001) and A. falcata (χ2 = 10.6, d.f. = 2, P < 0.005). For A. browniana, significantly more seeds germinated at 15°C (83%) than at 20°C (19%) or 25°C (0%). For A. truncata, significantly more seeds germinated at 15°C (90%) than at 20°C (25%) or 25°C (3%), and at 20°C there was a higher proportion of germination than at 25°C. The pattern was the same for A. aphylla (Fig. 8d) with 25°C having a significantly lower germination percentage than at the other two temperatures using pairwise comparisons; however, the overall model declared a non-statistically significant difference (P = 0.0599) in the influence of temperature. The model for A. falcata was a poor fit and we can assume that in the biological sense any HW treatment significantly increases germination in this species.
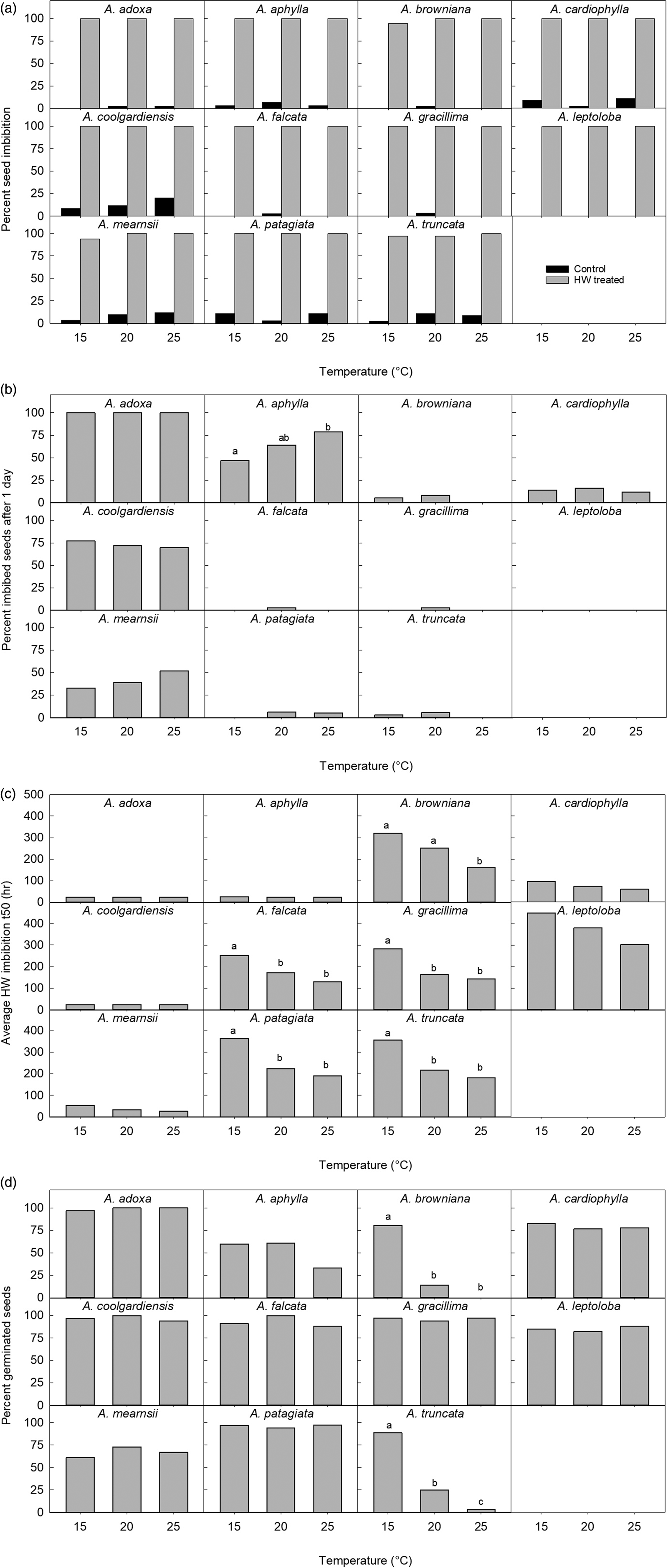
Fig. 8. Various responses of the seeds of 11 Acacia species at three temperatures: (a) end of experiment per cent seed imbibition for non-treated and hot water-treated seeds, (b) per cent imbibed 24 h after hot water treatment, (c) average t 50 imbibition after hot water treatment, and (d) per cent germination after hot water treatment, at end of the experiment. Treatments that have the same superscript letters do not have different averages (c) or percentages (a, b, d). Graphs with no superscript letters are for species where the responses were not significantly different between treatments or species where models could not be fitted to the data.
Seed moisture content
No significant difference in average seed moisture content was recorded between species with short and long HW imbibition t 50 values (average 8.2 and 8.7%, respectively) (t = 0.43; d.f. = 4,5; P = 0.67). Moisture content ranged from an average of 5.7% (A. adoxa) to 11.1% (A. mearnsii).
Discussion
Imbibition – non-treated seeds
This study has shown that Australian Acacia species have widely differing patterns of seed imbibition in both control and HW-treated seeds. In control seeds imbibition was either very low over an extended time period (almost all species) or all seeds imbibed within 6–24 h (two soft-seeded species). In large scale records of Acacia germination experiments Auld (Reference Auld1986), Cavanagh (Reference Cavanagh and Langkamp1987), Bell et al. (Reference Bell, Plummer and Taylor1993) and Cromer (Reference Cromer2007) recorded average control germination of 10–15%, which is very similar to control imbibition percentages in the present study. Imbibition of non-treated seeds of hard-seeded species occurred for two main reasons, pre-popped lenses (see later section) and larvae exit holes in the seed coat. Various authors have noted that insect exit holes can be relatively common in Acacia seed (Cavanagh, Reference Cavanagh1980; Southgate, Reference Southgate1983; Auld, Reference Auld1986; Baskin and Baskin, Reference Baskin and Baskin2014). Small exit holes in A. glaucoptera seeds were smaller in diameter than a popped lens but still allowed seeds to imbibe in less than 24 h (G. Burrows, unpublished data).
The present study used seeds from distinct localities, but they were probably bulked from at least several plants per locality. Ferreras et al. (Reference Ferreras, Zeballos and Funes2017) examined the imbibition of non-treated seeds of Vachellia (Acacia) aroma from four populations (10 to 11 individuals per population) along a precipitation gradient in Argentina. Overall imbibition per population was low (<10%) but in three populations a few individuals had >20% imbibition. Ferreras et al. (Reference Ferreras, Zeballos and Funes2017) discussed this intra-population variability in terms of rainfall but did not examine the seeds for seed coat damage, pre-popped lenses or lenses that popped during the month that the experiment ran.
Imbibition – HW-treated seeds
Almost all (97% median) HW-treated seeds imbibed, showing that a relatively short exposure to HW breaks PY in seeds of the studied species. For HW-treated seeds, a wide range of imbibition patterns was recorded, from at least 50% of seeds imbibed with 30 h (12 species) to species where imbibition t 50 was several weeks. The major finding of this study was that where average cumulative percentage imbibition gradually increased over time (e.g. Figs 3, 5 and 6), some seeds in a replicate would imbibe near the start of the experiment while others would remain unimbibed for an extended period and then become completely imbibed in less than 24 h (Figs 4 and 7). In a similar manner, unpublished data of Ganesha Liyange (personal communication) indicates that seeds of A. linifolia had a much earlier and rapid imbibition (t 50 approximately 110 h) than A. ulicifolia (t 50 approximately 240 h) (3 × 11–20 seed replicates, visually assessed every 2 days over a period of 30 days).
As noted, most previous studies of imbibition in the Caesalpinioideae and the Mimosoideae have been of mechanically scarified seeds over a few days. In addition, seeds in replicates were usually weighed together, meaning it was not possible to determine if imbibition occurred as described in the present study. The results of the present imbibition study indicate that HW-treated seeds of some Acacia species should germinate almost as quickly as mechanically scarified seeds, while in other species accumulative germination should slowly and gradually increase to levels achieved with scarification. Below are some examples indicating this has been described in the literature.
A good example is Doran and Gunn (Reference Doran, Gunn and Turnbull1987) who studied the germination of eight Acacia species after 10 treatments (control, nicking, acid and seven HW or BW treatments). In seven of eight species, nicking gave the earliest germination, the greatest germination rate and, in most species, the highest final germination percentage (see their fig. 3). The seven HW or BW treatments produced a wide range of germination curves. In several species the most effective BW or HW treatments showed a relatively slow and constant germination rate over the 27 days of the experiment and eventually produced similar final germination percentages to nicking, e.g. A. ampliceps, A. crassicarpa, A. melanoxylon and A. stenophylla. However, there were some species where the most effective BW treatments gave similar germination rates as nicking, e.g. A. aneura, A. mearnsii and A. mangium. Other recordings of gradual germination after HW or BW treatments in the Caesalpinioideae and Mimosoideae have been made by Clemens et al. (Reference Clemens, Jones and Gilbert1977), Tran (Reference Tran1979; A. sophorae), Odoemena (Reference Odoemena1988), Burrows (Reference Burrows1991), Khasa (Reference Khasa1993) and Burrows et al. (Reference Burrows, Virgona and Heady2009). In contrast, other studies have shown rapid imbibition after HW or BW treatments, e.g. Tran (Reference Tran1979; A. longifolia), Dell (Reference Dell1980), Rodrigues-Junior et al. (Reference Rodrigues-Junior, Faria, Vaz, Nakamura and José2014), and Erickson (Reference Erickson2015).
In the Faboideae, Taylor (Reference Taylor2005) noted that soft seeds of the majority of annual pasture legume species fully imbibe within a few days after contact with water. He also noted that some soft seeds of Trifolium campestre (Russi et al., Reference Russi, Cocks and Roberts1992) and Medicago polymorpha cv. Serena (Taylor, Reference Taylor1996) can take several days to imbibe. The most extensive investigations of delayed imbibition in pasture legumes are for yellow serradella (Ornithopus compressus), especially Charno and Santorini cultivars. Taylor (Reference Taylor2005; p. 41) suggested that ‘the simplest explanation for slow imbibition is the seed becomes permeable through a minute opening that only allows slow entry of water …’. He speculated that the minute opening would be somewhere other than the lens, with the micropyle and hilum suggested. Taylor (Reference Taylor2004) considered that a gradual build-up of moisture in tissues on the underside of the lens might cause its rupture, which would then be followed by rapid imbibition. As per the present study Taylor (Reference Taylor2004; p. 42) also mentions the ‘sudden appearance of fully imbibed seeds’. This two-site imbibition hypothesis would not apply to Acacia as the lens is fully open a few seconds after HW treatment.
The lens as a constriction
At a functional level, Baskin et al. (Reference Baskin, Baskin and Li2000; p. 144) considered that the ‘lens may act as a regulator of the rate of water entry into the seed, thereby affecting seedling vigour’. Slow imbibition results in maximum seedling vigour and avoids imbibitional damage through leakage of cellular solutes (Baskin et al., Reference Baskin, Davis, Baskin, Gleason and Cordell2004; Manning and Van Staden, Reference Manning and Van Staden1987). Baskin and Baskin (Reference Baskin and Baskin2014) also noted that slow imbibition in BW-treated seeds indicates that the water gap acts as a ‘rain gauge’ (prevents germination of all seeds in a population during false starts in rainfall). At a physical level, various authors have suggested that slower rates of imbibition are related to smaller lens diameters (Tran, Reference Tran1979; Cavanagh, Reference Cavanagh1980; Taylor, Reference Taylor2004; Burrows et al., Reference Burrows, Virgona and Heady2009). The present study shows that, at least for Australian acacias, this is not the full story. Burrows et al. (Reference Burrows, Virgona and Heady2009, Reference Burrows, Alden and Robinson2018) and Erickson et al. (Reference Erickson, Merritt and Turner2016) found a wide variation in popped lens structure in Acacia. There was no statistically significant correlation between the morphology of the popped lens and t 50 imbibition. Some species (e.g. A. falcata, A. leptoloba) with fully popped tube-type lenses had many seeds with delayed imbibition, while some species (e.g. A. aphylla, A. coolgardiensis) with dome-shaped lenses had uniformly early imbibition. There was weak but significant negative correlation between popped lens area and HW t 50 imbibition.
Xylem in the testa
Van Staden et al. (Reference van Staden, Manning, Kelly, Stirton and Zarucchi1989; p. 241) noted that in Caesalpinioideae and Mimosoideae seeds the ‘vasculature consists of an unbranched vascular bundle that forms a loop extending almost completely around the seed’. This vascular bundle enters the seed at the hilum, then descends deep into the seed coat before curving outwards to come just under the lens and then passing inwards into the testa again (Burrows et al., Reference Burrows, Alden and Robinson2018, and references within). There have been varying reports on whether the xylem cells in the vascular bundle are functional or blocked (Dell, Reference Dell1980; Hanna, Reference Hanna1984). Likewise, there are conflicting reports as to whether the vascular bundle that passes just under the lens (e.g. Burrows et al., Reference Burrows, Alden and Robinson2018; fig. 1f) helps in distributing water during imbibition (Dell, Reference Dell1980; Tran and Cavanagh, Reference Tran, Cavanagh and Murray1984; Van Staden et al., Reference van Staden, Manning, Kelly, Stirton and Zarucchi1989). Dell (Reference Dell1980) indicated that the sub-lens cavity placed some initial constraint on water entry, but this was only of the order of a few hours, not the extended times recorded in this study. Many authors have noted that BW- or HW-treated seeds swell from the hilum end (e.g. Cavanagh, Reference Cavanagh1980) and there can be a sharp wetting or swelling front between wetted cells and those about to be wetted (e.g. Bewley and Black, Reference Bewley and Black1994; Rodrigues-Junior et al., Reference Rodrigues-Junior, Faria, Vaz, Nakamura and José2014). In the present study various seeds were observed when partially imbibed and a distinct wetting front was observed (Fig. 2), which does not seem to correlate with the main water distribution occurring via the vascular bundle.
Palisade thickness
The relationship of testa or palisade thickness in legume seeds with water impermeability is complex (e.g. Taylor, Reference Taylor2005; Zeng et al., Reference Zeng, Cocks, Kailis and Kuo2005). Rodrigues-Junior et al. (Reference Rodrigues-Junior, Mello, Baskin, Baskin, Oliveira and Garcia2018b; p. 2) state ‘it is rather difficult to detect a relationship between seed coat thickness and level of dormancy’. Some studies of Caesalpinioideae and Mimosoideae species have related palisade thickness to water impermeability (e.g. Venier et al., Reference Venier, Funes and García2012; Pound et al., Reference Pound, Ainsley and Facelli2014). The present study, in combination with Burrows et al. (Reference Burrows, Alden and Robinson2018), shows that seeds with relatively short palisade cells can maintain PY in non-treated seeds in a continually moist environment at 25°C for many weeks (see A. glaucoptera, A. gilbertii and A. patagiata with average palisade lengths of 32, 31 and 28 µm, respectively).
There are two ways to view the interaction between impermeability and testa or palisade thickness. Firstly, in A. harpophylla (no palisade layer) and A. cambagei (palisade cells very short, average 12 µm), control seeds could imbibe quickly all over the seed (no wetting front) indicating that palisade thickness is correlated with imbibition. Secondly, and in contrast, all the other species (palisade thickness average 42 µm) control seeds remained unimbibed after several days or weeks on a moist substrate. This shows that what could be considered a relatively thin palisade layer can be very effective in maintaining PY.
Imbibition of two provenances of Acacia melanoxylon
This experiment shows that dormant Acacia seeds are long lived. For both provenances, after a further 12–13 years storage, seed germination still averaged above 80%. The good correlation of the 2003–2005 germination data (from seeds in potting mix in a glasshouse) with the imbibition data (Petri dishes in incubators) of the current study shows that the gradual increase in accumulative germination (Burrows et al., Reference Burrows, Virgona and Heady2009) was due to delayed non-uniform imbibition rather than the internal diameter of the popped lenses.
Ecology – soft-seeded/non-dormant species
Burrows et al. (Reference Burrows, Alden and Robinson2018) indicated the presence of two forms of non-treated, non-dormant Acacia seeds: soft-seeded species where the palisade layer is non-existent or poorly developed and the lens is non-functional, as well as hard-seeded species where the palisade layer is well developed but the lens can pop without a strong stimulus. There would appear to be a much smaller number of species with truly soft seeds compared with species where the lens can pop without a strong stimulus such as BW or the heat from a fire.
Acacia harpophylla and A. cambagei are well known to have non-dormant seeds that have a relatively short viability (Coaldrake, Reference Coaldrake1971; Reichman et al., Reference Reichman, Bellairs and Mulligan2006). Johnson (Reference Johnson1964) noted that this allows establishment after spasmodic rain which was then followed by hot, dry periods. The current study shows that control seeds imbibed water very rapidly, while in A. harpophylla relatively short exposure to HW killed the embryo. Danthu et al. (Reference Danthu, Roussel, Dia and Sarr1992) recorded a very similar result for the soft-seeded Acacia (Senegalia) senegal where seeds were killed by 10 s or longer of BW. Interestingly, A. harpophylla and A. cambagei had the flattest seeds (greatest surface-to-volume ratio) in a study of 51 Acacia species by Burrows et al. (Reference Burrows, Alden and Robinson2018). Other authors have noted that a larger surface-to-volume ratio in seeds may be associated with decreased heat tolerance (Morrison et al., Reference Morrison, Auld, Rish, Porter and McClay1992; Ruprecht et al., Reference Ruprecht, Fenesi, Fodor, Kuhn and Tökölyi2015).
Auld (Reference Auld1990) found that of the 42% of A. oswaldii seeds that were released from the fruits in a dormant state, most were non-dormant within a year and with sufficient rainfall germinated without the need for fire. Likewise, Pound et al. (Reference Pound, Ainsley and Facelli2014) found that non-treated seeds of A. oswaldii had a gradual and ongoing germination response over time and in the present study some lenses of this species popped from just being on a moistened paper towel. As noted in Burrows et al. (Reference Burrows, Alden and Robinson2018), all the non-dormant seed producing Acacia s. str. species are native to the arid central part of Australia. Several of these species have been noted to have fresh non-dormant seeds, high and quick germination and low storage ‘rain-ready’ seed banks (Auld, Reference Auld1993; Owens et al., Reference Owens, Wallace and Archer1995; Nano et al., Reference Nano, Bowland and Pavey2013). This type of seed can germinate without wildfire, but small rain events can trigger germination but not necessarily seedling establishment.
Ecology – hard-seeded species
Many Acacia species flourish in a post-fire environment with increased soil fertility and reduced competition for resources such as light and water. After fire a proportion of Acacia seeds would have their PY broken, while seeds in the litter layer would probably be consumed by the fire and deeply buried seed would remain dormant. The now non-dormant seeds then require a rainfall event or events to imbibe and germinate. The results of the present study indicate that only a relatively small portion of the non-dormant seeds of many Australian Acacia species would imbibe and germinate after a small amount of rain, leaving many quiescent seeds in the ground. With more rainfall, the soil would remain moist for longer and more seeds would imbibe and germinate. Thus many Acacia species seem to have a way of assessing how much rain there has been so as to reduce the risk of all non-dormant seed imbibing and germinating with a single small rainfall event. Slow or variable imbibition can prevent germination until there is a relatively long period of moist soil, with the lens acting as a ‘soil moisture gauge’ (Herranz et al., Reference Herranz, Ferrandis and Martínez-Sánchez1998; Baskin and Baskin, Reference Baskin and Baskin2014). Cavanagh (Reference Cavanagh1980) referred to several studies where BW-treated Acacia seeds (with popped lenses) can be stored for long periods with no loss of viability. It is unknown whether this would apply to the viability of seeds in the field. In short, for many Acacia species it appears that the lens acts as a ‘fire gauge’, with some other part of the testa acting as a ‘rain gauge’.
Conclusions
PY can vary in various ways for populations of seeds produced by a single plant. Within a single population some seeds can be soft-seeded and other hard-seeded, even at the time of release (e.g. Hudson et al., Reference Hudson, Ayre and Ooi2015; Ferreras et al., Reference Ferreras, Zeballos and Funes2017). Even when all seeds are hard, variability can be present in various forms. Rodrigues-Junior et al. (Reference Rodrigues-Junior, Baskin, Baskin and Garcia2018a) found that seed size of Senna multijuga can play a role in PY, where with 1.5 years dry storage more large seeds than small seeds had become sensitive. Hellum (Reference Hellum1990) found that for Acacia holosericea, mainly small seeds could germinate without pre-treatment, while Liyanage and Ooi (Reference Liyanage and Ooi2015) found intra-population variation in dormancy-breaking temperature thresholds. This study demonstrates another form of variability, one that may prevent all seeds with broken PY germinating after an isolated and minor rainfall event. However, the expression of delayed imbibition was very variable between the species studied and even within provenances of a single species. The next step is to determine what delays water reaching the embryo after the lens has popped, i.e. is it possible to examine a seed to determine if it is a seed that would imbibe promptly or whether it will have delayed imbibition after PY is broken?
Acknowledgements
We thank Jason Condon for preparing the graphs.
Author contributions
G.B. planned and designed the research. G.B. and R.A. performed the experiments. G.B. and W.R. analysed the data. G.B. wrote the text. All authors read, commented on and approved the final version of the manuscript.
Financial support
This work was supported by the Australian Flora Foundation.











