Introduction
The rupture of a thoracic aortic aneurysm (TAA) is a rare and life-threatening event that requires early detection, risk stratification, and surgical intervention.Reference Elefteriades 1 Point-of-care ultrasound (POCUS) has the potential to evaluate aortic aneurysmal disease.Reference Taylor, Oliva and Van Tonder 2 , Reference Nazerian, Vanni and Morello 3 We present an unusual case where POCUS led to the early detection of a pericardial effusion with an associated ascending TAA in a 29-year-old female.
Case Report
A 29-year-old previously healthy female patient presented to the emergency department (ED) with a four-day history of pleuritic retrosternal chest pain. She had a viral upper respiratory tract infection two weeks prior to presentation. Her only medication was the oral contraceptive pill. She denied other symptoms on review of systems.
On examination, the patient appeared well, was afebrile, and had normal vital signs with a heart rate of 77, blood pressure of 114/55, respiratory rate of 16, and room air oxygen saturation of 100%. Cardiac exam revealed normal heart sounds with a grade 2 diastolic murmur at the lower sternal border. Jugular venous pressure was elevated at 6 cm above the sternal angle. She also had water-hammer pulses and Quincke sign. The rest of the physical examination was unremarkable.
Lab testing, including troponin, was unremarkable. Electrocardiogram revealed normal sinus rhythm, and chest X-ray showed an enlarged cardiac silhouette (Figure 1). This prompted the emergency physician (EP) to perform a bedside ultrasound, which revealed a pericardial effusion (Figure 2).
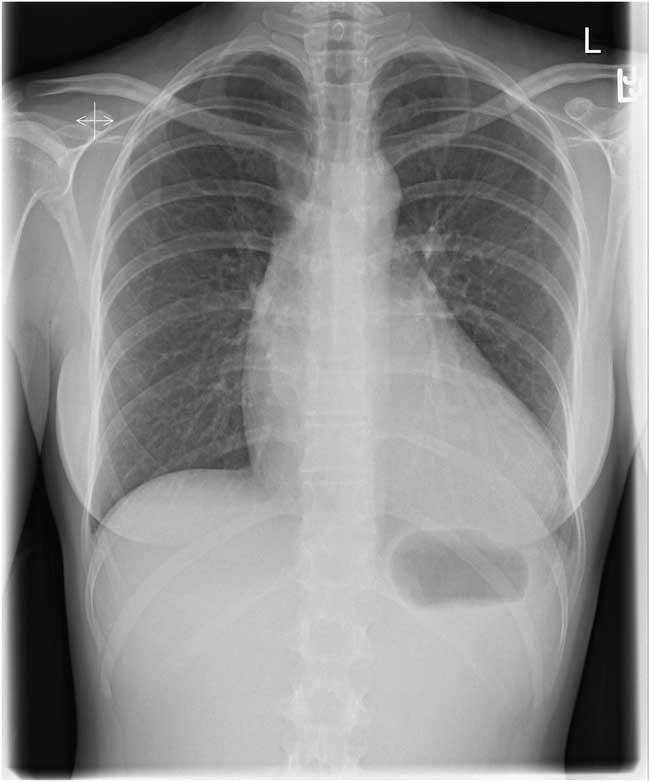
Figure 1 Chest X-ray showing an enlarged cardiac silhouette.
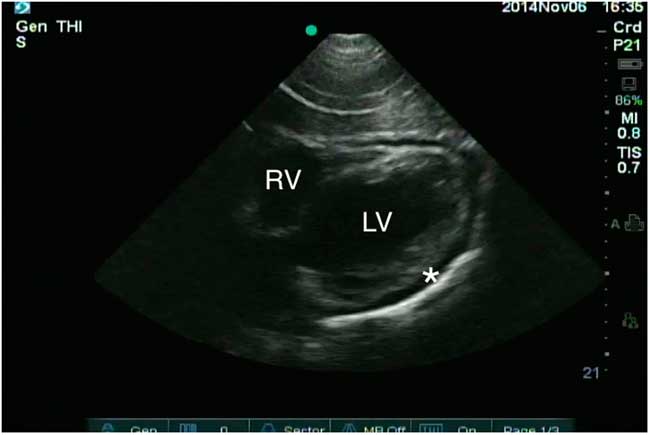
Figure 2 Subxiphoid window reveals a pericardial effusion (asterisk). The aortic root is not visible in this window. The left ventricle (LV) and right ventricle (RV) are also marked.
The EP consulted the emergency ultrasound fellow to scan the patient. The parasternal long axis (PSLA) window revealed a dilated ascending aorta measuring a maximum of 60 mm at the sinotubular aorta (Figure 3). The previously identified circumferential pericardial effusion measured 13 mm at the apex. There was no evidence of tamponade physiology such as right atrial or right ventricular collapse. The inferior vena cava had preserved respiratory variation, and interrogation of the abdominal aorta revealed no further aneurysmal disease.
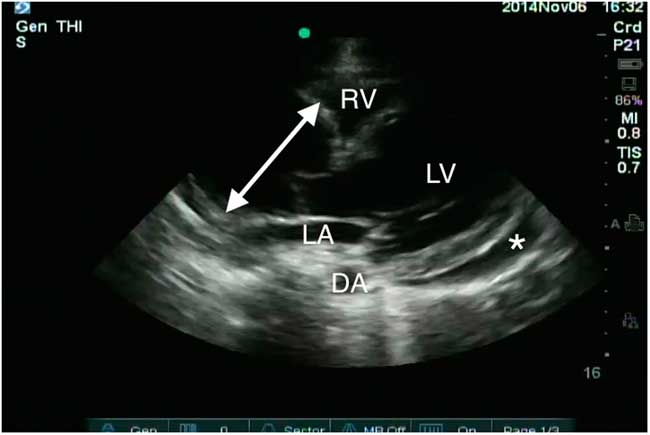
Figure 3 Parasternal long axis window demonstrates a dilated aortic root measuring 60 mm at the sinotubular junction (double-headed arrow) and a pericardial effusion (asterisk). The left ventricle (LV), left atrium (LA), right ventricle (RV), and descending aorta (DA) are also marked.
Consultative transthoracic echocardiography (TTE) confirmed the findings of the POCUS scan and also identified severe aortic insufficiency. Computed tomography angiogram (CTA) of the chest showed a dilated aortic root measuring a maximum of 80 mm and a large pericardial effusion of unclear density (Figure 4). There was no aortic dissection.
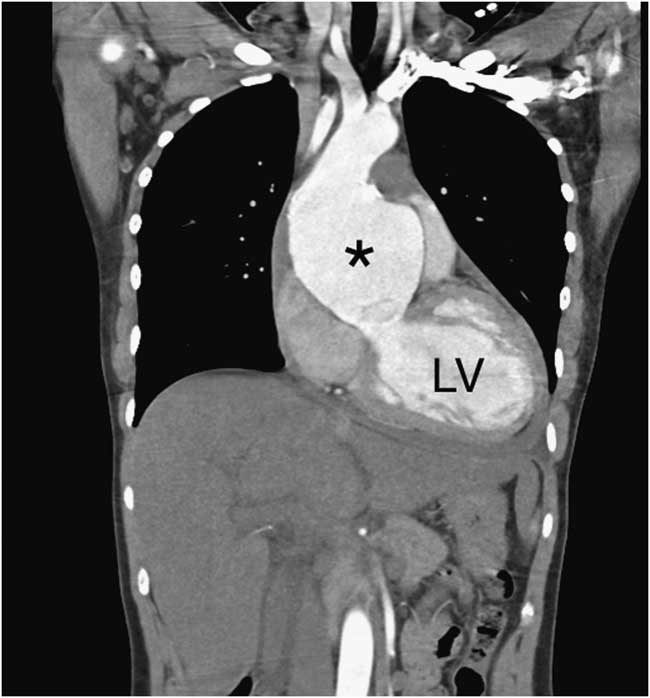
Figure 4 Coronal cut from the computed tomography angiogram demonstrates an aneurysmal ascending aorta (asterisk) with no dissection. The left ventricle (LV) is also marked.
The patient was given a presumptive diagnosis of pericarditis with effusion and an incidental ascending TAA. Screening bloodwork for autoimmune and inflammatory disease was negative. She did not meet clinical criteria for the diagnosis of any connective tissue disorders, including Marfan, Loeys-Dietz, and Ehlers-Danlos syndromes. She was admitted by the cardiovascular surgery team for further evaluation and a semi-elective repair of the aortic valve and annulus.
On the patient’s third admission day, she experienced a presyncopal episode secondary to obstructive shock necessitating emergency operative repair. A cardiology-performed TTE at that time confirmed significant tamponade physiology, so she was emergently taken to the operating room. The pericardium was opened and drained dark, old blood. The aorta was found to be very thin at the greater curvature, and a small puncture was seen in the aorta. These findings suggest the diagnosis of a contained ascending TAA rupture. She underwent successful operative repair and was ultimately discharged home in stable condition. Her native aortic valve and aorta were sent for pathologic analysis, which revealed nonspecific fibromyxoid degeneration and cystic degeneration with superimposed atherosclerosis, respectively.
Discussion
An aneurysmal vessel is one that is dilated more than 150% for what is expected based on age, sex, and body weight.Reference Elefteriades 1 TAAs have an estimated incidence of 5.6–10.4 cases per 100,000 patient years.Reference Clouse, Hallett and Schaff 4 Various etiologies can cause a TAA, including degeneration associated with increased age, atherosclerosis, genetically triggered syndromes (like bicuspid aortic valve, Marfan, Loeys-Dietz, Ehlers-Danlos, and Turner syndromes), aortitis, trauma, and chronic aortic dissection.Reference Booher and Eagle 5 The most dangerous complications are aortic rupture and aortic dissection, which become higher risk entities when the diameter of the ascending aorta increases above 50–60 mm.Reference Elefteriades and Farkas 6
A contained rupture of an ascending TAA is a very rare occurrence. The incidence of this event is estimated to be 5 in 100,000.Reference Johansson, Markström and Swedenborg 7 According to some reports, only about 41% of patients with a ruptured TAA are alive on arrival to hospital. Unfortunately, this vascular catastrophe is almost always fatal with a mortality of 97%–100%, even in the hands of very experienced cardiovascular surgeons.Reference Johansson, Markström and Swedenborg 7 Whereas overt, free rupture leads to massive bleeding, contained rupture is characterized by a perivascular hematoma that is sealed off by periaortic structures, such as the pleura, pericardium, and surrounding organs. As a result, patients with contained aortic rupture are hemodynamically stable.Reference Erbel, Aboyans and Boileau 8 A finding of ascending TAA with a pericardial effusion should always raise a high clinical suspicion for aortic rupture or aortic dissection and prompt immediate surgical evaluation.Reference Hiratzka, Bakris and Beckman 9
The PSLA approach is the most useful window for diagnosing an ascending TAA using ultrasound. A phased array cardiac probe is selected because it has a small footprint that allows ultrasound waves to be directed between the ribs. The PSLA window is generated by placing the probe in the fourth or fifth intercostal space adjacent to the sternum with the probe indicator pointing towards the patient’s right shoulder at 9 or 10 o’clock. Using the emergency medicine convention, the probe marker will be on the left side of the screen. If using the cardiology scanning convention, the probe marker will be on the right of the screen. The resulting image will be flipped in the horizontal plane. The operator can use either approach, provided that he or she understands the echocardiographic anatomy. This window will visualize the majority of the left ventricle, right ventricular outflow tract, left atrium, mitral and aortic valves, and aortic root. The probe can then be moved up one or two interspaces to better visualize the ascending aorta.Reference Peterson and Arntfield 10
Transesophageal echocardiography (TEE) is preferred over TTE for imaging the ascending aorta, but it may not be feasible, depending on adequate airway protection, need for sedation, and operator experience. TTE is often more practical in emergency situations because it is noninvasive, rapid, repeatable, and can be learned with a moderate amount of training.Reference Peterson and Arntfield 10 , Reference Evangelista, Flachskampf and Erbel 11 Aortic dilation is defined as an aortic diameter equal to or greater than 40 mm and aneurysm as a diameter equal to or greater than 45 mm.Reference Erbel, Aboyans and Boileau 8 These measurements should be taken in end-diastole using the leading edge technique (diameter measured from leading edge to leading edge) at the largest visible portion of the ascending aorta.Reference Taylor, Oliva and Van Tonder 2 , Reference Nazerian, Vanni and Morello 3 , Reference Vriz, Driussi and Bettio 12 , Reference Roman, Devereux and Kramer-Fox 13 POCUS demonstrates good agreement with CTA measurements of maximal thoracic aortic diameter. Using a cutoff of 40 mm, POCUS has a sensitivity of 77%–79% and a specificity of 93%–95% for thoracic aortic dilation.Reference Taylor, Oliva and Van Tonder 2 , Reference Nazerian, Vanni and Morello 3
Thoracic CTA is the current gold-standard imaging modality to evaluate patients who are suspected of having a TAA because it produces images that both quantify the aneurysm and allow for surgical planning.Reference Erbel, Aboyans and Boileau 8 , Reference Hiratzka, Bakris and Beckman 9 Ultrasound is primarily used as a surveillance tool in evaluating patients with connective tissue disease who are at higher risk for TAA.Reference Hiratzka, Bakris and Beckman 9 However, in the ED, EPs can use POCUS as an initial screening tool in the assessment of patients who present with a clinical picture concerning for TAA.Reference Taylor, Oliva and Van Tonder 2 , Reference Nazerian, Vanni and Morello 3
Conclusion
POCUS is a useful adjunct for evaluating high-risk patients with chest pain and can help identify lethal intrathoracic pathology, such as pericardial effusion or TAA at the bedside. CTA remains the imaging modality of choice for the diagnosis, evaluation, and surgical planning of a suspected TAA. However, in emergency situations, cardiac POCUS using a PSLA window can provide rapid recognition of an ascending TAA. This may allow for earlier diagnosis and expedited surgical management.
Competing interests: None declared.





