Introduction
There are significant geographical variations in the prevalence of attention-deficit/hyperactivity disorder (ADHD) drug use in children and adolescents (Kovess et al. Reference Kovess, Choppin, Gao, Pivette, Husky and Leray2015; Beau-Lejdstrom et al. Reference Beau-Lejdstrom, Douglas, Evans and Smeeth2016; Burcu et al. Reference Burcu, Zito, Metcalfe, Underwood and Safer2016; Piovani et al. Reference Piovani, Clavenna, Cartabia and Bonati2016; Furu et al. Reference Furu, Karlstad, Zoega, Martikainen, Bahmanyar, Kieler and Pottegard2017; Wang et al. Reference Wang, Lee, Yuan, Yang, Yang, Huang, Chou, Chou, Lee, Lee and Shyu2017), although the prevalence of ADHD does not vary as a function of geographical location (Thomas et al. Reference Thomas, Sanders, Doust, Beller and Glasziou2015). Understanding the geographical discrepancy in drug use will provide insights on potential over- or undermedication in the population. However, little is known about ADHD drug use in the Japanese population. Thus, we aimed to estimate the prevalence, incidence and persistence of ADHD drug use in children and adolescents in Japan.
Methods
Data source
A retrospective cohort study was conducted using the National Database of Health Insurance Claim Information and Specified Medical Checkups (NDB) that covered all electronically issued claims covered by public health insurance in Japan (Ministry of Health, Labour & Welfare, 2013; Okumura et al. Reference Okumura, Sakata, Takahashi, Nishi and Tachimori2017). As of April 2014, the proportion of electronically issued claims relative to all claims was 99.9% in hospitals, 95.9% in clinics and 99.9% in pharmacies. Claims for recipients of public assistance were not included in the NDB, comprising 286 048 inhabitants aged 0–19 years in 2014 (approximately 1% of the population). The NDB included information on clinical and procedural characteristics such as patient identification numbers, sex, age group, prescription date, drug codes, days of drug supply and dosage.
Settings
Japan has 21 001 000 inhabitants aged ≤18 years (Ministry of Internal Affairs & Communications, 2015). Atomoxetine (ATX) (available since June 2009) and the osmotic-controlled release oral delivery system methylphenidate (OROS-MPH) (available since December 2007) were the only drugs approved for ADHD in the fiscal year of 2014 (between April 2014 and March 2015). OROS-MPH can be prescribed by only licensed physicians with expertise in ADHD treatment. However, immediate-release methylphenidate (IR-MPH) is not approved for ADHD treatment in Japan.
In general, the universal health insurance system of Japan pays for 70% of the medical treatment costs and the other healthcare systems (i.e. the System of Medical Payment for Services and Supports for Persons with Disabilities; and the Medical Subsidy for Children and Infants) pay for the remaining 20–30% for children and adolescents.
Statistical analyses
We identified patients aged ≤18 years who were given at least one prescription of ADHD drug in the fiscal year of 2014. The annual prevalence of ADHD drug use was defined as the number of prevalent users per 1000 inhabitants.
Next, we identified the subgroup focusing on the incident and persistent users of ADHD drugs. First, we defined the index date as the date on which the ADHD drug was first prescribed to the patient during the fiscal year of 2014. We included the patients who had been enrolled in the database at least 180 days before and after the index date, as in previous studies (Lawson et al. Reference Lawson, Johnsrud, Hodgkins, Sasane and Crismon2012; Palli et al. Reference Palli, Kamble, Chen and Aparasu2012). Second, we excluded those who had a bundled payment claim within 180 days before and after the index date, in which the prescription status was not recorded. Finally, we excluded those who had a prescription of ADHD drugs within 180 days before the index date. All patients were followed up using an identification number (Kubo et al. Reference Kubo, Noda, Myojin, Nishioka, Higashino, Matsui, Kato and Imamura2018). The annual incidence of ADHD drug use was defined as the number of incident users per 1000 inhabitants. The percentage of persistent ADHD drug use was defined as the number of persistent users at least 150 days after the index date per 100 incident users, as in a previous study (Lawson et al. Reference Lawson, Johnsrud, Hodgkins, Sasane and Crismon2012). Discontinuation (non-persistence) was designated when a prescription medication for ADHD was not refilled within an interval defined by the days of drug supply plus a grace period of 30 days.
Subgroup analyses were conducted by sex and age groups. Age was classified into four groups: 0–6, 7–12, 13–15 and 16–18 years according to the school system (preschool, elementary school, junior high school and high school, respectively). All estimates were calculated with 95% confidence intervals (CI). All analyses were conducted using R version 3.4.1.
Results
There were 86 756 prevalent and 30 449 incident users of ADHD drugs in the database (Table 1). The annual prevalence per 1000 inhabitants was 4.1 with a peak of 7–12 years for both sexes (Table 2). Of the prevalent users, 64% used OROS-MPH (Table 1). Of the incident users, 61% still continued drug treatment at 150 days after the index date (Fig. 1). The persistence rate was much higher among those aged 7–12 years than among those aged 16–18 years (65 v. 43%).

Fig. 1. Percentage of persistent ADHD drug users.
Table 1. Sample characteristics of patients
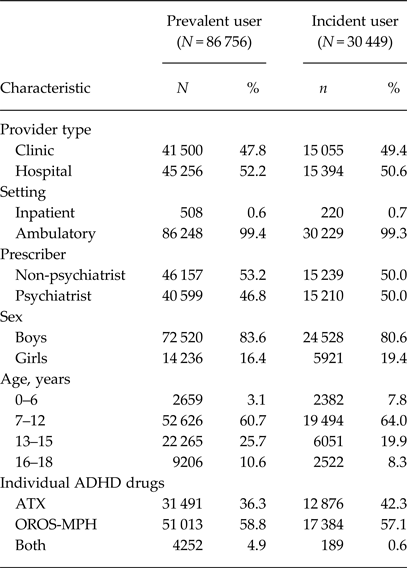
ADHD, attention-deficit/hyperactivity disorder; ATX, atomoxetine; OROS-MPH, osmotic-controlled release oral delivery system methylphenidate.
Table 2. Prevalence, incidence and persistence of ADHD drug users in children and adolescents
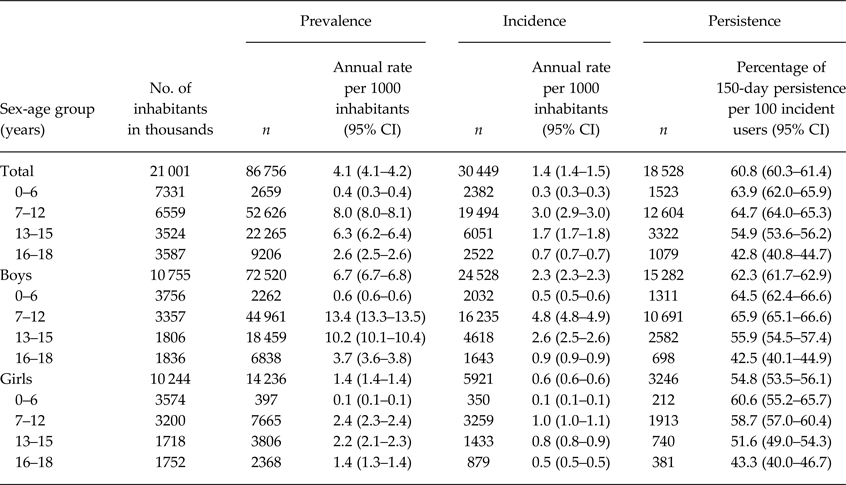
ADHD, attention-deficit/hyperactivity disorder; CI, confidence interval.
Discussion
This is the first study to establish the representative prescribing practices of ADHD drugs in Japan. The prevalence of ADHD drug use in children and adolescents in Japan (0.4%) is much lower than that in the USA (5.3%) (Burcu et al. Reference Burcu, Zito, Metcalfe, Underwood and Safer2016) and Norway (1.4%) (Furu et al. Reference Furu, Karlstad, Zoega, Martikainen, Bahmanyar, Kieler and Pottegard2017), while it is similar to that in Italy (0.2%) (Piovani et al. Reference Piovani, Clavenna, Cartabia and Bonati2016), France (0.2%) (Kovess et al. Reference Kovess, Choppin, Gao, Pivette, Husky and Leray2015) and the UK (0.5%) (Beau-Lejdstrom et al. Reference Beau-Lejdstrom, Douglas, Evans and Smeeth2016). As in Japan, the countries with a similar prevalence have some restriction policies for prescribing ADHD drugs. In Italy, ATX and IR-MPH can be initiated only by specialists with expertise in ADHD treatment after a standardised diagnostic process (Piovani et al. Reference Piovani, Clavenna, Cartabia and Bonati2016). These drugs can be re-filled by a general practitioner; however, specialists must compile an individual therapeutic plan that contains all the details on dosage and duration of therapy. In France, IR-MPH and OROS-MPH must be initiated by specialists such as child psychiatrists (Kovess et al. Reference Kovess, Choppin, Gao, Pivette, Husky and Leray2015). In the UK, ATX, dexamphetamine, guanfacine, lisdexamfetamine, IR-MPH and OROS-MPH are recommended to be initiated only by professionals with expertise in ADHD treatment (National Institute for Health & Care Excellence, 2006). Such a restriction policy may contribute to a relatively low prevalence of ADHD drug use in the population.
Our results indicate that children and adolescents with ADHD may be currently undermedicated in Japan. The National Institute for Health and Care Excellence guidelines recommend that pharmacological treatment should be initiated when ADHD symptoms are persistent and still causing significant impairment in at least one domain every day despite the implementation and review of environmental modifications (National Institute for Health & Care Excellence, 2018). The Regional ADHD Registry in Italy found that 44% of children and adolescents with ADHD had a severe impairment as indicated by a Clinical Global Impressions-Severity score of 5 or higher (Bonati et al. Reference Bonati, Reale, Zanetti, Cartabia, Fortinguerra, Capovilla, Chiappedi, Costantino, Effedri, Luoni, Martinelli, Molteni, Ottolini, Saccani and Lombardyin press). Given the ADHD prevalence of 3.4–7.2% (Polanczyk et al. Reference Polanczyk, Salum, Sugaya, Caye and Rohde2015; Thomas et al. Reference Thomas, Sanders, Doust, Beller and Glasziou2015) and the ratio of severely impaired ADHD (Bonati et al. Reference Bonati, Reale, Zanetti, Cartabia, Fortinguerra, Capovilla, Chiappedi, Costantino, Effedri, Luoni, Martinelli, Molteni, Ottolini, Saccani and Lombardyin press), 1.5–3.2% of children and adolescents would benefit from pharmacological treatment.
Among ADHD drug users, the percentage of methylphenidate use is much lower in Japan (64%) than in the UK (94%) (Beau-Lejdstrom et al. Reference Beau-Lejdstrom, Douglas, Evans and Smeeth2016), Norway (94%) (Furu et al. Reference Furu, Karlstad, Zoega, Martikainen, Bahmanyar, Kieler and Pottegard2017) and Germany (75–100%) (Bachmann et al. Reference Bachmann, Philipsen and Hoffmann2017). This may be partly explained by the fact that the restriction policy for prescribing ADHD drugs in Japan is only applied to OROS-MPH. For instance, ATX can be prescribed by any physician in Japan. This imbalance in the restriction policy may contribute to a decrease in the use of OROS-MPH. Other explanations for the low prevalence of methylphenidate prescriptions are that IR-MPH is not approved for ADHD treatment in Japan and that both ATX and OROS-MPH are considered as the first-line drugs for ADHD treatment in the Japanese clinical practice guideline (Saito, Reference Saito2016).
In addition, the persistence rate of ADHD drug use is much higher in Japan (61%) than that in the USA (10–29% at 150 days) (Lawson et al. Reference Lawson, Johnsrud, Hodgkins, Sasane and Crismon2012), while it is similar to that in the UK (66% at 1 year) according to the study conducted by Beau-Lejdstrom et al. who used a threefold wider grace period (Beau-Lejdstrom et al. Reference Beau-Lejdstrom, Douglas, Evans and Smeeth2016). We also observed a substantially lower persistence of ADHD drugs among patients who started taking drugs at an older age. These findings were consistent with those of previous studies (Beau-Lejdstrom et al. Reference Beau-Lejdstrom, Douglas, Evans and Smeeth2016; Wang et al. Reference Wang, Yang, Lee, Yang, Huang, Lee, Yuan and Shyu2016). Future research should clarify the reason for early cessation of ADHD drugs, particularly focusing on high school grades.
The main limitation of this study is that the entire population was not accounted for in the database, which comprised 1–2% of all inhabitants. Nevertheless, our study provides representative evidence on the treatment pattern of ADHD drug use in children and adolescents in Japan.
Acknowledgements
We are grateful to Dr Daniele Piovani, Dr Raphaelle Beau-Lejdstrom and Dr Thierry Trenque for providing us with information on the restriction policies for ADHD drugs. We would like to thank Editage (www.editage.jp) for English language editing.
Financial Support
This work was supported by a grant from the Japan Society for the Promotion of Science (number: 18K09991).
Conflict of Interest
During the past 3 years, YO received personal fees from Merck & Co., Inc.; Janssen Pharmaceuticals Inc.; Medical Technology Association; Cando Inc.; and the Japan Medical Data Center. YO has also received research grants from the Japan Agency for Medical Research and Development; Ministry of Health, Labour and Welfare; Japan Society for the Promotion of Science; Institute for Health Economics and Policy; and Mental Health and Morita Therapy. MU received personal fees from Janssen Pharmaceutical Inc.; and Eli Lilly Japan. MU has also received grants from the National Center for Global Health and Medicine; and the Agency for Medical Research and Development. TO received personal fees from Janssen Pharmaceutical Inc.; Eli Lilly Japan; Shionogi & Co. Ltd.; Shire Japan Co. Ltd.; Otsuka Pharmaceutical Company; Meiji Seika Pharma Co., Ltd.; Taisho Pharmaceutical Co., Ltd.; Mochida Pharmaceutical Co., Ltd.; Yoshitomiyakuhin Corporation; Chugai Pharmaceutical Co., Ltd.; Eisai Co., Ltd.; MSD K.K.; Novartis Pharmaceutical Company; Sumitomo Dainippon Pharma Co., Ltd.; Mitsubishi Tanabe Pharmaceutical Company; and Kyowa Hakko Kirin Co., Ltd. TO also has received research grants Otsuka Pharmaceutical Company; Ministry of Health, Labour and Welfare; Ministry of Education, Culture, Sports, Science and Technology; Agency for Medical Research and Development; and the Japan Society for the Promotion of Science. TS received personal fees from Eli Lilly Japan; Janssen Pharmaceuticals Inc.; Otsuka Pharmaceutical Company; Sumitomo Dainippon Pharma; Shionogi & Co. Ltd.; and Shire Japan Co. Ltd. HN received personal fees from Janssen Pharmaceutical Inc.; Eli Lilly Japan; Shionogi & Co. Ltd.; Shire Japan Co. Ltd.; and Otsuka Pharmaceutical Company. NT received personal fees from Janssen Pharmaceuticals Inc.; Sumitomo Dainippon Pharma; Mitsubishi Tanabe Pharmaceutical Company; Yoshitomiyakuhin Corporation; GlaxoSmithKline plc; Kyowa Hakko Kirin Co., Ltd. NT received personal fees Janssen Pharmaceuticals Inc.; Sumitomo Dainippon Pharma Co., Ltd.; Mitsubishi Tanabe Pharmaceutical Company; Yoshitomiyakuhin Corporation; GlaxoSmithKline plc; and Kyowa Hakko Kirin Co., Ltd. JF has nothing to disclose. JI received personal fees from Janssen Pharmaceuticals Inc.; Eli Lily Japan; Shionogi & Co. Ltd.; Shire Japan Co. Ltd.; Otsuka Pharmaceutical Company; Mochida Pharmaceutical Co., Ltd.; MSD K.K.; Sumitomo Dainippon Pharma Co., Ltd. JI also has received grants from the Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare; and the Agency for Medical Research and Development.
Ethical Standards
The local institutional review board at the Institute for Health Economics and Policy approved our study protocol. The review board waived the requirement for informed consent due to the anonymous nature of data.




