Non-suicidal self-injury (NSSI) is a widespread phenomenon with a prevalence of 18% in adolescents. Reference Muehlenkamp, Claes, Havertape and Plener1 NSSI, predominantly skin-cutting, is also seen in 69–90% of female patients with borderline personality disorder, Reference Zanarini, Frankenburg, Reich, Fitzmaurice, Weinberg and Gunderson2 where it is closely related to emotion dysregulation. Reference Zanarini, Frankenburg, Reich, Fitzmaurice, Weinberg and Gunderson2,Reference Skodol, Gunderson, Pfohl, Widiger, Livesley and Siever3 Since the primary motive for NSSI in people with borderline personality disorder is to downregulate aversive tension, Reference Kleindienst, Bohus, Ludaescher, Limberger, Kuenkele and Ebner-Priemer4 self-injurious behaviour can be conceptualised as a dysfunctional emotion regulation attempt Reference Klonsky5 and may be maintained by negative reinforcement. Reference Chapman, Gratz and Brown6,Reference Nock and Prinstein7 Aberrant impulse control, another symptom of borderline personality disorder, could be a critical factor as well, so one might suggest that NSSI is frequently driven by poor inhibitory control and disproportionate responses to stressors. Reference Simeon, Stanley, Frances, Mann, Winchel and Stanley8 However, experimental evidence for this assumption is sparse. Strongly connected to NSSI are findings in people with borderline personality disorder showing reduced sensitivity to pain. Reference Ludaescher, Greffrath, Schmahl, Kleindienst, Kraus and Baumgaertner9,Reference Schmahl, Greffrath, Baumgaertner, Schlereth, Magerl and Philipsen10 This hyposensitivity is further increased under stress. Reference Bohus, Limberger, Ebner, Glocker, Schwarz and Wernz11,Reference Ludaescher, Bohus, Lieb, Philipsen, Jochims and Schmahl12 Research has found that painful stimulation is related to a deactivation of the perigenual anterior cingulate cortex as well as the amygdala, Reference Schmahl, Bohus, Esposito, Treede, Di Salle and Greffrath13 a region with increased activity during the presentation of emotionally aversive pictures in patients with borderline personality disorder compared with healthy controls. Reference Donegan, Sanislow, Blumberg, Fulbright, Lacadie and Skudlarski14–Reference Koenigsberg, Siever, Lee, Pizzarello, New and Goodman16 Simultaneously, painful stimulation led to increased activation of the dorsolateral prefrontal cortex in participants with borderline personality disorder. Reference Schmahl, Bohus, Esposito, Treede, Di Salle and Greffrath13 These findings led to the hypothesis that a potential neural mechanism behind NSSI in borderline personality disorder is a pain-mediated downregulation of elevated limbic activity by prefrontal cortex. This was supported in recent studies from our group, demonstrating that thermal stimuli led to a decrease in limbic activation induced by negative pictures in patients with borderline personality disorder Reference Niedtfeld, Schulze, Kirsch, Herpertz, Bohus and Schmahl17 and, more specifically, that painful stimulation enabled amygdala–prefrontal coupling. Reference Niedtfeld, Kirsch, Schulze, Herpertz, Bohus and Schmahl18 It was argued that this pattern of neural activation mirrors dysfunctional emotion regulation by means of an attentional shift, i.e. painful stimulation may serve to direct attention away from the emotional contents. Reference Ochsner and Gross19
Previous studies on pain processing in borderline personality disorder have used different types of non-damaging nociceptive thermal stimuli, such as heat, cold or laser-evoked pain. Reference Bohus, Limberger, Ebner, Glocker, Schwarz and Wernz11,Reference Russ, Roth, Lerman, Kakuma, Harrison and Shindledecker20 However, most patients use methods of NSSI that lead to skin lesions, by means of cutting or burning. Reference Kleindienst, Bohus, Ludaescher, Limberger, Kuenkele and Ebner-Priemer4,Reference Klonsky5 This is important because different neural processes are activated by mechanical compared with thermal nociceptive stimuli, suggesting that different stimuli may also lead to different brain responses. Reference Baumgaertner, Iannetti, Zambreanu, Stoeter, Treede and Tracey21 Therefore, one could argue that skin lesions in particular have an important role within the mechanisms of NSSI. A pain model that takes the aspect of the skin lesion into account is the incision–pain model, Reference Kawamata, Takahashi, Kozuka, Nawa, Nishikawa and Narimatsu22 where a 4 mm long incision with a scalpel is applied to the volar forearm. This model has been found acceptable by several ethics committees, because (a) pain levels are moderate, (b) bleeding is minimal and usually stops by itself and (c) healing is rapid and without visible scars. Reference Kawamata, Takahashi, Kozuka, Nawa, Nishikawa and Narimatsu22–Reference Reitz, Krause-Utz, Pogatzki-Zahn, Ebner-Priemer, Bohus and Schmahl24 Pogatzky-Zahn et al Reference Pogatzki-Zahn, Wagner, Meinhardt-Renner, Burgmer, Beste and Zahn23 investigated 44 right-handed male healthy volunteers with a functional magnetic resonance imaging (fMRI) block design. Fourteen out of 44 experienced a sham condition (pressing the handle bar of the scalpel on the skin of the right volar forearm), 30 underwent the incision procedure (as explained for our study). Dependent on painfulness, a ‘high-sensitivity’ and a ‘low-sensitivity-subgroup’ were analysed separately. In the latter, similar to our present borderline personality disorder sample and our earlier findings, Reference Schmahl, Bohus, Esposito, Treede, Di Salle and Greffrath13 a deactivation of the left amygdala after incision could be shown, whereas the high-sensitivity subgroup showed an activation of the amygdala. In a pilot study Reference Reitz, Krause-Utz, Pogatzki-Zahn, Ebner-Priemer, Bohus and Schmahl24 we confirmed the feasibility of the incision model and tested whether tissue damage reduces stress in patients with borderline personality disorder. The incision was applied after a stress induction Reference Dedovic, Renwick, Mahani, Engert, Lupien and Pruessner25 and resulted in a short-term increase in aversive tension in the healthy control group in contrast to a decrease in tension and heart rate in the borderline personality disorder group. In the present study, we aimed to investigate neural mechanisms of NSSI-associated tissue damage in borderline personality disorder by transferring the established incision paradigm into the fMRI environment. Specifically, we compared the effects of incision v. sham treatment following a stress induction while participants were undergoing fMRI. We hypothesised that subjective and objective stress responses would show a stronger decrease in the borderline personality disorder group compared with the healthy control group after incision, whereas in the sham condition, we expected the healthy control group to show a stronger decrease in stress measures (i.e. functional stress regulation) than the borderline personality disorder group. Furthermore, we hypothesised that incision after stress would lead to altered connectivity between the amygdala and prefrontal brain regions in the borderline personality disorder group. Given previous findings pointing to the relevance of the amygdala and its interaction with prefrontal areas in the context of stress regulation, we decided to focus on this brain region as a seed region of a functional connectivity analysis of the stress regulation phase after incision. On the basis of previous work, we also hypothesised that inhibitory coupling (i.e. negative connectivity between prefrontal and limbic regions) would be present in people with borderline personality disorder under the incision condition only, whereas it should be present in the healthy control group in the sham condition.
Method
Participants
Twenty-one female patients with borderline personality disorder and current NSSI (borderline personality disorder group) and 17 female healthy controls without any NSSI events in their history (control group) participated in this study. Both groups did not differ significantly in age (borderline personality disorder group: 25.95 (s.d. = 6.92), control group: 26.88 (s.d. = 8.29), t (36) = 0.38, P = 0.71) or in educational background (Z = 0.81, P = 0.53). For demographic characteristics see Table 1. Patients met DSM-IV 26 criteria for borderline personality disorder according to the International Personality Disorder Examination (IPDE). Reference Loranger27 Axis I comorbidity was assessed by the Structured Clinical Interview for Axis I disorders (SCID-I). Reference First, Spitzer, Gibbon, Williams and Benjamin28 Both interviews were administered by trained clinical psychologists. Interrater reliability for borderline personality disorder was kappa (κ) = 0.69 for the SCID-I (primary diagnosis) and κ = 0.77 for the IPDE. Exclusion criteria comprised a current episode of major depression, a lifetime diagnosis of schizophrenia, bipolar disorder, acute suicidal tendencies, major medical or neurological illness and psychotropic medication within the 4 weeks prior to the investigation. We only included patients who had shown NSSI at least once in the 6 months prior to study, as assessed with the structured self-rating Questionnaire for Non-Suicidal Self-Injury, Reference Kleindienst, Bohus, Ludaescher, Limberger, Kuenkele and Ebner-Priemer4 and who did not express a request for treatment. This questionnaire assesses frequency, motives and methods of self-injurious behaviour without the intent to die, preferred methods, as well as intensity and medical treatment of wounds. A total of 71% endorsed cutting as their preferred NSSI method. NSSI was mainly used to reduce aversive inner tension (57%) and negative emotions (21%). The healthy controls were excluded if they had any Axis I or Axis II lifetime morbidity or a history of NSSI.
TABLE 1 Demographics and comorbidities

| Borderline personality disorder group (n = 21) |
Control group (n = 17) |
|
|---|---|---|
| Age, years:Footnote a mean (s.d.) | 25.95 (6.92) | 26.88 (8.29) |
| Current body mass index, kg/m2: mean (s.d.) | 23.50 (6.29) | 22.80 (4.83) |
| Educational background, n (%) | ||
| Without graduation (0 years) | 1 (4.76) | 0 (0) |
| ≤9 years of school education | 4 (19.05) | 0 (0) |
| 10 years of school education | 3 (14.29) | 2 (11.76) |
| ≥12 years of school education | 13 (61.90) | 15 (88.24) |
| Professional position, n (%) | ||
| In education | 7 (33.33) | 12 (70.59) |
| Employed | 6 (28.57) | 3 (17.65) |
| Unemployed | 8 (38.10) | 2 (11.76) |
| Psychiatric comorbidities, n (%) | ||
| Major depressive disorder, current | 0 (0) | 0 (0) |
| Major depressive disorder, lifetime | 16 (76.19) | 0 (0) |
| Dysthymia | 2 (9.52) | 0 (0) |
| Substance misuse, lifetime | 5 (23.81) | 0 (0) |
| Substance dependence, lifetime | 5 (23.81) | 0 (0) |
| Panic disorder, current | 2 (9.52) | 0 (0) |
| Panic disorder, lifetime | 2 (9.52) | 0 (0) |
| Agoraphobia, current | 1 (4.76) | 0 (0) |
| Social phobia, current | 4 (19.05) | 0 (0) |
| Social phobia, lifetime | 2 (9.52) | 0 (0) |
| Specific phobia, current | 1 (4.76) | 0 (0) |
| Obsessive–compulsive disorder, current | 1 (4.76) | 0 (0) |
| Obsessive–compulsive disorder, lifetime | 2 (9.52) | 0 (0) |
| Post-traumatic stress disorder, current | 7 (33.33) | 0 (0) |
| Post-traumatic stress disorder, lifetime | 7 (33.33) | 0 (0) |
| Anorexia nervosa, current | 2 (9.52) | 0 (0) |
| Anorexia nervosa, lifetime | 4 (19.05) | 0 (0) |
| Bulimia nervosa, current | 2 (9.52) | 0 (0) |
| Bulimia nervosa, lifetime | 2 (9.52) | 0 (0) |
| Eating disorder, not otherwise specified | 2 (9.52) | 0 (0) |
| Number of psychiatric comorbidities, n (%) | ||
| 0 | 6 (28.57) | 17 (100) |
| 1 | 5 (23.81) | 0 (0) |
| 2 | 1 (4.76) | 0 (0) |
| 3 | 3 (14.29) | 0 (0) |
| 4 | 0 (0) | 0 (0) |
| 5 | 2 (9.52) | 0 (0) |
| Unknown | 4 (19.05) | 0 (0) |
a. The borderline personality disorder group did not differ significantly from the control group in age (t(36) = 0.38, P=0.71).
Participants were given a written as well as a verbal explanation of the task. Remaining questions were answered, and after that all participants gave written informed consent. No participant withdrew consent during the course of the investigation. The study was conducted in accordance with the Declaration of Helsinki and was approved by the ethics committee of the Medical Faculty Mannheim/University of Heidelberg (application no. 2008-234N-MA).
Stimulus material and procedure
Participants were investigated twice on two consecutive days. During fMRI, stress was induced in the first 21 min using the Montreal Imaging Stress Task (MIST), Reference Dedovic, Renwick, Mahani, Engert, Lupien and Pruessner25 which combines arithmetic with an algorithm causing disappointment. Both difficulty and time limit are manipulated to simulate poor performance (45–50% performance range). During the stress task, we employed a block design with three 7 min sessions of image acquisition.
After stress induction and disinfection with 70% alcohol, incision/sham was conducted according to the standardised incision protocol. Reference Kawamata, Takahashi, Kozuka, Nawa, Nishikawa and Narimatsu22,Reference Pogatzki-Zahn, Wagner, Meinhardt-Renner, Burgmer, Beste and Zahn23 With a sterile ceramic scalpel, a small 4 mm long and 5–7 mm deep incision through the skin, fascia, and muscle of the anterior aspect of the volar forearm was conducted by one of the authors (T.K.). Painfulness of this procedure is comparable with a venipuncture and incision was well tolerated by all participants. In all participants, bleeding stopped spontaneously within 1 min. For the sham condition, the participants' skin was touched with the blunt end of the scalpel without cutting. On both days in permutated order, participants were not told which treatment they would receive. They were told the incision could happen once or on both days of the experiment. After incision/sham, participants underwent three 7 min blocks of resting-state fMRI, during which they were asked to relax and look at a fixation cross. Figure 1 shows an overview of the study design.
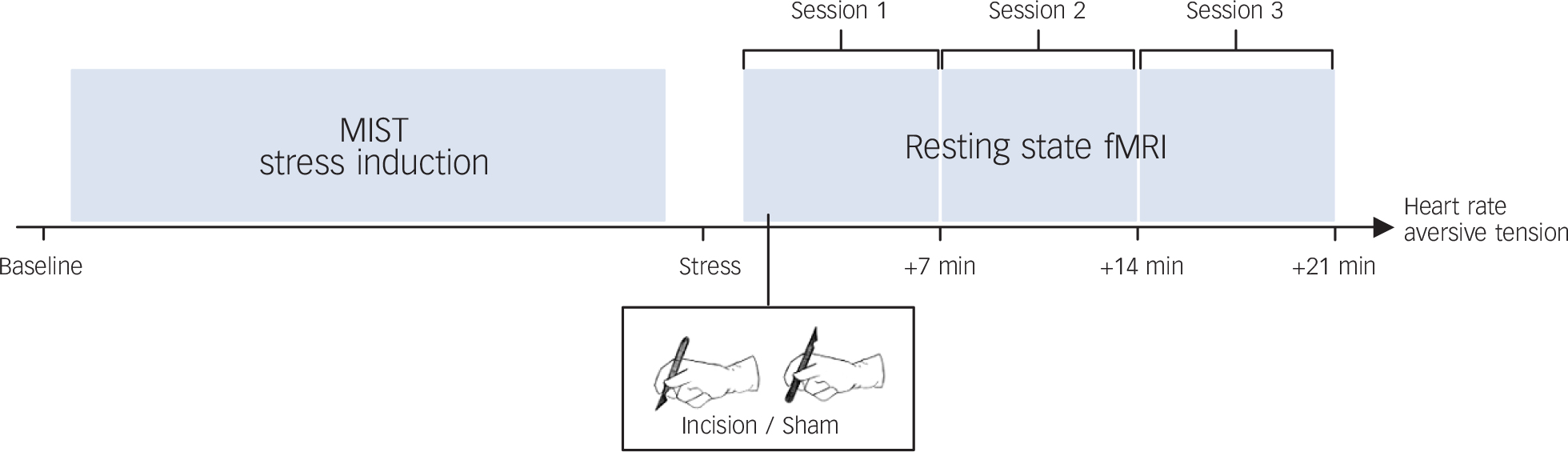
Fig. 1 Study design.
Following a stress induction (Montreal Imaging Stress Task, MIST) either an incision or a sham treatment was conducted, followed by three sessions of resting-state functional magnetic resonance imaging (fMRI). Heart rate and aversive tension were assessed throughout the experiment.
Subjective and objective assessment of stress levels
Participants rated their level of aversive tension at five time points, namely baseline, after stress induction and sessions 1 to 3 (7, 14 and 21 min post incision/sham, respectively) with a Self-Assessment Manikin (SAM) Reference Bradley and Lang29 on a visual analogue scale ranging from 1 (none) to 9 (extreme). Heart rate was calculated based on the analysis of the systolic peaks of the oximetry signal measured by finger mounted pulse oximetry. Heart rate was expressed as the number of beats per min and averaged for the rest and stress conditions of the MIST as well as the three time periods after incision/sham corresponding to the time intervals for which aversive tension was rated. Additionally, heart rate variability (HRV) was calculated non-parametrically with the HRV triangular index (HRV index; see Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology 30 ), and log-transformed because of violations of the normality assumption. Reliable HRV scores can be calculated based upon pulse oximetry data, Reference Schafer and Vagedes31 although compared with electrocardiogram data the derived parameter estimates have a lower precision because of a rather low sampling rate. As a result of technical problems and artefacts, heart rate and HRV data of four participants in the borderline personality disorder group and six in the control group had to be excluded from the analyses.
MRI acquisition
All imaging data were acquired using a 3-Tesla MRI scanner (TRIO, Siemens Medical Systems, Erlangen, Germany) equipped with a 32-channel head coil. In addition to a high resolution T 1-weighted structural scan, we acquired T 2*-weighted echo planar images with blood oxygenation level-dependent contrast (BOLD, repetition time (TR) = 2 s, echo time (TE) = 30 ms, 192 mm field of view, 64×64 matrix, 36 slices, 3 mm slice thickness, 3 mm slice gap; flip angle, 80°). As a result of technical problems or movement, we had to exclude two participants in the borderline personality disorder group and one in the control group. Imaging data were preprocessed using SPM 8 (www.fil.ion.ucl.ac.uk/spm, Wellcome Trust Centre for Neuroimaging, University College London, London, UK) according to standard procedures. The fMRI images pertaining to the three post-stress/incision/sham runs were further subjected to detrending using the REST toolbox version 1.7 (www.restfmri.net, Hangzhou Normal University, Zhejiang, China) in order to remove linear drifts.
Statistical analysis
Analysis of subjective and objective stress responses
To verify the effectiveness of the experimental stress induction through the MIST procedure, we computed a repeated measures analysis of variance (rm-ANOVA) for condition (incision, sham) and time (baseline, stress). Aversive tension, heart rate and HRV were analysed using a rm-ANOVA with the between-subjects factor group (borderline personality disorder, control) and the within-subjects factors condition and time. We included data for four time points: after stress induction, 7, 14 and 21 min after incision/sham. In case of significant effects, post hoc tests were computed, and effect sizes (Cohen's d) were reported. According to Cohen, Reference Cohen32 d = 0.2 reflects a small effect, d = 0.5 a medium and d = 0.8 a large effect size. All statistical analyses were carried out with SPSS for Windows (20.0.0).
MRI image analysis
In order to investigate functional connectivity post incision/sham, we extracted each participant's BOLD signal time courses from the three post-stress/incision/sham runs using in-house MATLAB code. For subsequent connectivity analyses, we extracted BOLD time signal from detrended data; for regression analyses of amygdala activity, we extracted BOLD time signal from non-detrended data. In both cases, bilateral amygdala was selected as a seed region and identified in each data-set using an anatomical mask defined by automated anatomical labeling (AAL) software (Groupe d'Imagerie Neuro-fonctionelle, Bordeaux Cedex, France). Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard and Delcroix33
First, to analyse changes in amygdala activity over time, regression analyses of the extracted BOLD time signal were computed for each person using simple linear regression (ordinary least squares) in MATLAB, and the resulting regression coefficient (gradient) for each person was entered into subsequent statistical analyses. For connectivity analyses, the resulting time courses were entered as regressors of interest into first-level analyses in SPM 8 to identify regions showing a time course correlated with amygdala activity. The first-level analyses also included eight regressors of no interest comprising the six movement parameters as well as regressors for white matter and cerebrospinal fluid. For each participant, we computed separate first-level analyses for each of the three post-stress sessions. The resulting contrast images were then subjected to a full factorial second-level analysis with the between-subjects factor group (borderline personality disorder, control group) and the within-subjects factors condition (sham, incision) and time (session 1–3 after stress).
To reduce the possibility of type I errors, we combined a statistical threshold of P<0.001 with the cluster extent correction procedure implemented in SPM 8, which computes the number of expected voxels per cluster according to random field theory. Reference Hayasaka and Nichols34 Based on this correction procedure, the minimum cluster size for the factorial analysis was determined to be 27 adjacent voxels. We focused on regions showing a group×condition interaction, i.e. effects that were stable over the post-stress course of the experiment.
Results
Effects of condition and stress induction on aversive tension and heart rate
Stress induction was successful in all participants, i.e. a 2×2×2 rm-ANOVA for the time points ‘baseline’ and ‘stress’ revealed an increase in aversive tension after the stress induction (F (1,36) = 69.9, P<0.001, f 2 = 1.39, Fig. 2(a)). A main effect of group (F (1,36) = 16.07, P<0.001, f 2 = 0.67) confirmed higher aversive tension in the borderline personality disorder group compared with the control group. There was no interaction effect time×group (F (1,36) = 046, P = 0.500, f 2 = 0.11). Heart rate increased with the induction of stress (main effect of time (F (1,26) = 59.02, P<0.001, f 2 = 1.51; Fig. 2(b)), but the data revealed neither a main effect of group (F (1,26) = 2.40, P = 0.133, f 2 = 0.30) nor an interaction effect of timegroup (F (1,26) = 0.04, P = 0.843, f 2 = 0.04). The HRV index showed a decrease during the stress induction (F (1,26) = 15.27, P = 0.001, f 2 = 0.77; Fig. 2(c)). The rm-ANOVA for HRV index further revealed a significant interaction effect time×group (F (1,26) = 4.23, P = 0.05, f 2 = 0.40) but no significant main effect of group (F (1,26) = 0.33, P = 0.572). For none of the aforementioned parameters was a main or interaction effect regarding condition (sham/incision) observed, implying a successful stress induction independent of the day or condition of the investigation.

Fig. 2 Stress levels.
(a) Ratings of aversive tension for five time points (baseline, stress and sessions 1–3), two conditions and two groups; (b) and (c) show heart rate values and heart rate variability, respectively, for the same time points, conditions and groups. Grey shaded areas mark the time when the Montreal Imaging Stress Task (MIST) and incision or sham treatment were conducted. Error bars reflect standard errors. SAM, Self-Assessment Manikin; BPD, borderline personality disorder; BPM, beats per minute.
Effects of incision on aversive tension and heart rate
In order to test the immediate effects of the incision, we computed a 2×2×2 rm-ANOVA, including condition (sham/incision), group (control/borderline personality disorder), and the two time points ‘stress’ and ‘session 1’. For aversive tension, we found a significant condition×time×group interaction (F (1,36) = 16.28, P<0.001, f 2 = 0.67; Fig. 2(a)) with the borderline personality disorder group revealing a stronger decrease after incision compared with the control group (t (36) = 2.19, P<0.05, d = 0.71). In contrast, the control group showed a stronger decrease after sham treatment compared with the borderline personality disorder group (t (36) = 2.17, P<0.05, d= 0.72). For heart rate, no immediate effect of incision could be confirmed statistically (condition×time× group: F (1,26) = 2.55, P = 0.123; incision: t (26) = 0.53, P = 0.603; sham: t (26) = 1.40, P = 0.173; Fig. 2(b)). However, the analysis of HRV showed a condition×time×group interaction (F (1,26) = 5.07, P<0.05, f 2 = 0.44), with a stronger increase in the HRV index in the control group after sham treatment compared with the borderline personality disorder group (t (26) = 3.27, P<0.01, d = 1.27). Contrarily, no significant group difference could be found regarding HRV for the effects of incision (t (26) = 0.51, P = 0.612; Fig. 2(c)).
In order to test the intermediate effects of the incision, we computed 4×2×2 rm-ANOVAs with the four time points after the stress induction. We found an interaction of condition×time×group (F (2.32,83.43) = 5.95, P<0.01, f 2 = 0.41) and condition×group (F (1,36) = 6.01, P<0.05, f 2 = 0.41) for aversive tension (Fig. 2(a)). After the stress induction, in particular during the first two sessions of resting state fMRI, those in the borderline personality disorder group stayed at a lower level of aversive tension after incision compared with sham (session 1: t (20) = 3.13, P<0.01, d = 0.60); session 2: t (20) = 4.64, P<0.001, d = 0.77). The analysis of heart rate (Fig. 2(b)) showed a trend effect of condition× time×group (F (2.19,56.88) = 2.49, P = 0.087, f 2 = 0.31). Analogous to aversive tension, heart rate remained higher following the stress induction after sham compared with incision in the borderline personality disorder group. This effect was confirmed statistically for session 2 (t (16) = 2.74, P<0.05, d = 0.37), and found as a trend for session 1 (t (16) = 2.07, P = 0.055, d = 0.38) and session 3 (t (16) = 2.02, P= 0.061, d= 0.30). HRV again revealed a condition× time×group effect (F (1.83,47.49) = 3.64, P<0.05, f 2 = 0.37; Fig. 2c). Specifically, the control group showed a higher value on the HRV index in session 2 after sham compared with incision (t (10) = 2.51, P≤0.05, d = 0.64).
Effects of incision on amygdala BOLD time series
In order to analyse bilateral amygdala activity over time, we computed a 2×2 ANOVA with the regression coefficient for amygdala activity in the resting-state session after the stress induction of each person as dependent variable and the factors group and condition as independent variable. This resulted in a main effect for group (F (1,66) = 6.73, P<0.05, f 2 = 0.09), showing that amygdala activity decreased more in the borderline personality disorder group than in the control group. More specifically, we found that regression coefficients were positive for the control group (mean = 0.014, s.d. = 0.029), pointing to an increase over time. In the borderline personality disorder group, regression coefficients were negative (mean = −0.015, s.d. = 0.059), pointing to an overall decrease of amygdala activity (T (68) = 2.581, P = 0.012) (Fig. 3(b)).

Fig. 3 Amygdala activity and connectivity analysis.
(a) Shows a cluster within the amygdala (white) that was used as a seed region in our functional connectivity analysis as well as a cluster in the superior frontal gyrus/Brodmann area 8 (black). (b) Depicts regression coefficients for amygdala activity after incision or sham treatment with positive values standing for an increase and negative values for a decrease over time. As depicted in (c) functional connectivity between these two brain regions was higher in the control group following sham treatment, whereas the borderline personality disorder (BPD) group showed higher connectivity after incision compared with sham. Error bars reflect standard errors. MNI, Montreal Neurological Institute.
Effects of incision on functional connectivity
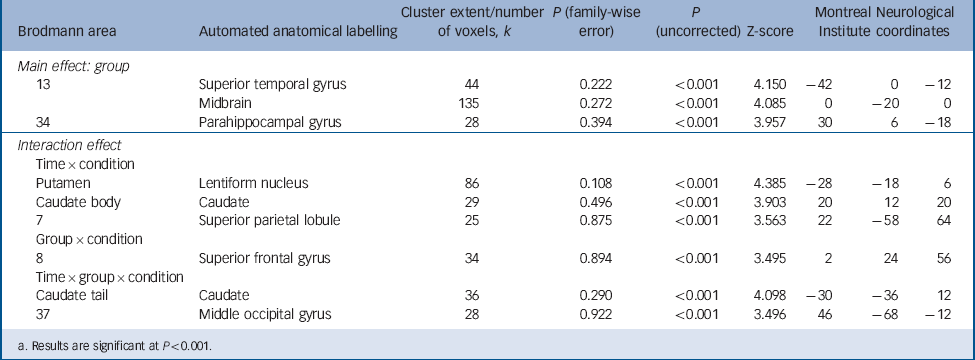
The seed-based correlation analysis of fMRI data with the bilateral amygdala revealed a group×condition interaction effect in Brodmann area 8/superior frontal gyrus (Montreal Neurological Institute (MNI) coordinates 2, 24, 56; P<0.001, k = 34, Z = 3.50, Fig. 3(a)). Post hoc t-tests showed a significant difference of amygdala functional connectivity in this region between the borderline personality disorder and control groups following incision (t (33) = 3.57, P<0.001, d = 1.22). Additional post hoc tests comparing amygdala superior frontal gyrus connectivity between the sham and incision condition in each group separately revealed a significant difference between conditions for the control group (t (15) = 2.77, P<0.05, d = 0.99) and a statistical trend for the borderline personality disorder group (t (18) = 2.03, P = 0.057, d = 0.70; Fig. 3(c)). Particularly, the control group showed reduced connectivity in response to incision compared with sham, whereas connectivity of amygdala with superior frontal gyrus was increased in the borderline personality disorder group after incision compared with sham. In order to ascertain that this effect was not caused by a difference in the amount of head motion, Reference Power, Barnes, Snyder, Schlaggar and Petersen35 we additionally performed a 3×2×2 rm-ANOVA for realignment parameters. This revealed no significant group×condition interactions (translation: P = 0.557; rotation: P = 0.481). For complete data of amygdala connectivity see Table 2.
TABLE 2 Full factorial analysis of seed-based connectivity with the bilateral amygdala
| Brodmann area | Automated anatomical labelling | Cluster extent/number of voxels, k |
P (family-wise error) |
P
(uncorrected |
Z-score | Montreal Neurological Institute coordinates |
||
|---|---|---|---|---|---|---|---|---|
| Main effect: group | ||||||||
| 13 | Superior temporal gyrus | 44 | 0.222 | <0.001 | 4.150 | −42 | 0 | −12 |
| Midbrain | 135 | 0.272 | <0.001 | 4.085 | 0 | −20 | 0 | |
| 34 | Parahippocampal gyrus | 28 | 0.394 | <0.001 | 3.957 | 30 | 6 | −18 |
| Interaction effect | ||||||||
| Time × condition | ||||||||
| Putamen | Lentiform nucleus | 86 | 0.108 | <0.001 | 4.385 | −28 | −18 | 6 |
| Caudate body | Caudate | 29 | 0.496 | <0.001 | 3.903 | 20 | 12 | 20 |
| 7 | Superior parietal lobule | 25 | 0.875 | <0.001 | 3.563 | 22 | −58 | 64 |
| Group × condition | ||||||||
| 8 | Superior frontal gyrus | 34 | 0.894 | <0.001 | 3.495 | 2 | 24 | 56 |
| Time × group × condition | ||||||||
| Caudate tail | Caudate | 36 | 0.290 | <0.001 | 4.098 | −30 | −36 | 12 |
| 37 | Middle occipital gyrus | 28 | 0.922 | <0.001 | 3.496 | 46 | −68 | −12 |
a. Results are significant at P<0.001.
Discussion
Main findings
This is the first study to explore the effects of incision-induced pain and/or skin lesions on neural stress regulation in patients with borderline personality disorder. Our findings revealed a stress-reducing effect of tissue damage that affects subjective experience, psychophysiological functioning and cerebral processing (i.e. amygdala activity and connectivity) after stress selectively in the borderline personality disorder group. This offers a deeper understanding of the mechanisms underlying NSSI in people with borderline personality disorder.
Analysing subjective tension ratings, heart rate data and amygdala activity, we have replicated and extended our previous findings of decreased stress levels in people with borderline personality disorder in response to tissue damage. Reference Reitz, Krause-Utz, Pogatzki-Zahn, Ebner-Priemer, Bohus and Schmahl24 Given that subjective tension associated with an increased reactivity to stress is one of the most aversive feelings for those with borderline personality disorder, Reference Kleindienst, Bohus, Ludaescher, Limberger, Kuenkele and Ebner-Priemer4 our findings support the notion that tissue damage has a soothing effect for these individuals. Lower levels on the HRV index in the borderline personality disorder group are consistent with the suggestion that reduced HRV indices may be linked to dysregulated affective styles. Reference Beauchaine36 In general, HRV is influenced by the interaction of sympathetic and parasympathetic outputs of the central autonomic nervous system (ANS), with high HRV indicating a healthy and adaptive organism. Reference Thayer and Lane37 Changes in ANS tone are found in different mental disorders, for example in patients with depression. Reference Roose38 An increase in the range of HRV is related to a more vagally influenced heart rate (corresponding to an increased HRV index), whereas a decrease is related to a more sympathetically influenced heart rate (corresponding to a lower HRV index) and an increased risk of cardiac morbidity. Reference Roose38 Because it has been suggested that psychological distress in borderline personality disorder is related to an inability to regulate intense physiological arousal, Reference Stiglmayr, Shapiro, Stieglitz, Limberger and Bohus39 we interpret our finding of reduced HRV in the borderline personality disorder group in terms of revealing a higher sympathetic tone and reduced resources for stress adaptation.
By simulating NSSI with an experimental pain model involving mild skin lesions, a higher ecological validity should be achieved, thereby enhancing our understanding of the psychological and neural processes underlying self-injury. Furthermore, we used a powerful method of stress induction, resulting in high and sustained aversive tension. We found that amygdala activity, which has been repeatedly found to be elevated in patients with borderline personality disorder, Reference Krause-Utz, Winter, Niedtfeld and Schmahl40 decreased over time in the borderline personality disorder group, which was most pronounced after incision. Furthermore, we conducted an analysis of functional connectivity during the resting-state period following incision/sham. Here, the borderline personality disorder group showed a medium-sized effect (albeit only resulting in a statistical trend) for enhanced coupling between the amygdala and superior frontal gyrus (Brodmann area 8) following incision compared with sham. The opposite pattern was observed in the control group. This is in line with previous findings Reference Schmahl, Bohus, Esposito, Treede, Di Salle and Greffrath13,Reference Niedtfeld, Kirsch, Schulze, Herpertz, Bohus and Schmahl18 and provides further evidence for the conceptualisation of NSSI as a dysfunctional attempt to cope with dysregulated affect.
Other groups also investigated the intermediate influence of stress on resting-state functional connectivity of the amygdala in the recovery phase after stress. Reference Veer, Oei, Spinhoven, van Buchem, Elzinga and Rombouts41 They found a coupling between amygdala and prefrontal regions and interpreted this finding in terms of a downregulation of emotional states and an adaptive recovery from stress. In our study, functional connectivity between amygdala and superior frontal gyrus was enhanced in the borderline personality disorder group in the incision condition compared with sham, whereas control group showed modulatory coupling only for the sham condition. Given these similarities, we speculate that healthy people's ‘normal’ connectivity between the amygdala and superior frontal gyrus is disturbed by incision. In contrast, we conclude that in individuals with borderline personality disorder the downregulation of emotional states and a recovery from stress are promoted by incision. In accordance with this view, it has previously been shown that prefrontal regions modulate emotional responses via functional coupling to limbic regions like the amygdala. Reference Ochsner, Silvers and Buhle42 Activation in the superior frontal gyrus, or rather Brodmann area 8, has also repeatedly been observed during attentional distraction. Reference Mak, Hu, Zhang, Xiao and Lee43,Reference McRae, Hughes, Chopra, Gabrieli, Gross and Ochsner44 Therefore, our current findings may represent an enhanced prefrontal–limbic interaction through pain, which may have acted as an attentional distractor. Reference Niedtfeld, Kirsch, Schulze, Herpertz, Bohus and Schmahl18
Strengths and limitations
This study established the incision model in an fMRI environment and thereby links the elucidation of clinically relevant mechanisms with alterations in neuronal systems involved in NSSI in patients with borderline personality disorder. However, several important limitations must be kept in mind when interpreting our results. First, we did not use a conventional fMRI design, which is why we cannot draw inferences regarding brain activation patterns in response to a task/intervention, but only regarding brain activity over time and connectivity regarding resting state after stress. This approach was deemed necessary because incision treatment can only be applied once for ethical reasons. Second, aiming at a replication and extension of our prior results Reference Reitz, Krause-Utz, Pogatzki-Zahn, Ebner-Priemer, Bohus and Schmahl24 to brain activity, we used a similar study design. As a consequence, we analysed resting-state connectivity after stress with a focus on intermediate (10–20 min) effects. Thus, one could argue that we cannot infer direct effects of incision on stress, which is only possible when combining both experimental factors at the same time. Although the analysis of seed-voxel connectivity is an adequate method to detect connections between brain areas, it is based on correlations, and causal interpretations should be treated with caution. However, our conclusions are based on combined observations regarding decreased amygdala activity and enhanced connectivity in participants with borderline personality disorder after incision, allowing for a more complex interpretation than connectivity analyses alone. Nevertheless, future studies should test explicit models of the assumed interactions, for example by dynamic causal modelling.
Third, our method does not enable us to differentiate neuronal traits underlying NSSI from changes in neuronal connectivity. Therefore, our results may also be traced back to previously existing traits, such as impulsivity. Besides, although we provided a more realistic model of NSSI than earlier studies, we cannot disentangle the effects of painful stimulation and tissue damage. Further studies should focus on this differentiation as well as the difference between an injury applied by an investigator and a self-inflicted injury. Reference Haines, Williams, Brain and Wilson45 One might assume that self-inflicted pain implicates cognitive appraisal processes that, in turn, have an impact on actual pain processing. Fourth, although our sample size is appropriate to detect large interaction effects between condition and group, the statistical power was insufficient to detect medium effect sizes (1−β = 0.96 and 0.65, respectively). Especially with regard to our connectivity analyses, this could be an explanation why we found only a statistical trend in the borderline personality disorder group (with 1−β = 0.82), although this was related to a medium sized effect. Because we investigated only female participants, our results cannot be generalised to men with borderline personality disorder. Likewise, given that we excluded patients with borderline personality disorder taking medication, our findings pertain to a ‘borderline personality disorder subgroup’ and cannot be generalised to the average patient with borderline personality disorder. Interrater reliabilities for SCID-I and IPDE were relatively low. Finally, it has to be mentioned that NSSI not only occurs in people with borderline personality disorder, but also in depression and post-traumatic stress disorder. Reference Auerbach, Kim, Chango, Spiro, Cha and Gold46 Therefore, an important question for future studies is whether patients with other psychiatric disorders and who self-harm differ from those with borderline personality disorder.
Implications
Taken together, we found higher levels of inner tension and lower parasympathetic tone in patients with borderline personality disorder than in healthy controls. An experimental skin lesion normalised these symptoms and increased the connectivity between the amygdala and medial prefrontal brain regions. This was paralleled by decreased amygdala activity in people with borderline personality disorder in response to incision after a stress induction. Thus, we were able to confirm and extend previous findings on the important role of pain in emotion regulation in borderline personality disorder. Our data lend support to the assumption that NSSI may help to normalise disturbed emotion regulation circuits in borderline personality disorder and thus elucidate why these patients use NSSI to reduce high levels of stress.
Funding
The study was supported by the Deutsche Forschungsgemeinschaft (KFO 256).
Acknowledgements
The authors would like to thank Jens Pruessner for providing the Montreal Imaging Stress Task as well as Martin Jungkunz and Lars Schulze for their help with the figures.







eLetters
No eLetters have been published for this article.