Depressive disorders are highly prevalent in the general population. 1 They are associated with numerous negative outcomes for the individual and society, including an increased risk of suicide, Reference Chesney, Goodwin and Fazel2 and with major depressive disorder (MDD) alone accounting for 7.5% of all years lived with disability. Reference Vos, Abajobir and Abate3 Psychotherapy, pharmacotherapy and a combination of both are common first-line treatments for MDD, 4,Reference Gabriel, Melo, Fráguas, Leite-Santos, Silva and Ribeiro5 but their public health impact is limited. It has been estimated that only a third of the global disease burden of MDD can be averted, even if every patient were to receive evidence-based treatments under optimal conditions. Reference Andrews, Issakidis, Sanderson, Corry and Lapsley6
Early intervention in depression
One way to meet this challenge is to intervene before patients develop MDD. Individuals with subthreshold depression may be the most promising target group for such an approach. Subthreshold depression can be defined as depressive symptoms that do not (yet) meet the diagnostic criteria of MDD. Such symptoms affect roughly 11% of the general population. Reference Zhang, Peng and Song7 They are associated with elevated mortality, Reference Cuijpers, Vogelzangs, Twisk, Kleiboer, Li and Penninx8 increased healthcare utilisation, Reference Goldney, Fisher, Dal Grande and Taylor9 substantial economic costs Reference Cuijpers, Smit, Oostenbrink, De Graaf, Ten Have and Beekman10 and adverse effects on quality of life comparable to MDD. Reference Volz, Stirnweiß, Kasper, Möller and Seifritz11 Individuals with subthreshold depression are also three times more likely to develop MDD than healthy controls. Reference Zhang, Peng and Song7 This underlines the importance of early intervention research. Intervening in this target group could be helpful to reduce the incidence of new MDD cases, and to treat already existing symptoms.
Prior evidence
Meta-analytic evidence suggests that psychological interventions are effective in subthreshold depression, yielding small to moderate benefits, Reference Cuijpers, Koole, Dijke, Roca, Li and Reynolds12–Reference Rigabert, Motrico and Moreno-Peral16 and that they can reduce the incidence of MDD by 19–43%. Reference Cuijpers, Pineda and Quero17,Reference Buntrock, Harrer and Sprenger18 Almost all of this evidence is based on aggregate data meta-analyses. We are aware of only two meta-analyses using individual participant data (IPD), both conducted by our group. These previous studies were limited to digital intervention trials Reference Reins, Buntrock and Zimmermann15 and trials examining MDD incidence; Reference Buntrock, Harrer and Sprenger18 they included 7 and 30 trials, respectively.
Present study
A major strength of IPD meta-analyses (IPD-MAs) is that they can identify patient-specific effect modifiers. Reference Riley and Fisher19 This may be particularly attractive for psychological interventions in subthreshold depression, where findings on moderators remain inconclusive. Reference Cuijpers, Pineda and Quero17,Reference Conejo-Cerón, Bellón and Motrico20 Robust moderators identified using IPD-MA could allow the stratification of existing care, by prioritising psychological intervention among individuals with subthreshold depression who are most likely to benefit. Conversely, they could be used to develop better treatments for those at high risk of non-response. Furthermore, IPD-MA allows the analysis of rates of treatment response, remission and symptom deterioration in a consistent way across all studies. Effects on such secondary outcomes remain understudied in subthreshold depression populations, as are the potential benefits of treatment over several years.
We therefore conducted an IPD-MA of psychological intervention effects in subthreshold depression, focusing on depressive symptom severity, 50% symptom reduction, reliable improvement, reliable symptom deterioration and achieving close to symptom-free status. We analysed both the short- and long-term benefits up to 2 years. Furthermore, we examined participant- and study-level moderators of differential treatment effects.
Method
Registration and protocol
This study has been registered with PROSPERO (no. CRD42017058585), with further methodological information provided in a published protocol. Reference Ebert, Buntrock, Reins, Zimmermann and Cuijpers21 For the present IPD-MA, we also preregistered a detailed protocol addendum and statistical analysis plan (SAP; doi.org/10.17605/osf.io/vba7f). The SAP also documents all planned deviations from the protocol, and their justification. We report this study following the PRISMA-IPD statement. Reference Stewart, Clarke and Rovers22
Eligibility criteria
Eligible studies were randomised trials in which (a) a psychological intervention (see definition in Supplementary Material S1) was compared with a control group (waitlist, care as usual, psychoeducational material, placebo) with regard to (b) effects on depressive symptom severity, (c) as measured by a validated patient or clinician-rated instrument in (d) adults without MDD at baseline and (e) as confirmed by a standardised diagnostic interview.
We also included studies in which participants were eligible regardless of MDD status, but only when the diagnostic status was assessed at baseline, so that baseline MDD cases could be excluded. Individuals were considered to experience subthreshold depression when displaying at least mild depressive symptom severity at baseline. If trials did not employ inclusion cut-offs, individuals experiencing less than mild symptoms were removed, using a cut-off equivalent to a score of 5 on the Patient Health Questionnaire 9 (PHQ-9). Reference Kroenke, Spitzer and Williams23,Reference Wahl, Löwe and Bjorner24
Study identification
Eligible studies were identified by two independent researchers screening full texts of the Metapsy (www.metapsy.org) meta-analytic research domain for depression interventions (docs.metapsy.org/databases/depression-psyctr). This database is updated three times a year by a systematic literature search of the libraries PubMed, Embase, PsycINFO and Cochrane Central Register of Controlled Trials (see Supplementary Material S2 for search strings). During each update, two independent researchers screen the titles and abstracts of all articles and subsequently review full texts of eligible studies. We also screened previous reviews on the prevention of MDD Reference Cuijpers, Koole, Dijke, Roca, Li and Reynolds12–Reference Cuijpers, Pineda and Quero17,Reference Alonso, Liu and Evans-Lacko25,Reference Hao, Jia, Chen, Zou and Jiang26 and contacted senior researchers in the depression prevention field regarding other relevant trials.
The first database search we used to identify trials was conducted on 10 January 2017. The date of the first data extraction from retrieved full texts was not recorded. Requests for IPD from eligible trial authors began on 22 February 2017. From 2017 onward, search updates were screened annually to include IPD of trials that had been published in the interim. The most recent search we screened was conducted in January 2024, so that all studies published up to 1 January 2024 could be considered in this IPD-MA.
Data collection and harmonisation
Corresponding authors of eligible articles were contacted to request permission to use their IPD. Authors who responded were asked to provide data on demographic, clinical, outcome-related and intervention-related characteristics, if assessed. We included variables as putative moderators if they matched a pre-defined list of characteristics predictive of long-term outcomes in depression Reference Ebert, Buntrock, Reins, Zimmermann and Cuijpers21 (see Supplementary Material S3).
Depressive symptom severity measures were transformed into a ‘common metric’ to facilitate joint analyses Reference Wahl, Löwe and Bjorner24 (see Supplementary Material S4). Then, harmonised IPDs were merged into a single data-set following a standardised protocol. Reference Cuijpers, Miguel and Harrer27 Post-intervention assessments were treated as one assessment, and follow-ups were categorised based on their length (up to 6, 12 or 24 months). When eligible trials did not provide IPD, we extracted outcome data for an aggregate data meta-analysis from the published reports, if feasible.
Risk of bias
In each study, two independent reviewers assessed the risk of bias using Cochrane’s revised tool to assess risk of bias in randomised trials. Reference Sterne, Savović and Page28 We rated all studies as being at low risk of bias for the ‘missing outcome data’ criterion, because multiple imputation with auxiliary variables could be used to handle missing data consistently in this IPD-MA.
Outcomes
The primary outcome of this IPD-MA was depressive symptom severity, as measured by a validated patient- or clinician-rated instrument. From symptom severity scores we derived the following additional outcomes: (a) 50% symptom reduction compared with baseline (response); (b) close to symptom-free status (remission, defined as scores equivalent to PHQ-9 < 523); and (c) reliable improvement and (d) reliable deterioration in depressive symptoms. Reference Jacobson and Truax29
We focused exclusively on depressive symptom severity, as well as on indicators that can be derived from it. This was done to maximise the number of eligible trials, thus optimising the statistical power for our examination of treatment–covariate interactions. Among eligible trials, a smaller subset (k = 30) also reported MDD onset as confirmed by diagnostic interviews. These preventive outcomes were examined in a previous study. Reference Buntrock, Harrer and Sprenger18
Statistical analyses
All analyses were conducted according to the ‘intention-to-treat’ principle (treatment policy estimand Reference Clark, Kahan, Phillips, White and Carpenter30 ). Multilevel multiple imputation models with heteroscedastic errors were used to impute missing values. Bayesian one-stage IPD-MA models were used to pool effects on all outcomes at post-test and follow-ups. Effects were considered ‘significant’ when the 95% credibility interval (CrI) of the treatment coefficient did not include zero. As a sensitivity analysis, we (a) recalculated all effects using two-stage IPD-MA, (b) conducted a conventional meta-analysis that also included studies not providing IPD, (c) calculated effects excluding ‘bottom-up’ therapies Reference Cristea, Vecchi and Cuijpers31 and stepped-care interventions and (d) ran analyses adjusting for potential small-study effects and/or selective publication. Reference Rücker, Schwarzer, Carpenter, Binder and Schumacher32–Reference Duval and Tweedie34
Moderator analyses focused on participant-level variables, which are typically not available in conventional meta-analyses, and come with a lower risk of ecological bias than aggregated study-level characteristics. Moderators were examined by extending the one-stage IPD-MA models, with symptom severity at the first post-treatment assessment point available in each study serving as the outcome. Additionally, we also examined study-level moderators of the effect. Last, we used an additive mixed model Reference Cho, Preacher, Yaremych, Naveiras, Fuchs and Fuchs35,Reference Wood36 to examine potentially non-linear interactions between treatment effects and baseline PHQ-9 scores. A detailed description of all statistical analyses is provided in Supplementary Material S4.
Results
Of the 1131 full-text articles screened, 79 were eligible for present investigation. IPD could be obtained from K = 50 (63.29%) of all eligible trials. After enforcing all inclusion criteria, a total of N = 10 671 individuals (intervention, n = 5470; control, n = 5201) were included in the IPD-MA. Additional effect size data was available for k = 11 studies that did not provide IPD (1376 participants; intervention, 680; control, 696). Study references can be found in Supplementary Material S6. Supplementary Material S5 summarises the study search and inclusions.
Study characteristics
Table 1 provides characteristics of the included studies. The largest proportion of trials were conducted in general adult populations (k = 14, 28%), followed by older adults (k = 13, 26%). Cognitive-behavioral therapy (CBT) was the most frequently employed intervention (k = 24, 48%). Contents were most frequently delivered face to face (k = 22, 44%), followed by the Internet (k = 15, 30%). Participant-level characteristics and missing outcome data are given in Supplementary Material S7 and S8, respectively. Most participants (N = 7199, 68%) were female and the mean age was M = 52.79 (s.d. = 18.72). The mean PHQ-9 score at baseline (directly recorded or converted using the common metric) was 8.78 (s.d. = 4.32). Most studies received a low risk of bias assessment (62%, k = 31). Eleven (22%) showed high overall risk.
Table 1 Study characteristics
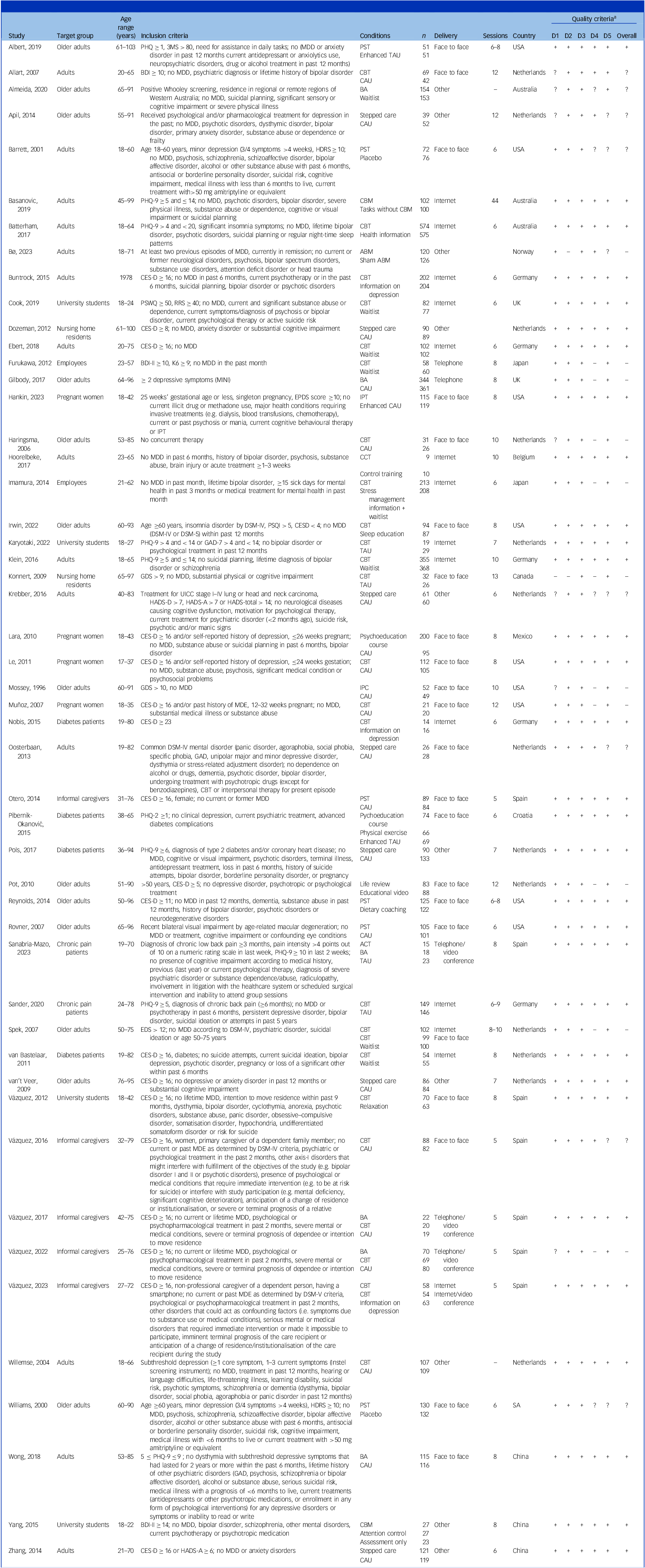
3MS, modified mini-mental state examination; ABM, attentional bias modification; ACT, acceptance and commitment therapy; BA, behavioural activation; BDI, Beck Depression Inventory; CAU, care as usual; CBM, cognitive bias modification; CBT, cognitive behaviour therapy; CES-D, Center for Epidemiological Studies’ Depression Scale; EPDS, Edinburgh Postnatal Depression Scale; GAD, generalised anxiety disorder; GDS, Geriatric Depression Scale; HADS-A/D, Hospital Anxiety and Depression Scale (anxiety/depression subscale); HDRS, Hamilton Rating Scale for Depression; IPC, interpersonal counselling; IPT, interpersonal psychotherapy; K6, Kessler Non‐specific Distress Scale; MDE, major depressive episode; MINI, mini-international neuropsychiatric interview; PHQ, Patient Health Questionnaire; PST, problem-solving therapy; PSQI, Pittsburgh Sleep Quality Index; RRS, Ruminative Response Scale; TAU, treatment as usual; UICC, Union for International Cancer Control.
a Signs in this column represent judgements based on the revised Cochrane risk-of-bias tool for randomised trials (RoB 2) on five domains: randomisation process (D1), deviation from the intervention (D2), missing outcome data (D3), measurement of the outcome (D4) and selection of the reported results (D5; in this order); the column ‘All’ represents the overall judgement regarding risk of bias. All studies were rated as fulfilling the ‘missing data’ criterion, because multiple imputation could be used in all studies to handle missing data. Rating options are ‘low risk of bias’ (+), ‘high risk of bias’ (–) or ‘some concerns’ (?).
Treatment effects
A forest plot displaying results on depressive symptom severity is given in Fig. 1. Psychological intervention reduced depressive symptom severity significantly at post-test (standardised mean difference [s.m.d.] = −0.48, 95% CrI = −0.63 to −0.33, k = 47), within 6 months (s.m.d. = −0.28, 95% CrI = −0.40 to −0.16, k = 39) and within 1 year (s.m.d. = −0.27, 95% CrI = −0.37 to −0.16, k = 33). No significant effect emerged among studies recording outcomes up to 2 years (s.m.d. = −0.18, 95% CrI = −0.41 to 0.06, k = 15). At post-test, our models indicate a >99% posterior probability that treatment effects surpass s.m.d. = −0.24. This effect was determined as a minimally important threshold that is still relevant from a patient perspective. Reference Cuijpers, Turner, Koole, van Dijke and Smit37 Up to 6 months, the probability of greater than minimally important effects was 75%, 71.3% up to 1 year and 28.9% up to 2 years.
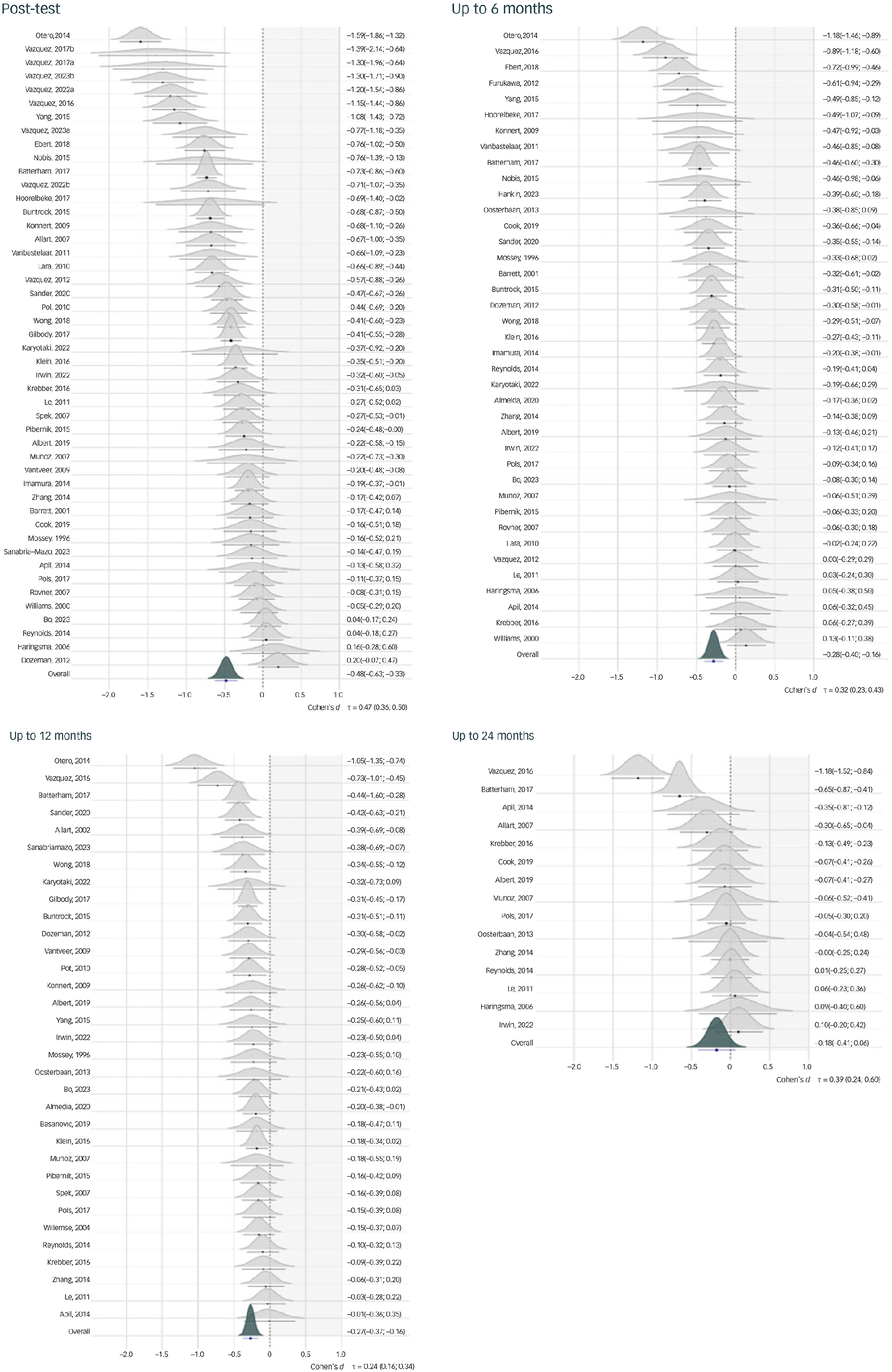
Fig. 1 Forest plot for effects on depressive symptom severity at different assessment points. All effects are expressed as the standardised mean difference (s.m.d.; Cohen’s d). Study densities represent the estimated model-based effect, not empirical values of the s.m.d. found in the original studies.
Similar findings emerged for all other outcomes. From post-test up to 12 months, we found positive effects on 50% symptom reduction, reliable improvement and achieving close to symptom-free status (relative risk = 1.23–1.91). Interventions also had a protective effect on reliable symptom deterioration, reducing the risk by 23–32%. No significant effects could be ascertained for any of these outcomes up to 24 months. For all favourable outcomes, control group event rates increased considerably at later follow-ups. For example, while only 32.6% of control group individuals achieved close to symptom-free status at post-test, this number was 52.4% up to 2 years. Table 2 details all one-stage IPD-MA results. Results of sensitivity analyses closely mirrored the main results, and we found no strong indications of publication bias (see Supplementary Material S9–S13).
Table 2 Pooled effects on depressive symptom severity, response and deterioration
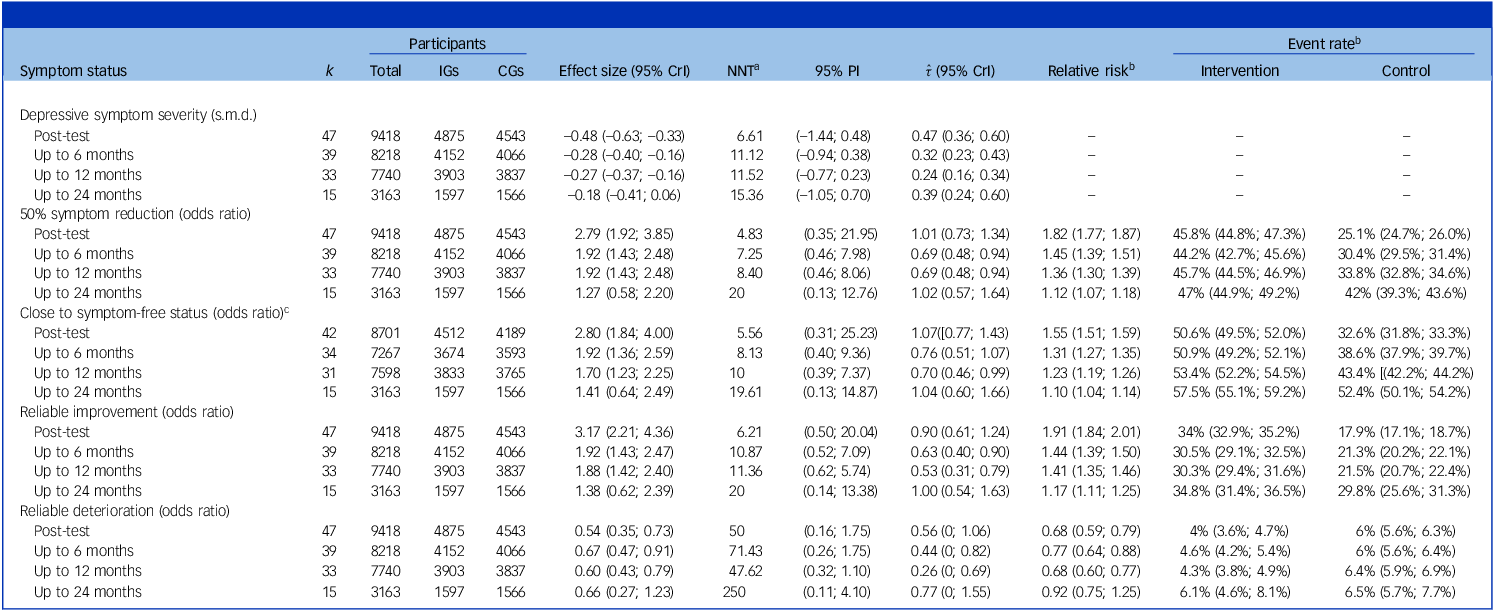
CGs, control groups; CrI, credibility interval; IGs, intervention groups; k, number of studies/effects; NNT, number needed to treat; PI, prediction interval; s.m.d., standardised mean difference.
a For effects on depressive symptom severity, NNTs were estimated using the method of Furukawa and Leucht Reference Furukawa and Leucht48 , with control group event rates (CERs) imputed from reliable improvement rates in the CGs.
b Calculated using regression standardisation (G-computation). Marginal risk ratios and their credible CrIs may diverge in their interpretation from the conditional ORs measured by the treatment indicator coefficient in the main one-stage IPD-MA model (see ‘Effect size’ column).
c Defined as scoring PHQ-9 < 5. This analysis included only studies using PHQ-9, or some other instrument that could be converted to PHQ-9 scores using the common metric of Wahl et al. Reference Wahl, Löwe and Bjorner24
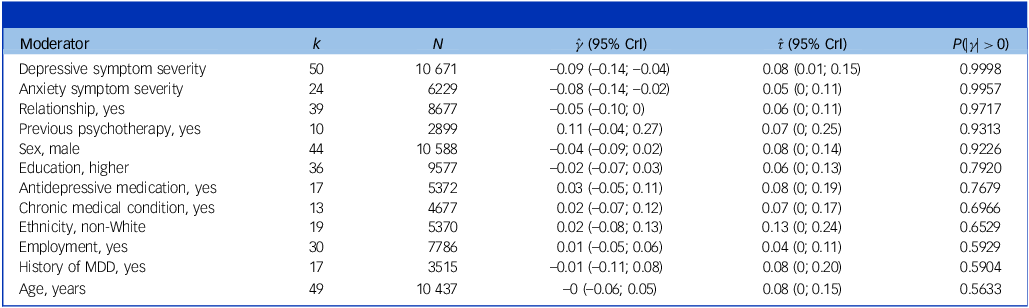
Effect modifiers
Table 3 shows the results for participant-level moderators. Only baseline depression and anxiety symptom severity emerged as highly credible effect modifiers (posterior tail probabilities >99%). For both, higher initial symptom severity predicted larger effects (
![]() $\hat \gamma $
= −0.09 to −0.08). Probabilities >90% were assigned to three additional variables: relationship status (higher benefits when in a relationship;
$\hat \gamma $
= −0.09 to −0.08). Probabilities >90% were assigned to three additional variables: relationship status (higher benefits when in a relationship;
![]() $\hat \gamma $
= −0.05, P = 0.97), psychotherapy in the past (predicting lower benefits;
$\hat \gamma $
= −0.05, P = 0.97), psychotherapy in the past (predicting lower benefits;
![]() $\hat \gamma \;$
= 0.11, P = 0.93) and sex (higher benefits in males,
$\hat \gamma \;$
= 0.11, P = 0.93) and sex (higher benefits in males,
![]() $\hat \gamma $
= −0.04, P = 0.92). Tests of study-level effect modifiers are given in Supplementary Material S14. Among study-level variables, only target group and intervention type were significant moderators. Smaller effects were found in general adult, older adult, chronic pain, diabetes and pregnant women populations (s.m.d. = −0.15 to −0.39), while higher benefits emerged in university students (s.m.d. = −0.59) and informal caregivers (s.m.d. = −1.35). Across intervention types, the largest effects were found for behavioural activation (s.m.d. = −0.72) and CBT-based treatments (s.m.d. = −0.56). The subgroup-specific effect among studies with a low revised Cochrane risk-of-bias tool for randomised trials (RoB) rating was s.m.d. = −0.51 (95% CrI = −0.70 to −0.33).
$\hat \gamma $
= −0.04, P = 0.92). Tests of study-level effect modifiers are given in Supplementary Material S14. Among study-level variables, only target group and intervention type were significant moderators. Smaller effects were found in general adult, older adult, chronic pain, diabetes and pregnant women populations (s.m.d. = −0.15 to −0.39), while higher benefits emerged in university students (s.m.d. = −0.59) and informal caregivers (s.m.d. = −1.35). Across intervention types, the largest effects were found for behavioural activation (s.m.d. = −0.72) and CBT-based treatments (s.m.d. = −0.56). The subgroup-specific effect among studies with a low revised Cochrane risk-of-bias tool for randomised trials (RoB) rating was s.m.d. = −0.51 (95% CrI = −0.70 to −0.33).
Table 3 Results of participant-level moderator analyses
![]() $\hat \gamma $
, Standardised pooled coefficient of the treatment–covariate interaction; k, number of studies providing data; N, number of participants included in the analysis; P(|
$\hat \gamma $
, Standardised pooled coefficient of the treatment–covariate interaction; k, number of studies providing data; N, number of participants included in the analysis; P(|
![]() $\gamma $
| > 0), posterior tail probability of
$\gamma $
| > 0), posterior tail probability of
![]() $\hat \gamma $
being greater/less than zero; MDD, major depressive disorder.
$\hat \gamma $
being greater/less than zero; MDD, major depressive disorder.
Figure 2 shows the predicted symptom severity (left) and treatment effect (right) conditional on baseline PHQ-9 values, as estimated by a non-linear interaction model. This analysis largely corroborated the main moderator model, showing that benefits rise with higher initial PHQ-9 values. Predicted treatment effects at established PHQ-9 cut-offs Reference Kroenke, Spitzer and Williams23 were s.m.d. = −0.33 (PHQ-9 = 5; lower cut-off for subthreshold depression), s.m.d. = −0.45 (PHQ-9 = 10; moderate subthreshold depression symptoms), s.m.d. = −0.51 (PHQ-9 = 15; moderately severe symptoms) and s.m.d. = −0.65 (PHQ-9 = 20; severe symptoms). Response and deterioration rates conditional on baseline PHQ-9 values are presented in supplementary material S15.
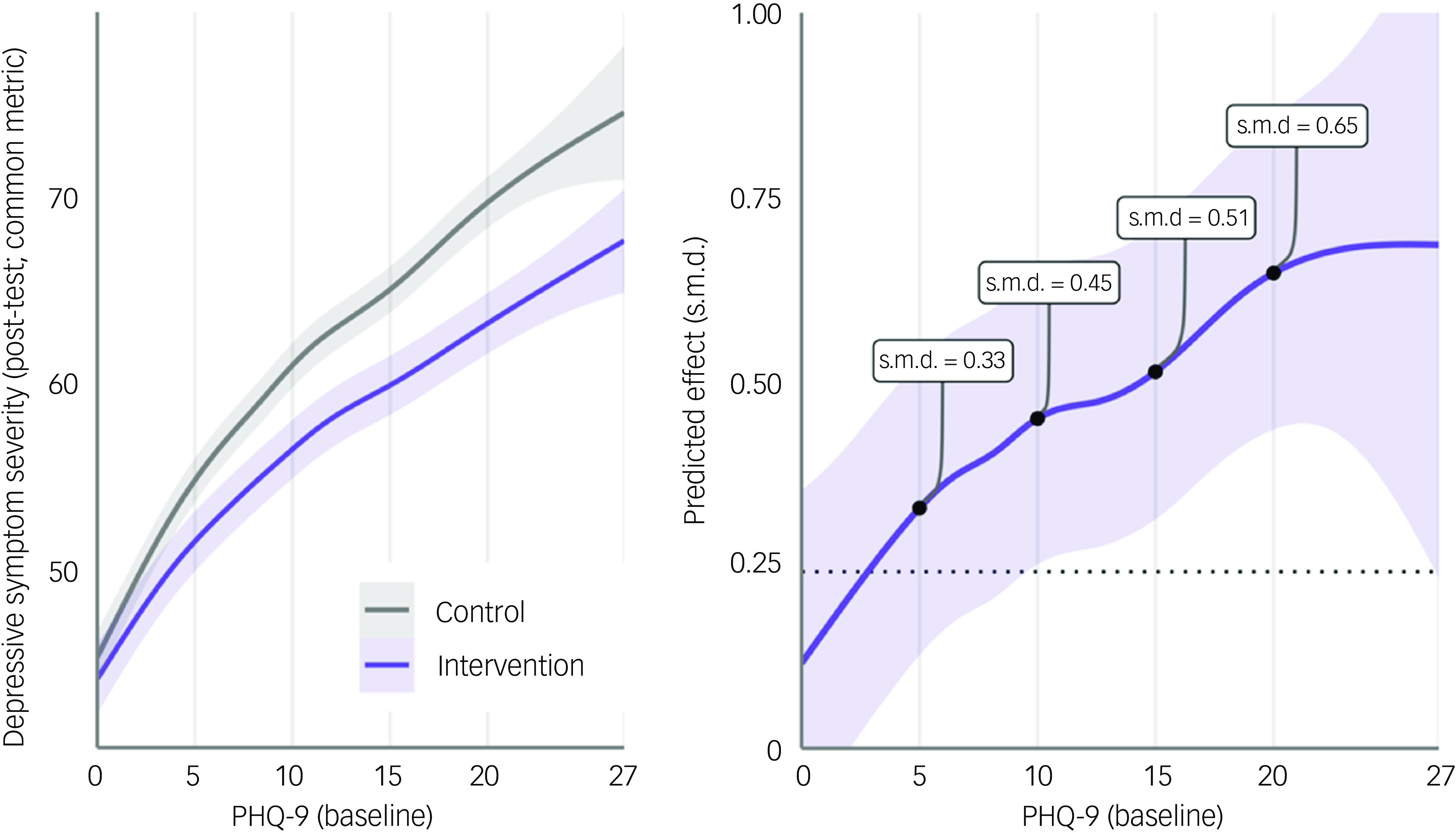
Fig. 2 Symptom severity and predicted treatment effects conditional on PHQ-9 baseline scores, based on a generalised additive mixed model with replicate cubic regression splines for both treatment groups (K = 10 basis functions). Models were fitted in the multiply imputed data and predictions obtained using the ‘predict-then-combine’/pool-last approach. Analyses were restricted to studies including assessments of PHQ-9 at baseline, or instruments convertible to PHQ-9 as per the common metric of Wahl et al. Reference Wahl, Löwe and Bjorner24 (k = 47, N = 9598). Signs of the predicted effect size were reversed, so that standardised mean difference (s.m.d.) values with a positive sign indicate favourable effects of the treatment. A population-level s.d. of 10 was assumed to standardise treatment effects, as implied by the common metric. Reference Wahl, Löwe and Bjorner24
Discussion
To our knowledge, this is the first IPD-MA to synthesise the effect of psychological intervention in subthreshold depression across all major treatment formats and target groups. We find that interventions yield significant benefits up to 12 months, which includes a protective effect on symptom deterioration. Baseline depression and anxiety severity emerged as the most credible effect modifiers, indicating that effects are greatest for individuals who already experience more severe symptoms.
Our pooled post-test effect (s.m.d. = −0.48) slightly exceeds estimates of previous meta-analyses (s.m.d. = −0.17 to −0.39). Reference Cuijpers, Koole, Dijke, Roca, Li and Reynolds12–Reference Rigabert, Motrico and Moreno-Peral16 It should be noted that our synthesis included a considerably larger number of trials than did these previous reviews (k = 50 v. 5–32), and that our IPD-MA approach also allowed the inclusion of trials with mixed populations. Furthermore, our results up to 6 months (s.m.d. = −0.28) and 12 months (s.m.d. = –0.27) also indicate somewhat weaker benefits. Nevertheless, we can conclude that psychological intervention is an effective method to address subthreshold depression for at least up to 1 year.
Intervention effects up to 24 months are less certain. We could not ascertain significance for any outcome within this time frame, and found only a 29% probability that effects on symptom severity were minimally important. We want to stress here that clinically irrelevant effects at a patient level may still be important at the population level. Looking at the control groups, for example, we find that 52 out of 100 individuals achieved close to symptom-free status up to 24 months, even without treatment. Provision of psychological interventions studied in this meta-analysis would lead to an additional five individuals being symptom free after 2 years. On a global scale, this would still mean that thousands of additional individuals could achieve remission, presuming that treatments are widely disseminated.
Nevertheless, given these very subtle effects (if existent at all) and the fact that an estimated 43% of individuals will not attain symptom-free status even when treated, long-term monitoring of the symptom course seems indicated, even when individuals with subthreshold depression can be motivated to partake in a one-time psychological intervention. Future research may also put a greater emphasis on long-term intervention strategies – for example, repeated booster sessions administered 1 year following the main treatment, to determine whether this helps to maintain effects over a longer period.
Individuals with subthreshold depression, by definition, do not (yet) suffer from a diagnosable MDD. Some individuals may also display only very mild symptoms, which do not necessarily transition into more severe symptoms, and can be transient. Reference Hermens, van Hout and Terluin38,Reference Musliner, Munk-Olsen, Eaton and Zandi39 This increases the importance of identifying those for whom a psychological intervention is particularly helpful. Such benefits must also be viewed in the context of available healthcare resources, given that subthreshold depression is even more prevalent than MDD Reference Cuijpers, de Graaf and van Dorsselaer40 , as well as potential risks of intervening, which includes the medicalisation of individuals without a diagnosable mental disorder. Reference Foulkes and Andrews41
To this end, one major benefit of this IPD-MA is its ability to explore moderators on a participant level. We found that initial symptom severity was the most robust predictor of treatment effects. Thus, in clinical practice, symptom severity may be the most relevant yardstick by which the benefits of intervention in individuals with subthreshold depression can be determined. We found the largest effect estimates in individuals with at least moderate symptoms (s.m.d. = −0.45 to −0.65, PHQ-9 ≥ 10). For such individuals, psychological intervention seems strongly indicated.
Minimally relevant benefits were predicted even for individuals with very mild symptoms (i.e. PHQ-9 = 5). However, effects at this symptom level correspond with a number needed to treat (NNT) of 11, meaning that almost a dozen individuals need to be treated to achieve one additional case of improvement. One could argue that reliable improvement is less relevant among individuals with mild symptoms, and that the prevention of symptom deterioration is more important. Nevertheless, we found that only a few individuals with low PHQ-9 scores reliably deteriorate (Supplementary Material S15), suggesting that the NNT for this outcome would be even higher.
For individuals with very mild symptoms, interventions in this IPD-MA would therefore need to be widely disseminated to have a meaningful impact at the population level. This may be challenging, given that most of the investigated treatments were face-to-face therapies with limited scalability. Digital interventions could be a more suitable option, and can be most easily disseminated as pure self-help, although often at the cost of lower effectiveness. Reference Moshe, Terhorst and Philippi42,Reference Terhorst, Kaiser and Brakemeier43 A more time-honoured approach for mild symptoms could be watchful waiting, whereby professionals monitor individuals’ symptoms over a longer period, Reference Cuijpers44 and to intervene only when symptoms persist or worsen. Some of the stepped-care interventions included in this IPD-MA already implement comparable methods.
Last, we also want to indicate some other variables for which we found tentative evidence of effect modification (>90% probability), including relationship status, treatment history and sex. Such characteristics could be used as additional stratification variables, but this would probably warrant further investigation in the context under study. No signs of effect modification were found for other relevant indicators, including age, ethnicity, medical comorbidities, antidepressant use and past MDD episodes. If true, this would underline the broad applicability of psychological interventions across various subthreshold depression populations.
Our study has several limitations. First, we could not obtain IPD from all eligible trials (29 out of 79 studies); however, our analysis including both IPD and aggregate-data trials largely corroborated our main analysis. Second, not all putative moderators defined in our initial protocol could be analysed due to their absence in most, or all, studies. This includes variables such as traumatic events (at baseline or post-randomisation), childhood adversity, self-esteem and diet. We also only examined moderators individually, rather than developing a more complex multivariable prediction model. Reference Efthimiou, Seo, Chalkou, Debray, Egger and Salanti45 However, such approaches also come with a greater risk of detecting spurious relationships Reference Steyerberg46 and can be difficult to interpret from a clinical perspective. Third, while all trials allowed unrestricted access to usual care, uptake of other treatments was recorded in only a small subset and was therefore not analysed. While baseline co-interventions (i.e. antidepressant use) did not appear to moderate the treatment effect, future studies could prioritise the assessment of long-term treatment utilisation. This would help determine, for example, whether early intervention in subthreshold depression influences future healthcare needs or how help-seeking behaviour relates to response and deterioration under usual care. Overall, such data would enable much more fine-grained longitudinal analyses of ‘natural’ recovery in subthreshold depression, which were not possible in this study. Last, we also note that 26% of our trials focused on older adults, and the mean age of our sample was therefore rather high (M = 52.8). This may restrict the generalisability of our findings to other populations. A more severe limitation is that only four studies (8%) were conducted in low- and middle-income countries (LMICs; China and Mexico). More evidence is needed to examine whether psychological interventions for subthreshold depression are equally effective in LMICs, where 80% of all people with mental disorders live. Reference Rathod, Pinninti and Irfan47
In sum, our findings support the routine provision of psychological interventions in individuals with subthreshold depression, especially those who already experience moderate depressive symptoms. Minimally important benefits may even emerge among individuals with very mild symptoms, but should be weighed against available healthcare resources and potential risks of intervening. More research is also needed on how treatment benefits can be sustained over several years.
Supplementary material
The supplementary material can be found at https://doi.org/10.1192/bjp.2025.56
Data availability
All extracted data are available in the manuscript and supplementary materials, as well as on the Metapsy website (docs.metapsy.org/databases/depression-psyctr). Individual-level data cannot be shared due to confidentiality agreements in the original studies. The corresponding author, M.H., may be contacted to determine whether specific data supporting our findings can be made available upon reasonable request. In most cases, data sharing will require a separate agreement between the requesting institution and the authors of the original studies for which data are requested.
Acknowledgements
We thank Lea Schuurmans, Stella Wernicke and Svenja Kratzke for their help with data extraction and independent risk-of-bias ratings.
Author contributions
D.D.E. and P.C. conceived the study. C.B. further contributed to study design. C.B., M.H., A.A.S. and S.I. selected the studies and extracted data. M.C.A., S.M.A., E.A., O.P.A., J.B., K.M.P.v.B., P.J.B., H.B., V.S., R.B., R.J.C., D.C., H.C., M.C., L.C., J.C., K.S.D., E.D., S.G., B.L.H., R.H., K.H., M.R.I., E.K., N.K., J.P.K., C.K., K.I., M.A.L., H.-N.L., D.L., J.V.L., S.M., J.M.M., R.F.M., A.M., S.N., R.O., P.O., M.P.-O., A.M.P., C.F.R.III, B.W.R., J.P.S.-M., L.B.S., V.S., P.S., L.S., Y.T., F.L.V., I.V.-d.L., E.W., W.Y., S.Y.S.W., P.C., C.B. and D.D.E. contributed individual participant data. M.H., A.A.S. and S.I. verified the data. M.H. analysed the data. M.H., T.A.F., P.C., S.L. and C.B. interpreted the results. M.H. wrote the first draft of the manuscript. All authors had access to all the data, provided critical input and revisions to the draft manuscripts and approved the final manuscript. M.H., D.D.E. and C.B. had final responsibility for the decision to submit for publication.
Funding
None.
Declaration of interest
M.H. is a statistical consultant of HelloBetter/Get. On Institut für Gesundheitstrainings GmbH, a company that implements digital mental health therapeutics in routine care. D.D.E. has served as a consultant to or on the scientific advisory boards of Sanofi, Novartis, Minddistrict, Lantern, Schoen Kliniken, Ideamed, German health insurance companies (BARMER, Techniker Krankenkasse) and a number of federal chambers for psychotherapy; and is a shareholder of HelloBetter/Get. On Institut für Gesundheitstrainings GmbH. M.S. is employed through a grant provided by the City of Nagoya; and receives personal fees from SONY outside the submitted work. F.J.S. has received consultation fees from Abbott and funding from Novo Nordisk and Sanofi outside this work. T.A.F. reports personal fees from Boehringer-Ingelheim, Daiichi Sankyo, DT Axis, Kyoto University Original, Shionogi, SONY and UpToDate, and a grant from DT Axis and Shionogi, outside the submitted work; in addition, T.A.F. has a patent (no. 7448125), a pending patent (no. 2022-082495) and intellectual properties for Kokoro-app licensed to Mitsubishi-Tanabe. In the past 3 years, S.L. has received honoraria for advising/consulting and/or for lectures and/or for educational material from Angelini, Apsen, Boehringer Ingelheim, Eisai, Ekademia, GedeonRichter, Janssen, Karuna, Kynexis, Lundbeck, Medichem, Medscape, Mitsubishi, Neurotorium, Otsuka, NovoNordisk, Recordati, Rovi and Teva. E.W. receives royalties from Guilford Press for a CBT treatment manual that he authored; and was an expert member of the recent UK NICE Guidelines for treatment of adult depression. N.K. and K.I. are employed at the Department of Digital Mental Health, an endowed department supported by a grant from 15 enterprises (https://dmh.m.u-tokyo.ac.jp/en), outside the submitted work. H.B. reports having received consultancy fees, fees for lectures or workshops from chambers of psychotherapists and training institutes for psychotherapists in the context of e-health research and licence fees for an Internet intervention. Outside of the submitted work, J.P.K. received funding for clinical trials (German Federal Ministry of Health, Servier), payments for presentations on psychological internet interventions (GAIA, Oberberg, Servier, Stillachhaus), consulting fees from developers and distributors of psychological internet interventions (all about me, Boehringer, Ethypharm, GAIA, sympatient) payments for workshops and books (Beltz, Elsevier, Hogrefe and Springer) on psychotherapy for chronic depression and on psychiatric emergencies. He is past president of the CBASP network (now DsG-CBASP) and serves as vice chairman of the chapter ‘Digital Psychiatry’ of the German Psychiatric Association (DGPPN). All other authors have no conflict of interest to declare.
P.C. and E.K. are members of the British Journal of Psychiatry editorial board. Neither P.C. nor E.K. took part in the review or decision-making process for this paper.








eLetters
No eLetters have been published for this article.