Introduction
Curative treatment of head and neck squamous cell carcinoma involves surgery and/or radiotherapy. Definitive radiotherapy is usually delivered to a dose of 70 Gy, in 2 Gy per fraction, with the entire treatment course aimed to complete over a 7-week period, often with concurrent chemotherapy. Definitive treatment is known to be associated with severe toxicities including dysphagia, xerostomia, dysgeusia, malnutrition and dehydration.Reference Chang, Gosling, Larsen, Powell, Scanlon and Chander1
Delivery of curative treatment may not always be possible, either due to patient frailty, tumour extent and/or presence of distant metastasis. In this patient cohort, palliative radiotherapy for local tumour control is usually considered for the purposes of improving or delaying potential symptoms such as pain and dysphagia that could affect patient’s quality of life. Several effective conventional palliative radiotherapy (CPRT) regimens have been developed with varying outcomes in tumour control and toxicity.Reference Corry, Peters and Costa2,Reference Porceddu, Rosser and Burmeister3 The QUAD SHOT regimen consisted of delivering 14 Gy in four fractions, delivered twice a day and at least 6 hours apart for 2 consecutive days. If there was no tumour progression, this was repeated at 4-weekly intervals for two further courses. Results show improved quality of life in 44% of patients. Median overall survival (OS) was 5·7 months while median progression-free survival (PFS) was 3·1 months.Reference Corry, Peters and Costa2 The Hypo Trial regimen is another well-established palliative regimen.Reference Porceddu, Rosser and Burmeister3 The Hypo Trial was a Phase II, single cohort study of 35 patients with head and neck cancers that were unsuitable for curative-intent treatment. Patients were treated using conventional radiotherapy techniques to a dose of 30 Gy in 5 fractions at two fractions per week (with an additional boost of 6 Gy for those with small volume disease). Overall, 80% of patients had an objective response. The median times to progression and death were 3·9 months and 6.1 months, respectively. Grade 3 mucositis was reported in 26% and grade 3 skin reaction in 11%.
Stereotactic ablative body radiotherapy (SABR) is a technique that may offer important benefits over the previously described CPRT regimens. SABR refers to the precise delivery of highly conformal and image-guided hypofractionated external beam radiotherapy, delivered in a single or few fractions, with doses at least biologically equivalent to a radical curative course.Reference Sahgal, Roberge and Schellenberg4 With millimetric precision and rapid dose fall off with SABR techniques, radiation doses to surrounding normal organs may be significantly reduced compared to more CPRT techniques.
SABR is now well established with proven clinical efficacy in many body sites including lung, liver and spine.Reference Sahgal, Roberge and Schellenberg4 SABR for head and neck malignancies has primarily been employed in the setting of recurrence or second primary cancer following past curative radiotherapy. Multiple studies have shown that this alone or in combination with cetuximab is an effective treatment for recurrent and second primary head and neck cancer.Reference Ling, Vargo and Ferris5–Reference Cengiz, Ozyigit and Yazici7 A phase 2 prospective clinical trial is currently in progress investigating the use of SABR in previously untreated elderly and/or inoperable head and neck cancer.Reference Lee8 There are otherwise limited studies in the literature reporting the use of SABR in patients with previously untreated head and neck cancer. However, these have so far demonstrated toxicity profiles at least similar to that of CPRT, with a possible advantage of superior tumour control.Reference Khan, Tjong and Raziee9–Reference Al-Assaf, Erler and Karam12
There are currently no clinical or radiobiological modelling studies in the literature comparing outcomes for SABR against CPRT in the palliative management of patients with previously untreated head and neck cancer. We hoped to evaluate the role of SABR by performing a biological modelling study, comparing SABR to CPRT and curative-dose volumetric-modulated arc therapy (CD-VMAT). The rationale for the comparison to CD-VMAT is that this provides the gold standard radiotherapy regimen for maximising local control rates, while having a toxicity profile that’s usually considered to be too severe for palliation.
We hypothesise that SABR plans can be made that satisfy the dosimetric objectives in this study, and furthermore, has superior tumour control probability (TCP) to CPRT while having similar normal tissue complication probability (NTCP). We further hypothesise that SABR provides similar TCP to CD-VMAT with lower NTCP. Overall, we hypothesise that SABR has the best therapeutic ratio as measured by the uncomplicated tumour control probability (UTCP) amongst the three radiotherapy strategies evaluated in this study.
Methods
Eight patients diagnosed with head and neck cancer who were referred for radiotherapy at the Central Coast Cancer Centre between 2017 and 2020 were randomly selected for inclusion in this study. Inclusion criteria included biopsy-proven squamous cell carcinoma, with no single gross tumour volume (GTV) exceeding 5 cm in maximum dimension, and with no more than three separate GTVs. Exclusion criteria included those with laryngeal or hypopharyngeal primaries. Target volumes and organs at risk (OAR) were independently contoured by a head and neck radiation oncologist. This study was approved by the local human research ethics committee.
Radiotherapy treatment planning
Treatment plans were generated using Eclipse version 15.6 using a 6-megavoltage photon beam model from a Varian Clinac iX (Varian Medical Systems, Palo Alto, CA, USA). Three radiotherapy treatment plans were generated for each patient: SABR, CPRT and CD-VMAT. The GTV was defined based on all available clinical and imaging information for all three plans.
For SABR plans, a dose of 45 Gy in 5 fractions with an overall treatment time of 10 days was prescribed. A clinical target volume, 30 Gy (CTV30) was created by expanding the GTV by 5mm, and clipping it at anatomical boundaries. A clinical target volume, 25 Gy (CTV25) was created for a limited elective nodal volume, similar to that described in past studies on volume de-escalation of elective nodal volumes.Reference Sher, Pham and Shah13 Depending on the primary site CTV25 was created according to the following: For oral cavity primaries, the ipsilateral levels IA and IB were included for well-lateralised tumours and bilateral levels IA and IB were included for non-well-lateralised tumours. For well-lateralised tonsillar or oral cavity primaries, the ipsilateral level II was included. For other oral cavity, oropharyngeal, or nasopharyngeal primaries, bilateral level II were included. A planning target volume, 45 Gy (PTV45) was created by expanding the GTV isotropically by 3mm. A planning target volume, 30 Gy (PTV30) was created by expanding the CTV30 by 3mm. A planning target volume, 25 Gy (PTV25) was created by expanding the CTV25 by 3mm. The target objectives and OAR constraints are listed in Table 1. The three dose levels (45 Gy, 30 Gy and 25 Gy) were treated using simultaneous integrated boost (SIB) with volumetric-modulated arc therapy (VMAT) with 1-2 coplanar arcs.
Table 1. Summary of target objectives and OAR dose constraints for SABR plans

Abbreviations: OAR = organ at risk; PRV = planning organ at risk volume (3 mm used for the structures defined in the table); Dmax = the maximum absorbed dose as specified by a single calculation point; Dmean = the mean dose; D99% = the dose received by 99% of the volume; D0·1cc = the dose received by 0·1cc of the volume.
A dose of 30 Gy in 5 fractions with an overall treatment time of 10 days was prescribed for the CPRT plans. The CTV30 was defined in the same way as for SABR plans. The PTV30 was created by expanding the CTV30 by 5 mm. Simple two- or three-dimensional radiotherapy plans were created (in order to make the results comparable to the Hypo trial, which used this regimen clinically,Reference Porceddu, Rosser and Burmeister3 covering as much of the PTV as possible, while excluding the spinal cord to limit its dose to 25·3 Gy.
For CD-VMAT plans, we prescribed a dose of 70 Gy in 35 fractions with an overall treatment time of 47 days. The clinical target volume, 70 Gy (CTV70) was defined by expanding the GTV by 5 mm, and clipping it at anatomical boundaries. The clinical target volume, 56 Gy (CTV56) was defined as per consensus guidelines for elective nodal volumes.Reference Biau, Lapeyre and Troussier14,Reference Porceddu, Daniels and Yom15 The two dose levels (70 Gy and 56 Gy) were treated using a SIB technique with VMAT with a single coplanar arc. Target objectives and OAR constraints were as per the TROG 12·01 protocol and summarised in Table 2.16
Table 2. Summary of target objectives and OAR dose constraints for CD-VMAT plans, adapted from TROG 12·01

Abbreviations: OAR = organ at risk; PRV = planning organ at risk volume (3mm used for the structures defined in the table); Dmax = the maximum absorbed dose as specified by a single calculation point; Dmean = the mean dose; D98% = the dose received by 98% of the volume; D1% = the dose received by 1% of the volume.
Biological Modelling
TCP values were calculated using a modified Poisson model.Reference Webb and Nahum17 Parameters used were α/β = 10, α = 0·396, σα = 0·07 and clonogenic cell density = 107 clonogens/cm3. Tumour kinetics were also considered in the calculation with tumour kick-off time (Tk) of 28 days and potential doubling time (Tpot) of 3 days.
Two NTCP models were selected for the biological modelling in this study. NTCPsaliva for patient-rated xerostomia and sticky saliva were calculated using the Beetz logistic regression formula: NTCP = (1 + e-S)-1, where S = -1·443 + (mean dose contralateral parotid gland × 0·047) + (baseline xerostomia score × 0·720).Reference Beetz, Schilstra and van der Schaaf18 Baseline xerostomia score was standardised as 0 for all patients. NTCPswallow for swallowing dysfunction was calculated using the Christianen formula: NTCP = (1 + e-S)-1, where S = -6·09 + (mean dose superior pharyngeal constrictor muscle × 0·057) + (mean dose supraglottic larynx × 0·037).Reference Christianen, van der Schaaf and van der Laan19 Doses were converted to 2 Gy per fraction using the linear-quadratic model with an α/β ratio of 3 for the parotid gland, superior pharyngeal constrictor muscle and supraglottic larynx.
The Agren model was used to calculate UTCP.Reference Agren, Brahme and Turesson20 This calculation combined the TCP and NTCP values for the two organs using σ = 0·2.
All biological modelling calculations were performed using the in-house developed RADBIOMOD software.Reference Chang, Gehrke and Prabhakar21 RADBIOMOD is a platform that incorporates several biological modelling formulas including the modified Poisson and Agren models to allow ease of calculations within a single program.
Statistical analysis
One-way analysis of variance (ANOVA) and post hoc Tukey honest significant difference (HSD) were performed on the various radiobiological metrics for the three treatment plans. Statistical significance was taken at p < 0·05. This was performed with SPSS for Windows version 27 (IBM, Armonk, NY, USA).
Results
The patient characteristics for the 8 patients are summarised in Table 3. The median age of diagnosis was 66 years old (range: 48–81 years), and there was an equal distribution of male and female patients. The mean tumour volume was 23·19 cm3 (range: 5·32–37·57 cm3). Target objectives and OAR constraints as defined in the methods section were met in all treatment plans.
Table 3. Baseline patient characteristics

Abbreviations: SCC = squamous cell carcinoma.
The mean TCP values for SABR, CPRT and CD-VMAT were 100%, 81% and 93%, respectively. Therefore, mean TCP values for SABR were 19% and 7% higher than CPRT and CD-VMAT, respectively. The difference in means were statistically significant (f-ratio = 17·09, p < 0.001). Post Hoc Tukey HSD showed statistically significant differences in the means between SABR and CPRT (p < 0·001) as well as CPRT and CD-VMAT (p = 0·003). The difference between SABR and CD-VMAT was not statistically significant (p = 0·135)
The mean NTCPsaliva for SABR, CPRT and CD-VMAT were 27%, 41% and 36%, respectively. The differences in means were not statistically significant (f-ratio=2·67, p = 0·093).
Similarly, there was no statistically significant difference in means for NTCPswallow. The mean values for SABR, CPRT and CD-VMAT were 9%, 12% and 23%, respectively (f-ratio-2·69, p = 0·091).
Finally, the mean UTCP values for SABR, CPRT and CD-VMAT were 66%, 42% and 49%, respectively. The mean UTCP value for SABR was 24% and 17% higher than that of CPRT and CD-VMAT, respectively. This showed a statistically significant difference in means (f-ratio=7·25, p = 0·004). Post hoc Tukey HSD showed statistically significant difference between both SABR vs CPRT (p = 0·004), as well as SABR vs CD-VMAT (p = 0·04). The difference in mean UTCP values was not statistically significant between CPRT and CD-VMAT (p = 0·542).
The results of mean TCP, NTCP and UTCP values are summarised in Table 4. The mean values were rounded to the nearest whole number to reflect the uncertainties of these calculations in biological modelling.
Table 4. TCP, NTCP and UTCP results
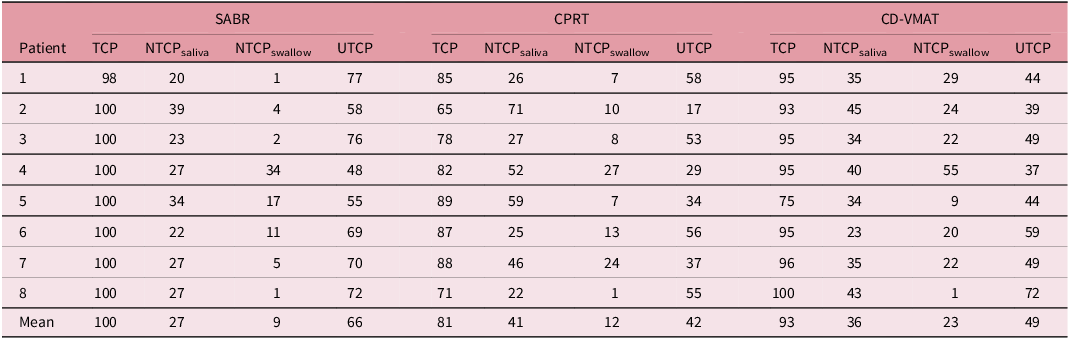
Abbreviations: SABR = stereotactic ablative radiotherapy; CPRT = conventional palliative radiotherapy; CD-VMAT = curative-dose volumetric arc therapy; TCP = tumour control probability; NTCPsaliva = normal tissue complication probability for sticky saliva and xerostomia; NTCPswallow = normal tissue complication probability for swallow dysfunction; UTCP = uncomplicated tumour control probability.
Values were rounded down to 0 decimal places to reflect the uncertainties of these calculations.
Discussion
In this biological modelling study, we have shown that SABR has the best therapeutic ratio (as measured by the UTCP) of the three plans analysed. This was the result of SABR having a statistically significantly higher mean TCP than the other two plans, and mean NTCPs that were numerically lower than the other two plans (despite not showing statistical significance). We have also shown that the dose constraints as described in this study are achievable. A colourwash representation of the dose distribution of the three different plans for patient 1 is depicted in Figure 1. Visually, we can observe that in the SABR plan, there is a very conformal high-dose region within the GTV with rapid dose fall off, while in the CPRT plan, homogenous high-dose wash results in increased dose to the midline structures and parotid glands. The CD-VMAT plan was also highly conformal, however, included a larger area of elective nodal volumes resulting in increased dose to surrounding OARs.

Figure 1. Colourwash representation of the dose distribution of the different plans for patient 1. The three treatment plans shown are: (a) SABR, (b) CPRT, (c) CD-VMAT. The blue contour denotes the GTV which is identical for all three plans. The red contour denotes the high dose PTV for each plan. The dose distribution is represented by the colour, with red representing high doses and blue representing low doses. As seen in plan (a), high dose region is concentrated within the GTV with a rapid dose drop off beyond the target volume. Plan (b) depicts a parallel opposed beam plan which as expected, has homogenous high dose wash through the midline structures and contralateral parotid gland. Plan (c) depicts a VMAT plan that also includes elective nodal volumes.
These results are consistent with the clinical results obtained in previous studies of SABR and CPRT in palliative patients.
There is currently no consensus in international guidelines regarding the optimal palliative radiotherapy regimen that should be used. The CPRT regimen used in this study was based on a slight modification of the Hypo Trial.Reference Porceddu, Rosser and Burmeister3 This regimen was chosen as Porceddu et al. reported a good response rate at 80% with an acceptable side-effect profile. Other attempts at dose escalation have resulted in improved local control but were limited by unacceptably high rates of toxicity. This includes a cohort study by Agarwal et al. where patients with unresectable head and neck cancer were treated to a dose of 40 Gy in 16 fractions over 3.5 weeks with an option of dose escalation to 50 Gy in 20 fractions in patients showing initial disease regression and acceptable toxicity. While response rate was excellent with 55% of patients demonstrating progression-free survival at 1 year, toxicities were high with 14% of patients developing grade III skin toxicity, 63% and 3% of cases with grade III and IV mucosal toxicities, respectively.Reference Agarwal, Nemade and Murthy22
With recent technological advances, SABR may overcome historical limitations in dose escalation. The SABR regimen used in this study was based on a retrospective single institution experience by Al-Assaf et al. of 114 patients including four distinct clinical groups (48 patients with previously untreated head and neck cancer, 19 patients with recurrent, never irradiated head and neck cancer, 17 patients with oligometastatic non-head and neck cancer primaries and 33 patients with previously irradiated head and neck cancer).Reference Al-Assaf, Erler and Karam12 Patients were treated to a dose of 35 to 50 Gy in 4–6 fractions using a SABR technique. The overall response rate was 84·6%. The median progression-free survival for each clinical group were 23·7, 14·8, 10·5 and 7·8 months, respectively. Grade 3 or higher acute mucositis and dermatitis were noted in 20·5% and 32·5%, respectively. The median overall survival was 13.6 months. This study demonstrated longer progression-free survival (especially in the most comparable cohort: the previously untreated head and neck cancer cohort) than the CPRT study (the Hypo trial),Reference Porceddu, Rosser and Burmeister3 while showing similar levels of grade 3 toxicity, which is consistent with the results of our study. Clinical studies of 70 Gy in 35 fractions were not performed in the same cohort of patients, so were generally not comparable. While we await the results of the SHINE phase 2 trial,Reference Lee23 prospective evidence for the use of SABR in the de-novo setting is currently lacking. A meta-analysis by Malik et al. included a total of 9 studies, 7 of which are retrospective.Reference Malik, Kim and Chen24 The 2 prospective studies included radiotherapy regimens that exceeded 10 fractions. The authors concluded that SABR may be effective and safe in the de-novo setting with high local control rates of 73.5% and overall survival of 50% at 3 years. Toxicity rates were acceptable, with 3% reported late grade 3 or higher toxicity.
As with all planning/biological modelling studies, a number of assumptions were made in this study that may not be representative of real-world clinical practice. Because the original Hypo TrialReference Porceddu, Rosser and Burmeister3 used conventional radiotherapy techniques, we also used conventional radiotherapy for the CPRT plans rather than more modern techniques as VMAT. This makes the results more comparable to the clinical results reported in the original Hypo Trial, however, would make the NTCP values in the CPRT plans higher than what could be achieved with more modern techniques. To simplify the calculations, the effects of concurrent chemotherapy were not modelled in the CD-VMAT plans which theoretically would have made the treatment have both higher TCP and higher NTCP. We chose only two NTCP models to evaluate in this study: that for salivary function and that for swallowing dysfunction. A number of other organs can potentially be modelled; however, we chose these two because they have been clinically validated, and we believe these are the most clinically relevant toxicities in the palliative radiotherapy cohort.Reference Chang, Wada and Anderson25 NTCP models for soft tissue necrosis and carotid blowout would have been interesting to explore; however, we could not find reliable models for those endpoints for this study. Data on carotid dosimetry and risk of carotid blowout syndrome are limited in current literature. However, in the setting of re-irradiation of head and neck cancers with SABR, events of carotid blowout are uncommon, especially if fractions are delivered over non-consecutive days and D0.1cc was <47·6 Gy.Reference Gebhardt, Vargo and Ling26 In the retrospective study, we based our SABR regimen on, there were no reported cases of carotid blowout.Reference Al-Assaf, Erler and Karam12 The TCP model likely overestimated the true TCP, yielding an average TCP of 100% for SABR. Part of the reason for this may be the fact that we chose to use the linear-quadratic model (rather than one of the models designed for hypofractionated radiotherapy) for the purposes of simplicity, which may have shortcomings when dealing with hypofractionated radiotherapy.Reference Kirkpatrick, Meyer and Marks27,Reference Brenner28
This study is important because to our knowledge, this is the first to compare SABR and CPRT in the de novo, previously unirradiated head and neck cancer patient cohort. This study at least establishes a theoretical basis for this technique, before we embark on a prospective clinical trial on this topic. While imperfect, the radiobiological modelling provides more clinically relevant endpoints than could be achieved by a planning study that only has dosimetric endpoints. We plan on following up this study with a prospective clinical trial on SABR for the palliation of head and neck cancer patients.
Conclusion
It is feasible to create SABR plans that satisfy the dosimetric objectives in this study. Based on radiobiological modelling, SABR has superior TCP and similar NTCP, leading to a better therapeutic ratio than CPRT and CD-VMAT.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
All authors have indicated that they do not have any conflicts of interest in relation to this work.
Ethics approval statement
This study was approved by the local human research ethics committee.







