Significant outcomes
-
Both aspirin and atorvastatin can improve depressive symptoms in patients with major affective disorders and inflammatory dysregulation.
-
Both medications also improved overall function in such patients.
-
Only atorvastatin increased anti-inflammatory cytokine soluble IL-2 receptor (sIL-2R) levels.
Limitations
-
Only patients with mild or residual depression were enrolled in the present study.
-
The present study was an add-on aspirin/atorvastatin clinical trial but not a medication-free clinical trial.
-
The present study assessed the levels of only three cytokines – C-reactive protein, sIL-2R, and soluble tumour necrosis factor-α receptor 1.
Introduction
Research has increasingly highlighted the crucial role of inflammatory dysregulation in the pathomechanisms of bipolar disorder (BD) and major depressive disorder (MDD) (Bai et al., Reference Bai, Chen, Hsu, Huang, Tu, Chang, Su, Li, Lin and Tsai2020, Chen et al., Reference Chen, Kao, Chang, Tu, Hsu, Huang, Su, Li, Lin, Tsai and Bai2020, Huang et al., Reference Huang, Chen, Chan, Li, Tsai, Bai and Su2022a, Huang et al., Reference Huang, Chen, Hsu, Huang, Tsai, Su and Bai2022b). Studies have identified the harmful effects of inflammatory dysregulation, such as obesity and elevated levels of C-reactive protein (CRP) and soluble tumour necrosis factor-α receptor 1 (sTNF-αR1), on clinical symptoms and cognitive function in patients with major affective disorders (Bai et al., Reference Bai, Chen, Hsu, Huang, Tu, Chang, Su, Li, Lin and Tsai2020, Huang et al., Reference Huang, Chen, Chan, Li, Tsai, Bai and Su2022a, Huang et al., Reference Huang, Chen, Hsu, Huang, Tsai, Su and Bai2022b, Chen et al., Reference Chen, Hsu, Huang, Tsai, Su, Li, Lin, Tu and Bai2021). Elevated CRP and sTNF-αR1 levels have been specifically linked to suicidal symptoms in both patients with BD and those with MDD (Huang et al., Reference Huang, Chen, Chan, Li, Tsai, Bai and Su2022a, Huang et al., Reference Huang, Chen, Hsu, Huang, Tsai, Su and Bai2022b). These markers have also been strongly associated with executive function deficits regardless of the type of major affective disorder (Chen et al., Reference Chen, Kao, Chang, Tu, Hsu, Huang, Su, Li, Lin, Tsai and Bai2020, Chen et al., Reference Chen, Hsu, Huang, Tsai, Su, Li, Lin, Tu and Bai2021, Chen et al., Reference Chen, Hsu, Huang, Tsai, Tu and Bai2023).
The therapeutic effects of anti-inflammatory medications, such as non-steroidal anti-inflammatory drugs (e.g., aspirin) and statins, on major affective disorders remain uncertain (Berk et al., Reference Bauer, Green, Colpo, Teixeira, Selvaraj, Durkin, Zunta-Soares and Soares2018, Bauer et al., Reference Berk, Mohebbi, Dean, Cotton, Chanen, Dodd, Ratheesh, Amminger, Phelan, Weller, Mackinnon, Giorlando, Baird, Incerti, Brodie, Ferguson, Rice, Schäfer, Mullen, Hetrick, Kerr, Harrigan, Quinn, Mazza, McGorry and Davey2020). For example, Bauer et al. reported that a 16-week combination of aspirin (1,000 mg/day) and N-acetylcysteine (NAC, 1,000 mg/day) produced a substantial antidepressant effect in patients with BD relative to placebo (Bauer et al., Reference Bauer, Green, Colpo, Teixeira, Selvaraj, Durkin, Zunta-Soares and Soares2018). Additionally, a 2-year follow-up study of 40 patients with MDD randomly assigned to treatment with escitalopram or duloxetine with or without aspirin (100 mg/day) indicated higher remission rates in the duloxetine + aspirin group than in the escitalopram + placebo group (Zdanowicz et al., Reference Zdanowicz, Reynaert, Jacques, Lepiece and Dubois2017). However, a randomised, triple-blind, placebo-controlled trial of 130 young individuals with moderate to severe MDD demonstrated that 12-week add-on treatments of either aspirin (100 mg/day) or rosuvastatin (10 mg/day) did not provide additional benefits over routine depression treatment (Berk et al., Reference Berk, Mohebbi, Dean, Cotton, Chanen, Dodd, Ratheesh, Amminger, Phelan, Weller, Mackinnon, Giorlando, Baird, Incerti, Brodie, Ferguson, Rice, Schäfer, Mullen, Hetrick, Kerr, Harrigan, Quinn, Mazza, McGorry and Davey2020). Raison et al. further demonstrated that the antidepressant effect of the TNF antagonist infliximab (5 mg/kg) was observed only in patients with MDD with high baseline CRP levels (>5 mg/L), but not in those without elevated CRP levels (Raison et al., Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake, Haroon and Miller2013). However, these studies have been conducted in Western countries, limiting their generalisability to Asian populations.
The present study investigated the antidepressant effects of a 12-week add-on treatment with aspirin (100 mg/day) and atorvastatin (10 mg/day) in patients with major affective disorder and inflammatory dysregulation. The therapeutic effects of aspirin and atorvastatin on overall function were also evaluated. Additionally, the influence of these medications on proinflammatory and anti-inflammatory cytokine levels was assessed. The present study hypothesised that both aspirin and atorvastatin would reduce depressive symptoms and improve overall function compared with placebo in patients with major affective disorder and inflammatory dysregulation. This study further hypothesised that aspirin and atorvastatin would reduce proinflammatory cytokine levels and increase anti-inflammatory cytokine levels.
Methods
Participants and study procedure
The study subjects met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for major depressive disorder (MDD) or bipolar disorder (BD) and exhibited inflammatory dysregulation. All participants scored Clinical Global Impressions scale ≤3, indicating that they only experienced mild or residual depressive states. We excluded those in manic or hypomanic states. Based on the findings of Verma et al’s, Lumeng et al’s and our previous studies (Verma et al., Reference Verma, Bhatta, Davies, Deanfield, Garvey, Jensen, Kandler, Kushner, Rubino and Kosiborod2023, Bai et al., Reference Bai, Su, Li, Tsai, Chen, Tu and Chiou2015, Lumeng & Saltiel, Reference Lumeng and Saltiel2011), those who met each criterion listed behind were regarded as those with inflammatory dysregulation. The definition of inflammatory dysregulation includes CRP levels ≥ 1,000 ng/ml, TNF-αR1 ≥ 800 pg/ml, body mass index (BMI) >25, and abdominal obesity (waist circumference ≥90 cm in men and ≥80 cm in women) (Verma et al., Reference Verma, Bhatta, Davies, Deanfield, Garvey, Jensen, Kandler, Kushner, Rubino and Kosiborod2023, Bai et al., Reference Bai, Su, Li, Tsai, Chen, Tu and Chiou2015, Lumeng & Saltiel, Reference Lumeng and Saltiel2011). Individuals who had a lifetime history of schizophrenia or any other psychotic disorders, organic mental disorders, intellectual disability, or alcohol or substance use disorders were excluded. Individuals who had a medical history of diabetes and other severe physical diseases, such as cancers, stroke, myocardial infarction, major autoimmune diseases, asthma, epilepsy, and chronic liver and kidney diseases, were excluded. We also excluded those with current infectious diseases and those who took any anti-inflammatory medications or antibiotics. Finally, we randomly assigned 48 patients, 23 with BD and 25 with MDD, to three add-on treatment groups: the placebo group, the 12-week aspirin (100 mg/day) group, and the 12-week atorvastatin (10 mg/day) group. Throughout the trial, all concomitant medicines used by the participants were kept the same and did not alter. We examined the patients’ clinical symptoms and overall functioning at baseline, week 4, week 8, and week 12 (study end) using the 17-item Hamilton Depression Rating Scale (HDRS), Montgomery-Åsberg Depression Rating Scale (MADRS), Young Mania Rating Scale (YMRS), Hamilton Anxiety Rating Scale (HAM-A), Personal and Social Performance Scale (PSP), and Global Assessment of Functioning Scale (GAF) (Hamilton, Reference Hamilton1960, Montgomery et al., Reference Montgomery, Rani, Mcauley, Roy and Montgomery1981, Young et al., Reference Young, Biggs, Ziegler and Meyer1978, Endicott et al., Reference Endicott, Spitzer, Fleiss and Cohen1976, Dasberg, Reference Dasberg1975, Nasrallah et al., Reference Nasrallah, Morosini and Gagnon2008). Proinflammatory (CRP and sTNF-αR1) and anti-inflammatory (soluble IL-2 receptor, sIL-2R) cytokines were assessed at baseline and on week 12. The Taipei Veterans General Hospital’s Institutional Review Board approved this study, which followed the Declaration of Helsinki. Before participating in this study, all participants provided written informed consent. Clinical trial registration: NCT04685642.
Laboratory measurement
The fasting serum samples were collected between 8:00 a.m. and 10:00 a.m. All samples were then stored at 80C until use. Enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) were applied to examine pro-inflammatory cytokines, including CRP, sIL-2R and sTNF-α R1. All assays were performed in accordance with the manufacturer’s instructions. The final absorbance of the mixture was measured at 450 nm, and the mixture was analysed using an ELISA plate reader with Bio-Tek Power Wave Xs and Bio-Tek’s KC junior software (Winooski, VT, USA). The standard range was considered as per the manufacturer’s instructions. A linear regression R-square value of ≥0.95 represented a reliable standard curve.
Statistical analyses
For between-group comparisons, the F-test was used for continuous variables and Pearson’s test was used for categorical variables. After adjusting for age, sex, diagnoses, and baseline clinical score, generalised estimating equation (GEE) models with the autoregressive method for correlations of repeated measures for the same individual over time was used to examine the effect of interventions on overall depressive symptoms (total HDRS and MADRS scores) and overall function (GAF) during the study period (baseline to week 12) with the group (aspirin vs. atorvastatin vs. placebo) as a between-patient factor, time as a within-patient factor, and baseline depressive symptoms and function scores as between-patient predictors as well as all possible interactions, respectively. After adjusting for age, sex, BMI, diagnoses, and baseline cytokine levels, we further performed GLMs with gamma log link to assess the intervention effect on the proinflammatory cytokine levels on week 12. Finally, we used the GLMs to examine the associations between the changes and between depressive symptoms and overall function and between the proinflammatory cytokines after adjusting for intervention groups, age, sex, BMI, and diagnosis. A two-tailed P value of less than 0.05 was considered statistically significant. All data processing and statistical analyses were performed using the SPSS version 17 software (SPSS Inc., Chicago, IL).
Results
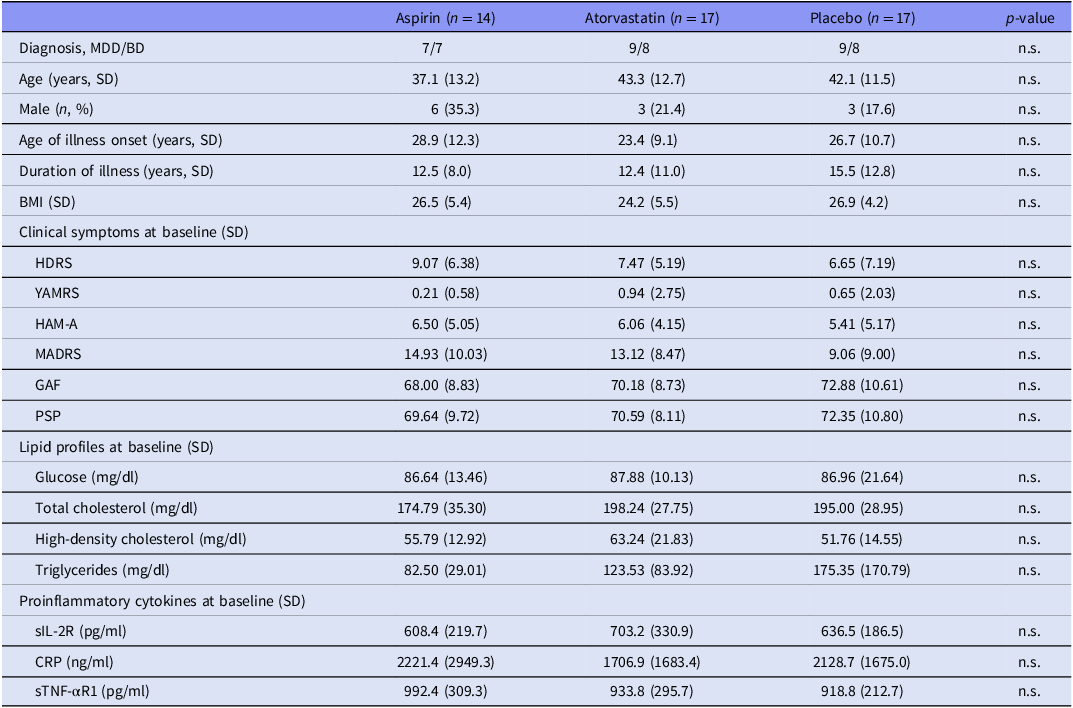
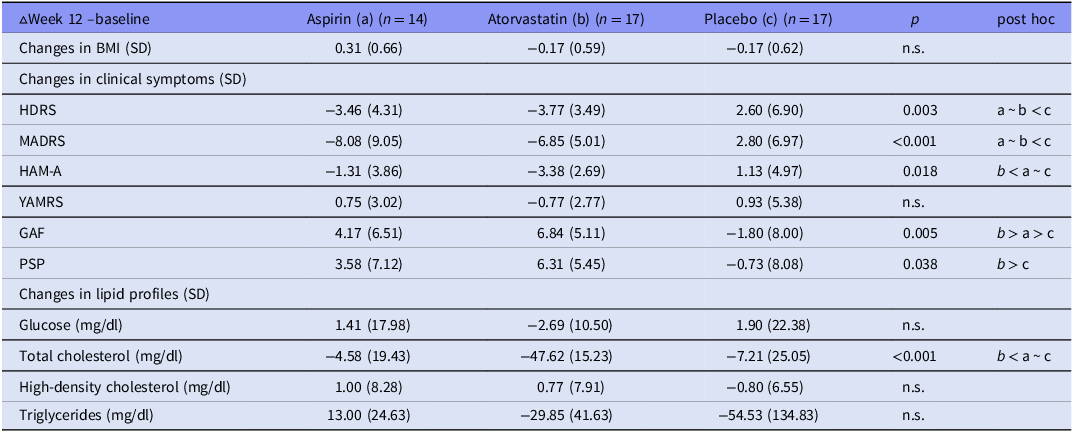
Overall, we randomly assigned 14 patients (7 with MDD, 7 with BD), 17 (9/8), and 17 (9/8) to three 12-week treatment groups, namely aspirin (100 mg/day), atorvastatin (10 mg/day), and placebo, respectively, without any differences in age, sex, BMI, clinical symptoms, lipid profiles, and proinflammatory cytokine levels (Table 1). Table 2 showed the unadjusted change rates of clinical symptoms, lipid profiles, and proinflammatory cytokine levels. Patients in both treatment groups exhibited the greater reduction in the HDRS (p = 0.003), MADRS (p < 0.001), and HAM-A (p = 0.018) scores between baseline and week 12 compared with the placebo group (Table 2). Patients in the atorvastatin group exhibited the most increase in the GAF (p = 0.005) and PSP (p = 0.038) scores compared with the other two groups (Table 2). Only the atorvastatin group had a significant reduction (p < 0.001) in the total cholesterol levels compared with the aspirin and placebo groups (Table 2).
Table 1. Baseline demographic, clinical, and inflammatory cytokine data between groups

MDD, major depressive disorder; BD, bipolar disorder; BMI, body mass index; SD, standard deviation; HDRS, 17-item Hamilton Depression Rating scale; HAM-A, Hamilton Anxiety Rating Scale; MADRS, Montgomery Åsberg Depression Rating Scale; GAF, Global Assessment of Functioning scale; PSP, Personal and Social Performance Scale; sIL-2R, soluble IL-2 receptor; CRP, C-reactive protein; sTNF-αR1, soluble tumour necrosis factor-α receptor 1; n.s., non-significant.
Table 2. Changes in clinical symptoms and lipid profiles between three treatment groups following a 12-week intervention

BMI, body mass index; SD, standard deviation; HDRS, 17-item Hamilton Depression Rating scale; HAM-A, Hamilton Anxiety Rating Scale; MADRS, Montgomery Åsberg Depression Rating Scale; GAF, Global Assessment of Functioning scale; PSP, Personal and Social Performance Scale.
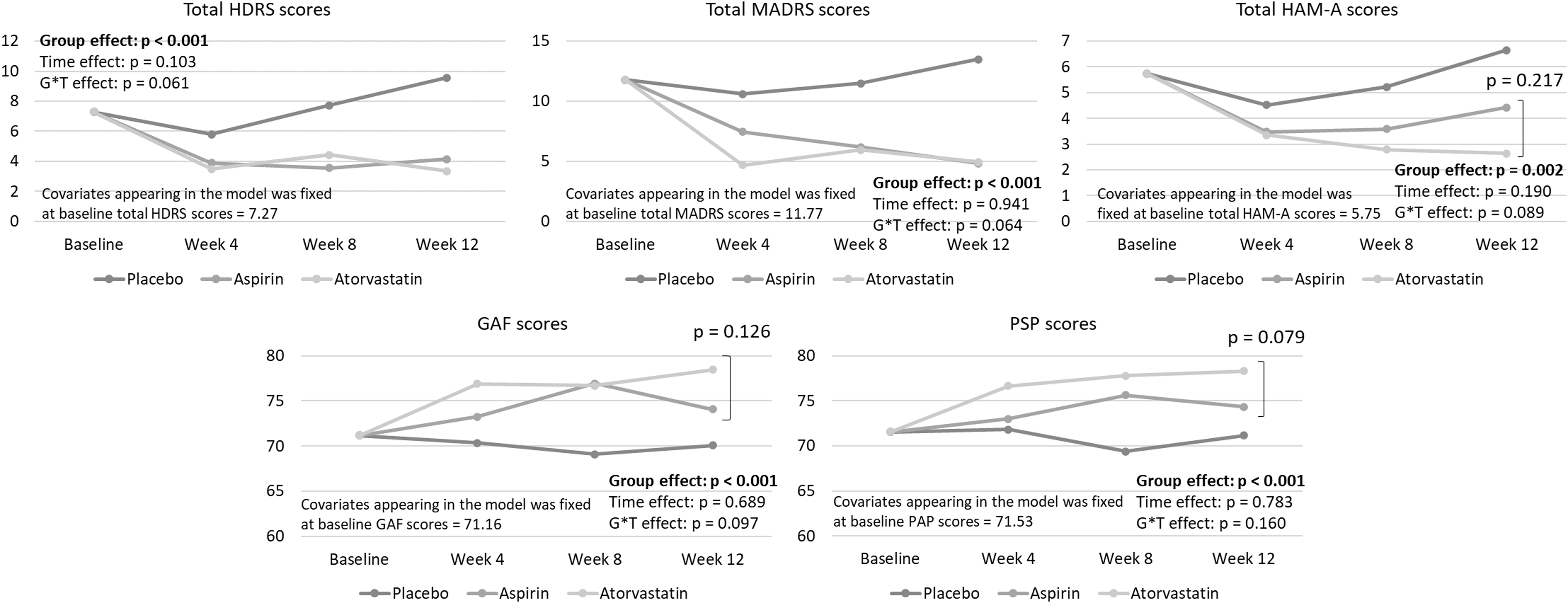
The GEE models with adjustment of age, sex, diagnoses, and baseline clinical score demonstrated that the total HDRS (p < 0.001), MADRS (p < 0.001) and HAM-A (p = 0.002) scores for the two treatment groups went down significantly compared with the placebo group (Figure 1). The GEE model found increasing GAF (p < 0.001) and PSP (p < 0.001) trajectories in the aspirin and atorvastatin groups compared with the placebo group, without a difference in the GAF and PSP scores between two treatment groups (Figure 1).

Figure 1. Generalised estimating equations for the estimated trajectory of clinical symptoms between groups after adjusting for age, sex, diagnoses, and baseline clinical scores. HDRS, 17-item Hamilton depression rating scale; HAM-A, Hamilton anxiety rating scale; MADRS, Montgomery Åsberg depression rating scale; GAF, global assessment of functioning scale; PSP, personal and social performance scale.
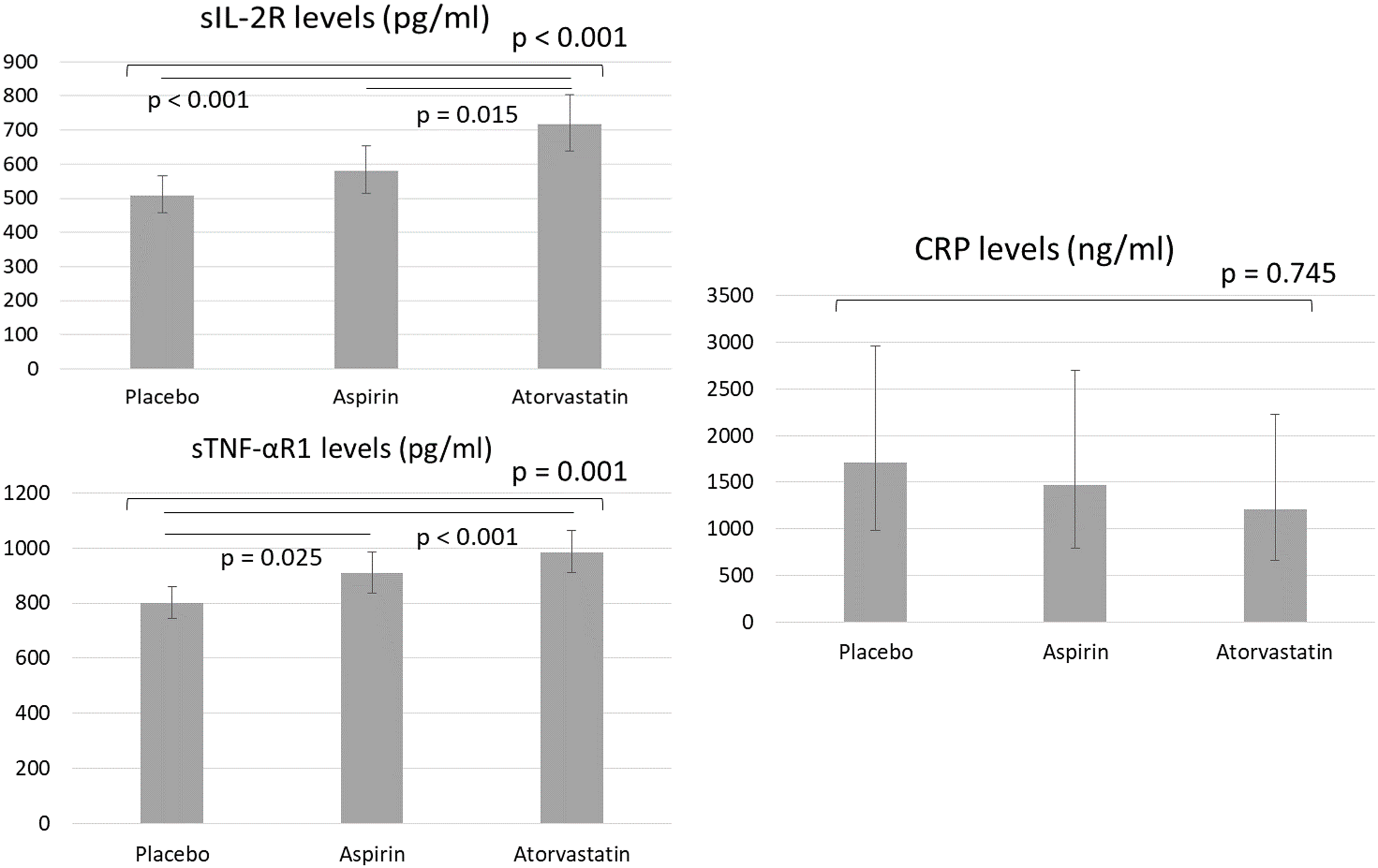
Figure 2 showed the proinflammatory cytokine levels between groups after adjusting for age, sex, BMI, diagnoses, and baseline cytokine levels. Patients in the atorvastatin group exhibited the highest levels of sIL-2R (p < 0.001) and sTNF-αR1 (p = 0.001) compared with the other two groups (Figure 2). Patients in the aspirin group had higher sTNF-αR1levels (p = 0.025) than the control group (Figure 2). CRP levels did not differ between groups (Figure 2). Finally, after adjusting for groups, age, sex, BMI, and diagnosis, we found a correlation between lower depressive symptoms and higher GAF (HDRS: B = −0.731, p < 0.001; MADRS: B = −0.601, p < 0.001) and PSP (HDRS: B = −0.754, p = 0.001; MADRS: B = −0.650, p < 0.001) scores and a correlation between higher sTNF-R1 levels and higher sIL-2R levels (B = 0.657, p < 0.001). We found no associations between changes in depressive symptoms and overall function and changes in cytokines (all p > 0.05).

Figure 2. Generalised linear models with gamma log link for estimated proinflammatory cytokines between groups after adjusting for age, sex, BMI, diagnoses, and baseline cytokine levels. BMI, body mass index; sIL-2R, soluble IL-2 receptor; CRP, C-reactive protein; sTNF-αR1, soluble tumour necrosis factor-α receptor 1.
Discussion
The present study’s findings partially supported the hypothesis that patients in both the aspirin and atorvastatin groups would experience substantial improvements in depressive and anxiety symptoms and overall function compared with the placebo group. Specifically, the increase in GAF and PSP scores was correlated with the expected reduction in depressive symptoms. However, only atorvastatin increased the levels of the anti-inflammatory cytokine sIL-2R. Nevertheless, both atorvastatin and aspirin elevated the levels of the proinflammatory cytokine sTNF-αR1. Furthermore, patients in the atorvastatin group experienced a significant reduction in total cholesterol.
The antidepressant and function-enhancing effects of anti-inflammatory medications, such as NSAIDs and statins, remain controversial (Berk et al., Reference Berk, Mohebbi, Dean, Cotton, Chanen, Dodd, Ratheesh, Amminger, Phelan, Weller, Mackinnon, Giorlando, Baird, Incerti, Brodie, Ferguson, Rice, Schäfer, Mullen, Hetrick, Kerr, Harrigan, Quinn, Mazza, McGorry and Davey2020, Bauer et al., Reference Bauer, Green, Colpo, Teixeira, Selvaraj, Durkin, Zunta-Soares and Soares2018). A meta-analysis of 36 randomised clinical trials (13 NSAIDs, 9 cytokine inhibitors, 7 statins, 3 minocycline, 2 pioglitazone, and 2 glucocorticoids) revealed that add-on anti-inflammatory agents improved depressive symptoms compared with placebo in patients experiencing a major depressive episode (Kohler-Forsberg et al., Reference Köhler‐Forsberg, N. Lydholm, Hjorthøj, Nordentoft, Mors and Benros2019). Nevertheless, Raison et al. suggested that the antidepressant effect of anti-inflammatory medications only occurred in patients with major affective disorders in a hyperinflammatory state (Raison et al., Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake, Haroon and Miller2013). However, Bauer et al. indicated that the combination of aspirin and NAC produced an antidepressant effect in patients with bipolar depression, regardless of CRP levels (Bauer et al., Reference Bauer, Green, Colpo, Teixeira, Selvaraj, Durkin, Zunta-Soares and Soares2018). The findings of the present study and that by Gougol et al.’s clinical trial support the antidepressant effects of add-on statins (atorvastatin and simvastatin) (Gougol et al., Reference Gougol, Zareh-Mohammadi, Raheb, Farokhnia, Salimi, Iranpour, Yekehtaz and Akhondzadeh2015). Additionally, Mendlewicz et al. reported that aspirin enhanced the antidepressant effects of selective reuptake inhibitors in patients with MDD (Mendlewicz et al., Reference Mendlewicz, Kriwin, Oswald, Souery, Alboni and Brunello2006). By contrast, a 12-week, double-blind, placebo-controlled trial of 165 patients with MDD did not support the antidepressant effects of add-on simvastatin, and changes in cytokine levels did not mediate the response to simvastatin (Husain et al., Reference Husain, Chaudhry, Khoso, Kiran, Khan, Ahmad, Hodsoll, Husain, Naqvi, Nizami, Chaudhry, Khan, Minhas, Meyer, Ansari, Mulsant, Husain and Young2023). Furthermore, Berk et al. demonstrated that adding rosuvastatin or aspirin to standard depression therapy in young individuals with MDD yielded no improvement in therapeutic benefits (Berk et al., Reference Berk, Mohebbi, Dean, Cotton, Chanen, Dodd, Ratheesh, Amminger, Phelan, Weller, Mackinnon, Giorlando, Baird, Incerti, Brodie, Ferguson, Rice, Schäfer, Mullen, Hetrick, Kerr, Harrigan, Quinn, Mazza, McGorry and Davey2020).
The association between anti-inflammatory medications and anxiety was also uncertain (Molero et al., Reference Molero, Cipriani, Larsson, Lichtenstein, D’Onofrio and Fazel2020, Yang et al., Reference Yang, Yang, Zeng, Jia and Sun2024). Two cohort studies from Molero et al. and Yang et al. investigated the potential effect of statin on the anxiety disorder risk and found inconsistent findings, indicating no association in Molero et al.’s study but a protective effect in Yang et al.’s study (Molero et al., Reference Molero, Cipriani, Larsson, Lichtenstein, D’Onofrio and Fazel2020, Yang et al., Reference Yang, Yang, Zeng, Jia and Sun2024). As previously mentioned, patients with MDD randomly assigned to add-on simvastatin or placebo treatment did not differ in either anxiety or depressive symptoms (Husain et al., Reference Husain, Chaudhry, Khoso, Kiran, Khan, Ahmad, Hodsoll, Husain, Naqvi, Nizami, Chaudhry, Khan, Minhas, Meyer, Ansari, Mulsant, Husain and Young2023). Furthermore, Hu et al. found conflicting findings of aspirin and non-aspirin NSAIDs on the risk of depression, anxiety, and stress-related disorders during the first year after cancer diagnosis (Hu et al., Reference Hu, Sjölander, Lu, Walker, Sloan, Fall, Valdimarsdóttir, Hall, Smedby and Fang2020). They demonstrated that the aspirin use was associated with a lower rate of depression, anxiety, and stress-related disorders (HR: 0.88, 95% CI: 0.81–0.97), whereas the use of non-aspirin NSAIDs was associated with a higher rate (1.24, 1.15–1.32) (Hu et al., Reference Hu, Sjölander, Lu, Walker, Sloan, Fall, Valdimarsdóttir, Hall, Smedby and Fang2020). Our findings identified that patients in the aspirin and atorvastatin groups had lower HAM-A scores during the clinical trial period than those in the placebo group.
A second major finding of our clinical trial was the cytokine modulation effects of aspirin and atorvastatin (Ghanizadeh & Hedayati, Reference Ghanizadeh and Hedayati2014). Both medications increased levels of the proinflammatory cytokine sTNF-αR1, despite the significant reduction in total cholesterol observed in the atorvastatin group (Ghanizadeh & Hedayati, Reference Ghanizadeh and Hedayati2014). Notably, only atorvastatin elevated the levels of the anti-inflammatory cytokine sIL-2R. IL-2 is crucial in stimulating the growth of natural killer cells and T lymphocytes, supporting anti-inflammatory processes and potentially exerting anti-malignancy effects (Berraondo et al., Reference Berraondo, Sanmamed, Ochoa, Etxeberria, Aznar, Pérez-Gracia, Rodríguez-Ruiz, Ponz-Sarvise, Castañón and Melero2019, de Rham et al., Reference de Rham, Ferrari-Lacraz, Jendly, Schneiter, Dayer and Villard2007). By contrast, TNF-α, is one of the most reliable proinflammatory markers in major affective disorders (Cakici et al., Reference Çakici, Sutterland, Penninx, Dalm, de Haan and van Beveren2020, Chen et al., Reference Chen, Li, Lin, Hong, Tu, Bai, Cheng and Su2018, Bai et al., Reference Bai, Chen, Hsu, Huang, Tu, Chang, Su, Li, Lin and Tsai2020, Chen et al., Reference Chen, Kao, Chang, Tu, Hsu, Huang, Su, Li, Lin, Tsai and Bai2020, Huang et al., Reference Huang, Chen, Hsu, Huang, Tsai, Su and Bai2022b, Huang et al., Reference Huang, Chen, Chan, Li, Tsai, Bai and Su2022a). For example, Raison et al. reported that the TNF antagonist infliximab reduced CRP levels in patients with MDD, who subsequently achieved a 50% reduction in HDRS scores (Raison et al., Reference Raison, Rutherford, Woolwine, Shuo, Schettler, Drake, Haroon and Miller2013). Additionally, Murata et al. discovered that adding the NSAID celecoxib had no effect on proinflammatory IL-1β levels in patients with BD (Murata et al., Reference Murata, Murphy, Hoppensteadt, Fareed, Welborn and Halaris2020). However, Ghanizadeh et al. discovered that aspirin negatively affects the balance between pro- and anti-inflammatory cytokines in mood disorders (Ghanizadeh & Hedayati, Reference Ghanizadeh and Hedayati2014), corroborating our findings that although aspirin and atorvastatin may exert antidepressant effects and improve clinical symptoms, they may not always reduce proinflammatory cytokine levels in patients with major affective disorders. Further trials are required to clarify the complex relationships between anti-inflammatory agents, cytokine levels, and antidepressant effects in these patients.
This study has several limitations. First, the study only included patients with mild or residual depression, which may limit the generalisability of the findings to those with severe depression. However, examining the therapeutic effects of anti-inflammatory medications in this group remains clinically valuable. Second, the study focused on add-on treatments without altering existing medications during the trial. Thus, the results should be interpreted as reflecting the combination of current and add-on medications. Third, the present study evaluated only three cytokines – CRP, sIL-2R, and sTNF-αR1. Future research should investigate whether atorvastatin and aspirin affect other cytokines associated with depression. Fourth, following the findings of Verma et al., Lumeng et al., and our previous studies (Verma et al., Reference Verma, Bhatta, Davies, Deanfield, Garvey, Jensen, Kandler, Kushner, Rubino and Kosiborod2023, Bai et al., Reference Bai, Su, Li, Tsai, Chen, Tu and Chiou2015, Lumeng & Saltiel, Reference Lumeng and Saltiel2011), the inflammatory dysregulation was defined based on the biochemical (CRP and TNF-αR1 levels) or phenotypic (BMI and abdominal obesity) inflammatory criteria. The levels of total cholesterol, HDL, and LDL were not used as an inflammatory criterion in the present study. Fifth, the inflammatory dysregulation status was only defined according to a cross-sectional measurement in the present study. Whether the inflammatory dysregulation status was acute, subacute, or chronic was not able to be exactly defined. In addition, the inflammatory condition was also related to liver function, although patients with major physical diseases, such as cirrhosis, were excluded in the present study.
In conclusion, atorvastatin and aspirin demonstrated antidepressant effects and improved overall function in patients with major affective disorders and inflammatory dysregulation compared with placebo. Atorvastatin increased both sIL-2R and sTNF-αR1 levels, whereas aspirin only increased sTNF-αR1. Further studies are required to validate these findings and clarify the complex relationships between cytokine levels and antidepressant effects. Given our finding that anti-inflammatory agents improved clinical symptoms and inflammatory markers, whether these agents also enhance cognitive function and modulate brain activity warrants investigation.
Data availability statement
The datasets generated during and/or analysed during the current study are not publicly available due to Taiwan’s clinical trial ethical regulati on but are available from the corresponding author on reasonable request.
Acknowledgements
The authors thank Mr. I-Fan Hu, MA (Courtauld Institute of Art, University of London; National Taiwan University) for his friendship and support. Mr. Hu declares no conflicts of interest.
Author contributions
Drs YMB and MHC designed the study, analysed the data, and drafted a manuscript; Drs YMB, JWH, YJL, and MHC enrolled the candidate patients and performed the literature reviews; all authors reviewed the final manuscript critically and agreed for its publication.
Funding statement
The study was supported by grant from Taipei Veterans General Hospital (V112C-111, V113C-060), Yen Tjing Ling Medical Foundation (CI-109-21, CI-109-22, CI-110-30, CI-113-30, CI-113-31, CI-113-32), and Ministry of Science and Technology, Taiwan (NSTC 112-2314-B-A49-025-MY3). The funding source had no role in any process of our study.
Competing interests
All authors have no financial relationships relevant to this article to disclose.
Ethical standards
The Taipei Veterans General Hospital’s Institutional Review Board approved this study, which followed the Declaration of Helsinki. Before participating in this study, all participants provided written informed consent. Clinical trial registration: NCT04685642.