Neuroleptic-induced extrapyramidal symptoms often lead to non-compliance and consequently poorer treatment outcome (Reference GerlachGerlach, 2002). Extrapyramidal symptoms may also occur with modern, atypical neuroleptic medication (Reference Tarsy, Baldessarini and TaraziTarsy et al, 2002). Risk factors include age, psychiatric diagnosis, psychopathology, and dosage and duration of neuroleptic exposure, but no clear-cut predictor has been identified as yet (Reference AydAyd, 1961; Reference SwettSwett, 1975; Reference Nasrallah, Churchill and Hamdan-AllenNasrallah et al, 1988; Reference Berardi, Giannelli and BiscioneBerardi et al, 2000; Reference Srinivasan, Thara and PadmavathiSrinivasan et al, 2001). More recently, genetic factors have been suggested to have a role in the susceptibility to extrapyramidal symptoms (Reference Basile, Masellis and PotkinBasile et al, 2002; Reference Segman, Heresco-Levy and YakirSegman et al, 2002). Given that a genetic predisposition may increase the susceptibility to such symptoms, a positive family history of primary movement disorders may be associated with their development. An increasing number of polymorphisms in dystonia and parkinsonism genes have been shown to be associated with primary movement disorders (Reference Klein and OzeliusKlein & Ozelius, 2002; Reference GasserGasser, 2003). We therefore investigated whether a family history of primary movement disorders might be a predictor of extrapyramidal symptoms in patients receiving typical and atypical neuroleptic medication.
METHOD
Patients and diagnosis
Participants were consecutively recruited from people admitted as in-patients of the Department of Psychiatry and Psychotherapy at the University of Lübeck. Each participant gave written informed consent after having been carefully informed about the study. The study was approved by the local ethics committee. Participants had to meet the following inclusion criteria:
-
(a) stable neuroleptic medication for at least 1 week;
-
(b) no neurological disease;
-
(c) no significant history of head trauma;
-
(d) no other medication that could potentially induce movement disorder.
Operational psychiatric lifetime diagnoses according to DSM–IV (American Psychiatric Association, 1994) were established using the German version of the Mini-International Neuropsychiatric Interview (MINI; Reference Sheehan, Lecrubier and SheehanSheehan et al, 1998) and the Structured Clinical Interview for Personality Disorders (SCID–II; Reference Spitzer and WilliamsSpitzer & Williams, 1987). Diagnoses were divided into four categories: organic psychiatric disorders, including all forms of alcohol and drug dependence; psychotic disorders; affective disorders; and other Axis I or Axis II disorders. Psychopathological symptom severity was rated on the Brief Psychiatric Rating Scale (BPRS; Reference Overall and GorhamOverall & Gorham, 1962). Each participant underwent a complete neurological examination with particular emphasis on specific signs of acute dystonic reaction, parkinsonism, akathisia and tardive dyskinesia. The following rating scales were used to assess the severity of extrapyramidal symptoms (Reference van Harten, Hoek and Matroosvan Harten et al, 1997): the Abnormal Involuntary Movement Scale (AIMS; National Institute of Mental Health, 1975); the Tsui Rating Scale for Cervical Dystonia (Reference Tsui, Eisen and StoesslTsui et al, 1986) and the Burke Rating Scale for Primary Torsion Dystonias (Reference Burke, Fahn and MarsdenBurke et al, 1985); Part III of the Unified Parkinson's Disease Rating Scale (UPDRS; Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease, 2003); the global score on the Hillside Scale (Reference Fleischhacker, Miller and SchettFleischhacker et al, 1991) and the Barnes Akathisia Scale (Reference BarnesBarnes, 1989). History of extrapyramidal symptoms on previous neuroleptic treatment was explored in a structured interview developed by our group, covering the typical symptoms of acute dystonic reaction, parkinsonism, akathisia and tardive dyskinesia. To check the reliability of their answers, participants were asked about the consequences of the development of their movement disorder (for example, change in medication or administration of biperiden). In addition, clinical records covering former treatment phases were reviewed to complete the information. Family history of movement disorders was obtained by means of a structured interview developed in our department, specifically covering symptoms of Parkinson's disease (stiffness of movement, gait problems, tremor, change in facial expression, lateralisation of symptoms), dystonia and psychiatric disorders in first-degree to third-degree relatives. This structured interview had previously been shown to provide reliable data in a large epidemiological study (Reference Klein, Vieregge and HagenahKlein et al, 1999). A diagnosis of a primary movement disorder was only made if the criteria for the disorder were clearly fulfilled by the symptom description given by the patient. Relatives were asked to also undergo the structured interview whenever possible.
Statistical procedure
Pearson's chi-squared tests for independence were performed to investigate the relationship between categorical outcome variables (lifetime occurrence of extrapyramidal symptoms, including reported and currently observed symptoms; reported extrapyramidal symptoms during previous treatment phases; currently observed extrapyramidal symptoms; extrapyramidal symptoms on typical neuroleptic medication; extrapyramidal symptoms on atypical neuroleptic medication; lifetime occurrence and currently observed acute dystonic reaction, parkinsonism, akathisia and tardive dyskinesia) and possible categorical predictor variables (gender; age; psychiatric diagnostic category; dosage range; duration of exposure to any neuroleptic medication; duration of exposure to typical neuroleptic medication; duration of exposure to atypical neuroleptic medication; positive family history of primary movement disorder). To achieve the required expected cell frequency of more than 5 in χ2-tests, we defined three different age groups: 18–40 years, 41–60 years and >60 years. For the same reason, duration of exposure to neuroleptic medication was categorised as <6 months, 6 months to 5 years, and >5 years. For easier comparison, drug dosages were defined as low, medium or high, based on current clinical practice. Chi-squared values are reported with two-tailed probabilities. Relationships between possible predictor and outcome variables with P<0.5 revealed by χ2-tests were entered in a step-wise logistic regression analysis to identify predicted probabilities for extrapyramidal symptoms (probability to enter at 0.05). By use of a logistic regression analysis, all predictor variables are considered within one testing procedure, including intercorrelations, which reduces the probability of type I errors. All statistical procedures were performed using the Statistical Package for the Social Sciences (version 11.0).
RESULTS
Demographic and clinical data are listed in Table 1. Most patients (62%) had been admitted for treatment of a psychotic disorder: schizophrenia or schizophreniform disorder (n=50), schizoaffective disorder (n=10) or delusional disorder (n=2). Fourteen patients were receiving neuroleptic treatment for an organic psychiatric disorder: substance-induced psychotic episodes (n=10), delirium (n=3) or organic delusional disorder (n=1). Eighteen patients were treated for an affective disorder: unipolar depressive disorder with psychotic symptoms (n=10), bipolar disorder with psychotic symptoms (n=8; five manic and three depressive episodes). Six patients had other psychiatric disorders: borderline or combined personality disorder (n=5) or dissociative disorder (n=1). Analysis of variance including post hoc comparisons revealed that patients with an affective disorder were significantly older than patients from the other groups (F=3.89, d.f.=3, P=0.01), who did not differ significantly with respect to age. The distribution of men and women differed significantly between the diagnostic groups (χ2=17.74, d.f.=1, P<0.01), whereas symptom severity measured by BPRS scores was similar in all groups. More patients had ever been treated with atypical neuroleptic medication (87%) than with typical neuroleptics (64%).
Table 1 Demographic and clinical characteristics of the sample

| n | Age, years | Gender (male/female) | BPRS score | Duration of exposure | |||
|---|---|---|---|---|---|---|---|
| Mean (s.d.) | n/n | Mean (s.d.) | <6 months n | 6 months to 5 years n | <5 years n | ||
| Psychiatric disorder | |||||||
| Organic disorder | 14 | 37.6 (14.9) | 12/2 | 34.2 (12.2) | |||
| Psychotic disorder | 62 | 38.5 (13.8) | 32/30 | 38.5 (10.7) | |||
| Affective disorder | 18 | 49.6 (15.8) | 3/15 | 39.7 (11.1) | |||
| Other Axis I or II | 6 | 31.5 (8.6) | 1/5 | 45.3 (9.1) | |||
| Total sample | 100 | 39.9 (14.7) | 48/52 | 38.5 (11.0) | |||
| Exposure to neuroleptic medication | |||||||
| Any neuroleptic | 100 | 38 | 38 | 24 | |||
| Typical neuroleptic | 64 | 39.1 (14.0) | 34/30 | 37.6 (10.7) | 27 | 19 | 18 |
| Atypical neuroleptic | 87 | 39.0 (14.6) | 42/45 | 38.3 (11.1) | 39 | 39 | 9 |
Lifetime prevalence of extrapyramidal symptoms
Lifetime prevalence of extrapyramidal symptoms, including both reported and currently observed symptoms, was 65% (Table 2). Acute dystonic reactions occurred most commonly (41%), followed by parkinsonism (37%), akathisia (19%) and tardive dyskinesia (4%). It should be noted that several patients suffered from more than one type of extrapyramidal symptom. Of those who had experienced such symptoms, more patients had been exposed to typical than to atypical medication. Details of mean age and distribution of gender are given in Table 2.
Table 2 Clinical characteristics and lifetime prevalences of extrapyramidal symptoms

| n | Age, years | Gender (male/female) | Duration of exposure | |||
|---|---|---|---|---|---|---|
| Mean (s.d.) | n/n | <6 months n | 6 months to 5 years n | <5 years n | ||
| Lifetime prevalence of | ||||||
| Any EPS | 65 | 37.6 (14.7) | 34/31 | |||
| Acute dystonic reaction | 41 | 35.6 (13.8) | 20/21 | |||
| Parkinsonism | 37 | 38.0 (13.5) | 20/17 | |||
| Akathisia | 19 | 35.8 (13.8) | 11/8 | |||
| Tardive dyskinesia | 4 | 36.8 (11.0) | 3/1 | |||
| Symptoms experienced under exposure to | ||||||
| Any neuroleptic | 65 | 15 | 30 | 20 | ||
| Typical neuroleptic | 47 | 38.7 (15.2) | 25/22 | 15 | 17 | 15 |
| Atypical neuroleptic | 34 | 37.3 (15.9) | 17/17 | 13 | 16 | 5 |
Currently observed extrapyramidal symptoms
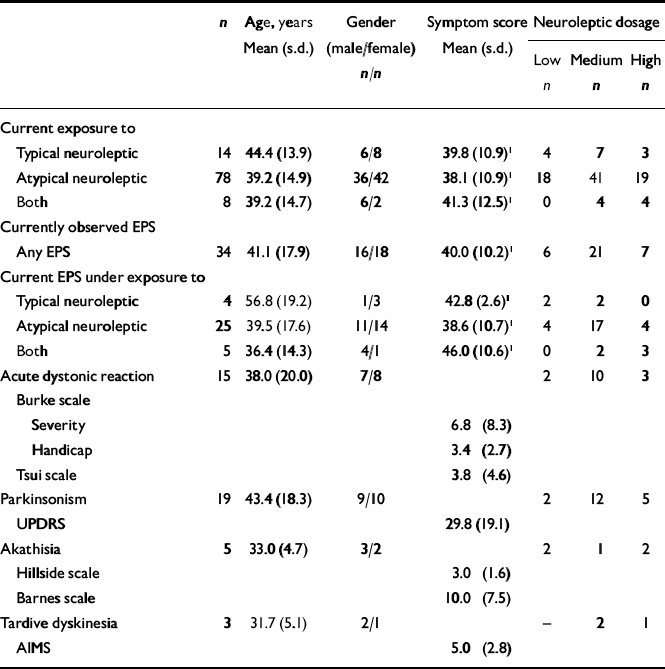
At the time of examination, most patients were taking an atypical neuroleptic medication (Table 3). Extrapyramidal symptoms were diagnosed in 34% of patients. The most commonly observed symptom was parkinsonism (19%), followed by acute dystonic reaction (15%), akathisia (5%) and tardive dyskinesia (3%). Again, some patients were diagnosed with more than one type of extrapyramidal symptom. Table 3 gives further clinical characteristics and details concerning dosages of neuroleptic medication.
Table 3 Clinical characteristics and prevalences of extrapyramidal symptoms at the time of examination
| n | Age, years Mean (s.d.) | Gender (male/female) n/n | Symptom score Mean (s.d.) | Neuroleptic dosage | |||
|---|---|---|---|---|---|---|---|
| Low n | Medium n | High n | |||||
| Current exposure to | |||||||
| Typical neuroleptic | 14 | 44.4 (13.9) | 6/8 | 39.8 (10.9)1 | 4 | 7 | 3 |
| Atypical neuroleptic | 78 | 39.2 (14.9) | 36/42 | 38.1 (10.9)1 | 18 | 41 | 19 |
| Both | 8 | 39.2 (14.7) | 6/2 | 41.3 (12.5)1 | 0 | 4 | 4 |
| Currently observed EPS | |||||||
| Any EPS | 34 | 41.1 (17.9) | 16/18 | 40.0 (10.2)1 | 6 | 21 | 7 |
| Current EPS under exposure to | |||||||
| Typical neuroleptic | 4 | 56.8 (19.2) | 1/3 | 42.8 (2.6)1 | 2 | 2 | 0 |
| Atypical neuroleptic | 25 | 39.5 (17.6) | 11/14 | 38.6 (10.7)1 | 4 | 17 | 4 |
| Both | 5 | 36.4 (14.3) | 4/1 | 46.0 (10.6)1 | 0 | 2 | 3 |
| Acute dystonic reaction | 15 | 38.0 (20.0) | 7/8 | 2 | 10 | 3 | |
| Burke scale | |||||||
| Severity | 6.8 (8.3) | ||||||
| Handicap | 3.4 (2.7) | ||||||
| Tsui scale | 3.8 (4.6) | ||||||
| Parkinsonism | 19 | 43.4 (18.3) | 9/10 | 2 | 12 | 5 | |
| UPDRS | 29.8 (19.1) | ||||||
| Akathisia | 5 | 33.0 (4.7) | 3/2 | 2 | 1 | 2 | |
| Hillside scale | 3.0 (1.6) | ||||||
| Barnes scale | 10.0 (7.5) | ||||||
| Tardive dyskinesia | 3 | 31.7 (5.1) | 2/1 | – | 2 | 1 | |
| AIMS | 5.0 (2.8) | ||||||
Reported symptoms and family history of primary movement disorders
Fifty-three patients (29 men and 24 women, mean age 36.3 years, s.d.=13.2) reported that they had experienced extrapyramidal symptoms during previous treatment phases. Information about the family history of primary movement disorders was available for 98 participants and could be assessed for a total of 1316 relatives, 438 of whom were first-degree relatives. Thirty-two of these 98 patients (20 men and 12 women) reported a positive family history, resulting in a total of 47 affected relatives, or a prevalence of a primary movement disorder of 3.5% among all relatives. Specifically, the prevalences were 1.1% (n=14) for Parkinson's disease, 1.6% (n=21) for tremor and 0.9% (n=2) for dystonia. Among relatives of patients with lifetime extrapyramidal symptoms (n=848), the prevalences were 1.4% (n=12) for Parkinson's disease, 2.4% (n=20) for tremor and 1.1% (n=9) for dystonia, whereas among the relatives of patients without lifetime extrapyramidal symptoms (n=468), the prevalences were 0.4% (n=2) for Parkinson's disease, 0.2% (n=1) for tremor and 0.6% (n=3) for dystonia. A subgroup analysis was performed using data from 27 (2.1%) first-degree relatives of 21 patients who were seen personally by one of the investigators. In all of them, the presence or absence of a primary movement disorder was confirmed as established by the family history interview (25 relatives without a primary movement disorder, 1 with dystonia and 1 with tremor). In order to avoid multiple inclusion of patients with more than one affected relative in further statistical analyses, we considered only the closest relative of those patients. This resulted in 12 patients with a relative with Parkinson's disease (5 first-degree relatives and 7 second-degree relatives), 11 patients with a relative with tremor (6 first-degree relatives and 5 second-degree relatives) and 9 patients with a relative with dystonia (7 first-degree relatives and 2 second-degree relatives).
Relationship between possible predictor and outcome variables
Family history of primary movement disorder
The family history of primary movement disorders was related to lifetime prevalence of extrapyramidal symptoms (EPS) (χ2=8.35, d.f.=1, P<0.01), currently observed EPS (χ2=8.05, d.f.=1, P<0.01), prevalence of reported EPS (χ2=6.75, d.f.=1, P<0.01) and lifetime prevalence of acute dystonic reaction (χ2=4.69, d.f.=1, P=0.03). Table 4 shows that the prevalence of these four related outcome variables was higher in participants with a positive family history than in patients with a negative history. Furthermore, lifetime prevalence of acute dystonic reaction was related to the subtype of primary movement disorders occurring in relatives (χ2=8.27, d.f.=3, P=0.04). Lifetime acute dystonic reaction occurred in 7 of 9 (78%) patients with a family history of dystonia, but only in 7 of 12 (58%) patients with a family history of Parkinson's disease, 4 of 11 (36%) patients with a family history of tremor and 22 of 44 (50%) patients with a negative family history.
Table 4 Distributions of outcome and possible predictor variables

| Possible predictor | Outcome variable | ||||||
|---|---|---|---|---|---|---|---|
| Lifetime EPS (yes/no) n/n | Reported EPS (yes/no) n/n | Currently observed EPS (yes/no) n/n | EPS with typical neuroleptic (yes/no) n/n | Lifetime ADR (yes/no) | Lifetime parkinsonism (yes/no) | Lifetime akathisia (yes/no) | |
| Family history1,2 | P>0.05 | P>0.05 | P>0.05 | ||||
| Positive | 27/5 | 23/9 | 17/15 | 18/14 | |||
| Negative | 36/30 | 29/37 | 16/50 | 22/44 | |||
| Age | P>0.05 | P>0.05 | P>0.05 | ||||
| 18–40 years | 46/11 | 40/17 | 31/26 | ||||
| 41–60 years | 12/18 | 9/21 | P>0.05 | 7/23 | |||
| > 60 years | 7/6 | 4/9 | 3/10 | ||||
| Lifetime exposure to any neuroleptic2 | P>0.05 | P>0.05 | |||||
| <6 months | 15/23 | 6/32 | 10/28 | 2/36 | |||
| 6 months to 5 years | 30/8 | 27/11 | P>0.05 | 13/25 | 9/29 | ||
| > 5 years | 20/4 | 20/4 | 14/10 | 8/16 | |||
| Lifetime exposure to typical neuroleptic2 | P>0.05 | ||||||
| <6 months | 17/10 | 14/13 | 15/12 | 11/16 | 6/21 | 5/22 | |
| 6 months to 5 years | 18/1 | 16/3 | 17/2 | 12/7 | 7/12 | 6/13 | |
| > 5 years | 15/3 | 15/3 | 15/3 | 10/8 | 13/5 | 6/12 | |
| No exposure | 15/21 | 8/28 | 8/28 | 11/25 | 2/34 | ||
| Lifetime exposure to atypical neuroleptic2 | P>0.05 | P>0.05 | P>0.05 | P>0.05 | P>0.05 | P>0.05 | |
| <6 months | 14/25 | ||||||
| 6 months to 5 years | 28/11 | ||||||
| > 5 years | 7/2 | ||||||
| No exposure | 4/9 | ||||||
Age
We further observed a strong relation between age and lifetime prevalence of EPS (χ2=15.13, d.f.=2, P<0.01), reported EPS (χ2=15.70, d.f.=2, P<0.01) and lifetime prevalence of acute dystonic reaction (χ2=9.82, d.f.=2, P<0.01). The prevalence of the three related outcome variables was higher in the youngest age group than in either of the other age groups (Table 4).
Duration of exposure to neuroleptic medication
The duration of exposure to any neuroleptic medication was related to the lifetime prevalence of EPS (χ2=17.86, d.f.=2, P<0.01), the prevalence of reported EPS (χ2=34.96, d.f.=2, P<0.01) as well as the lifetime occurrence of parkinsonism (χ2=6.67, d.f.=2, P=0.04) and akathisia (χ2=8.41, d.f.=2, P=0.02). More specifically, the duration of exposure to typical neuroleptics was associated with the lifetime prevalence of EPS (χ2=18.71, d.f.=3, P<0.01), the prevalence of reported EPS (χ2=27.78, d.f.=3, P<0.01), occurrence of EPS on typical neuroleptics (χ2=7.83, d.f.=2, P=0.02), lifetime prevalence of acute dystonia (χ2=10.68, d.f.=3, P=0.01), parkinsonism (χ2=12.75, d.f.=3, P<0.01) and akathisia (χ2=8.59, d.f.=2, P=0.04). The duration of the exposure to atypical neuroleptics was related to the prevalence of reported EPS (χ2=14.91, d.f.=3, P=0.01). As summarised in Table 4, we found the prevalence of all statistically related outcome variables to increase with longer duration of neuroleptic medication.
For all other relationships between possible predictor variables (gender, psychiatric diagnostic category or dosage range) and outcome variables considered in χ2 tests, P>0.05. Thus, only the following possible predictor variables were entered in a logistic regression analysis: family history of primary movement disorders; age; duration of exposure to any neuroleptic medication; duration of exposure to typical neuroleptics; and duration of exposure to atypical neuroleptic medication. Occurrence of tardive dyskinesias was excluded from outcome variables because of the small number of cases identified.
Predictors revealed by logistic regression analysis
We found that lifetime occurrence of EPS (yes or no) could be correctly predicted in 74% of all cases by knowing the duration of treatment with typical neuroleptics, and family history of primary movement disorders. Lifetime occurrence of acute dystonic reaction (yes or no) could be predicted in 65% of cases taking into account the exposure to typical neuroleptic medication and age. Both lifetime occurrence of parkinsonism (yes or no) and of akathisia (yes or no) were predicted correctly in 72% and 82%, respectively, by the duration of typical neuroleptic medication. Reported EPS (yes or no), regardless of the subtype, were correctly predicted in 82% of cases by the duration of exposure to typical neuroleptic medication, age and family history of primary movement disorders. Currently observed EPS (yes or no) were predicted in 68% of all cases by the family history of primary movement disorders. Extrapyramidal symptoms on typical neuroleptic medication (yes or no) could be predicted in 73% when considering the duration of exposure. As can be seen from the regression coefficients B in Table 5, the probability for the occurrence of symptoms always increased with the duration of exposure to neuroleptic medication, younger age and positive family history.
Table 5 Step-wise logistic regression analysis revealing significant predictor variables for occurrences of extrapyramidal symptoms (d.f.=1 in all tests)

| EPS | Predictor | B | s.e. | Wald | P |
|---|---|---|---|---|---|
| Lifetime occurrence of EPS | Duration of typical neuroleptic | 0.78 | 0.25 | 10.65 | <0.01 |
| Age | – 0.85 | 0.35 | 5.95 | 0.02 | |
| Positive family history | 1.30 | 0.60 | 4.67 | 0.03 | |
| Lifetime occurrence of acute dystonia | Duration of typical neuroleptic | 0.54 | 0.20 | 7.16 | <0.01 |
| Age | – 0.88 | 0.36 | 6.17 | 0.01 | |
| Lifetime occurrence of parkinsonism | Duration of typical neuroleptic | 0.53 | 0.20 | 7.16 | <0.01 |
| Lifetime occurrence of akathisia | Duration of typical neuroleptic | 0.63 | 0.24 | 6.81 | <0.01 |
| Reported EPS | Duration of any neuroleptic | 1.98 | 0.41 | 23.28 | <0.01 |
| Age | – 1.30 | 0.45 | 8.54 | <0.01 | |
| Positive family history | 1.45 | 0.62 | 5.57 | 0.02 | |
| Currently observed EPS | Positive family history | 1.27 | 0.46 | 7.69 | <0.01 |
| EPS under typical neuroleptics | Duration of typical neuroleptic | 0.90 | 0.40 | 4.97 | 0.03 |
DISCUSSION
The main purpose of our study was to identify predictor variables for neuroleptic-induced extrapyramidal symptoms, for the first time considering family history of primary movement disorders.
Prevalence rates for extrapyramidal symptoms and primary movement disorders
Based on a sample that represents a typical cohort of in-patients with acute psychotic symptoms, we observed a lifetime prevalence of extrapyramidal symptoms of 65% and a point prevalence of 34%, comparable to rates reported in large epidemiological studies (Reference AydAyd, 1961; Reference SwettSwett, 1975; Reference Owens and JohnstoneOwens & Johnstone, 1982). Compared with these studies, the mean age of our patients was low, and the rate of patients ever having been treated with atypical neuroleptics was high (87%). The percentage of patients who had been exposed to neuroleptics for less than 6 months was high (38%). The overall prevalence of about 3% for primary movement disorders in all relatives seems plausible. Higher prevalence rates of Parkinson's disease, tremor and dystonia among relatives of patients with lifetime extrapyramidal symptoms than among relatives of patients without such symptoms are comparable with results reported by others, although our study was not designed as a population-based case–control study (Reference Marder, Tang and MejiaMarder et al, 1996; Reference Louis, Levy and Mejia-SantanaLouis et al, 2003). The relatives' diagnoses could be confirmed in a small subgroup.
Predictors of extrapyramidal symptoms
In our sample, logistic regression analysis revealed that a positive family history had significant predictive value for the occurrence of extrapyramidal symptoms. The probability of observed symptoms at the time of examination, for which it was the only predictor, as well as of lifetime prevalence of symptoms and reported symptoms, was increased in patients who had a relative with a primary movement disorder. The strongest predictive value was found for the duration of treatment with typical neuroleptics (lifetime occurrence of EPS, acute dystonic reaction, parkinsonism, akathisia, and EPS on typical neuroleptics) as well as with any neuroleptic medication (reported EPS). The probability of extrapyramidal symptoms increased with longer duration of exposure. Furthermore, younger age was also a significant predictor for the occurrence of symptoms, especially for acute dystonic reaction.
Positive family history of primary movement disorders
The finding that a positive family history of primary movement disorders had a significant impact on the occurrence of extrapyramidal symptoms has two main implications. First, our results suggest that primary and secondary movement disorders may share common genetic factors. Second, the association with primary movement disorders observed in patients suffering from psychotic symptoms and developing extrapyramidal symptoms may represent a dysfunction within a common pathway of the dopaminergic system. This system is involved not only in primary and secondary movement disorders but also in psychotic disorders. Earlier hypotheses suggest that such symptoms might represent exacerbated involuntary movements that are directly related to cerebral dysfunctions underlying psychotic diseases – i.e. a dysfunction within the dopaminergic system (Reference AydAyd, 1961; Owen & Johnstone, 1982). Indeed, both Kraepelin (Reference Kraepelin, Barclay and Robertson1971) and Bleuler (Reference Bleuler and Zinkin1950) described ‘spasmodic phenomena in the musculature’ and ‘extraordinary movements of the tongue and lips’ in patients with psychosis long before the introduction of neuroleptic drugs. Other studies have confirmed this observation by the finding that prevalence and distribution of extrapyramidal symptoms were the same in treated and never-treated patients and depended only on the age at onset of the psychotic illness (Owen & Johnstone, 1982; Reference Srinivasan, Thara and PadmavathiSrinivasan et al, 2001). Further studies should address the question whether the occurrence of such symptoms represents an endophenotype for schizophrenia, as has been shown for other neurological dysfunctions in neuroleptic-naïve patients with psychosis (Reference Gottesman and GouldGottesman & Gould, 2003). Genetic association studies of candidate genes, such as dopamine receptor genes, will be the natural extension of our study to elucidate the hypothesised common underlying mechanism at the molecular level.
Duration of neuroleptic medication
We observed a strong effect of duration of exposure to neuroleptic medication, especially of typical neuroleptics, on the occurrence of extrapyramidal symptoms, including the subtypes acute dystonic reaction, parkinsonism and akathisia. Although many authors have proposed such an effect, retrospective studies rarely confirmed this notion (Reference MarsalekMarsalek, 2000). From our results, however, it can be assumed that the longer the exposure to neuroleptic drugs, the higher the prevalence of these symptoms. This finding may support the hypothesis of an accumulating toxic effect of neuroleptic drugs that is suggested to be higher in the typical drugs than in the atypicals, with the exception of clozapine (Reference Gil-ad, Shtaif and ShilohGil-ad et al, 2001). An effect of dosage on occurrence of extrapyramidal symptoms at the time of examination could not be confirmed.
None of the predictor variables entered in the logistic regression analysis was found to predict the occurrence of extrapyramidal symptoms in patients taking atypical neuroleptics. The significant relation observed in the χ2 test between reported symptoms and the duration of medication with atypical neuroleptics may be interpreted as a trend. However, the long-term effect of atypical neuroleptics on extrapyramidal symptoms may yet not have been verified, since only a small proportion of patients had been exposed to these drugs for more than 5 years (10%), and this aspect remains a matter of debate (Reference Tarsy, Baldessarini and TaraziTarsy et al, 2002).
Other possible predictors: age, gender and psychiatric diagnosis
We were able to validate young age as a predictor for the occurrence of extrapyramidal symptoms, especially of acute dystonia (Reference AydAyd, 1961; Reference SwettSwett, 1975). This finding gives further support to the genetic influence on occurrence of these symptoms. In contrast, there is no evidence from our data for either diagnostic specificity or gender as a predictor for the occurrence of symptoms.
Methodological limitations
Our study design must be regarded as somewhat explorative, since for most of the relatives the diagnosis of a primary movement disorder could only be established through the family history interview. This procedure might have reduced the validity and reliability of the data, although special efforts were made to rate a family history as positive only if the criteria were unambiguously fulfilled. In a clinical setting, this is also the most practical way to gather information and decide whether a patient is at risk of developing extrapyramidal symptoms because of a positive family history.
We are aware that the way in which we categorised neuroleptic dosages may appear arbitrary. By definition, atypical neuroleptic dosages cannot be converted to chlorpromazine equivalents. We therefore decided to use a categorisation based on clinical experience. The distribution of dosage ranges in our sample appears reasonable (22% on low, 52% on medium and 26% on high medication dosages). Exact dosages could only be assessed for the time of examination.
Genetic considerations
Our data imply two hypotheses. First, our findings underline the notion of genetic susceptibility for secondary extrapyramidal symptoms, and second, they suggest possible shared genetic factors in primary and secondary movement as well as in psychotic disorders.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Neuroleptic medication with a known low risk of extrapyramidal symptoms (i.e. an atypical neuroleptic) is preferable for patients with a family history of primary movement disorders, especially dystonia.
-
▪ Atypical neuroleptics are also preferable in patients under 40 years old, particularly to reduce the occurrence of painful acute dystonic reaction.
-
▪ Patients who have been exposed to long-term typical neuroleptic medication should be switched to an atypical agent even though extrapyramidal symptoms might not have been observed as yet.
LIMITATIONS
-
▪ Our study design must be regarded as somewhat explorative owing to the family history approach.
-
▪ Our data-set might have been too small to confirm any dosage effect.
-
▪ The small sample size might also have been the reason why we did not observe the gender effect described elsewhere of a higher prevalence of acute dystonia in men and of parkinsonism, akathisia and tardive dyskinesia in women.
Acknowledgements
We are grateful to Dr Inke König, Institute of Medical Biometry and Statistics, University of Lübeck, for statistical advice. The study was supported by the Deutsche Forschungsgemeinschaft (K1-1134/3-1) and the University of Lübeck (Grant N07-2000).










eLetters
No eLetters have been published for this article.