On June 6, 2017, a plutonium internal contamination accident involving 5 workers occurred during operations at the Plutonium Fuel Research Facility (PFRF) of the Japan Atomic Energy Agency (JAEA) Oarai Research and Development Center (currently renamed the Oarai Nuclear Engineering Institute).Reference Iwai, Sasaki and Higaki1, Reference Tatsuzaki, Tominaga and Kim2 Following the initial decontamination, the workers were transferred to the Nuclear Fuel Cycle Engineering Laboratories (NFCEL), another JAEA base, where lung measurements were conducted and Ca-DTPA was administered intravenously.
The next day, the National Institutes for Quantum Science and Technology (QST) (formerly known as the National Institute for Radiological Sciences [NIRS]), which is one of Japan’s Advanced Radiation Emergency Medical Support Centers, admitted the 5 workers to continue treatment and conducted individual monitoring for internal dose assessment. This case marked the first instance in Japan where Ca/Zn-DTPA treatment was administered to patients with actinide internal contamination as a medical intervention.
Approximately one month later, on July 10, QST published the internal dose assessment results for the 5 workers. To address uncertainties in the evaluation and safeguard the workers’ privacy, the results were presented as dose bands.Reference Tominaga, Shimomura and Tanosaki3 The worker with the highest internal dose was estimated to have received a dose band of 100-200 mSv.
The internal dose assessment had 2 main objectives: (1) to report the results to regulatory authorities in compliance with national laws and regulations, and (2) to refine biokinetic model parameters for improved realism in research. QST’s assessment was primarily focused on the first objective, employing conservative but reasonable assumptions based on individual monitoring results.
This paper provides a detailed account of the methods used in the internal dose assessment at the time, evaluates the validity and uncertainties of the assessment using subsequently obtained information, and reflects on lessons learned from the process. An in-depth analysis of the dose reduction effects achieved through Ca/Zn-DTPA treatment lies beyond the scope of this study.
Overview of the Accident
At approximately 11:15 a.m. on June 6, 2017, during inspection of a storage container holding nuclear materials inside a hood in Room 108 of PFRF (in a radiation-controlled area), a plastic bag inside the container ruptured. This accident created the potential for contamination of 5 workers present in the room. At the time, they were wearing coveralls, half-face masks, caps, double rubber gloves over cotton gloves, and shoe covers over boots.Reference Tatsuzaki, Tominaga and Kim2 To prevent the spread of contamination, the room was sealed, and a temporary enclosure, known as a “greenhouse,” was set up at the entrance. This greenhouse was a chamber made of plastic sheeting, designed to isolate the affected area and contain radioactive material. Contamination surveys of the bodies and nasal cavities of the workers were conducted sequentially. For 4 workers with confirmed contamination, decontamination via showering was performed, and all workers exited the controlled area at approximately 6:55 p.m. after decontamination was completed.
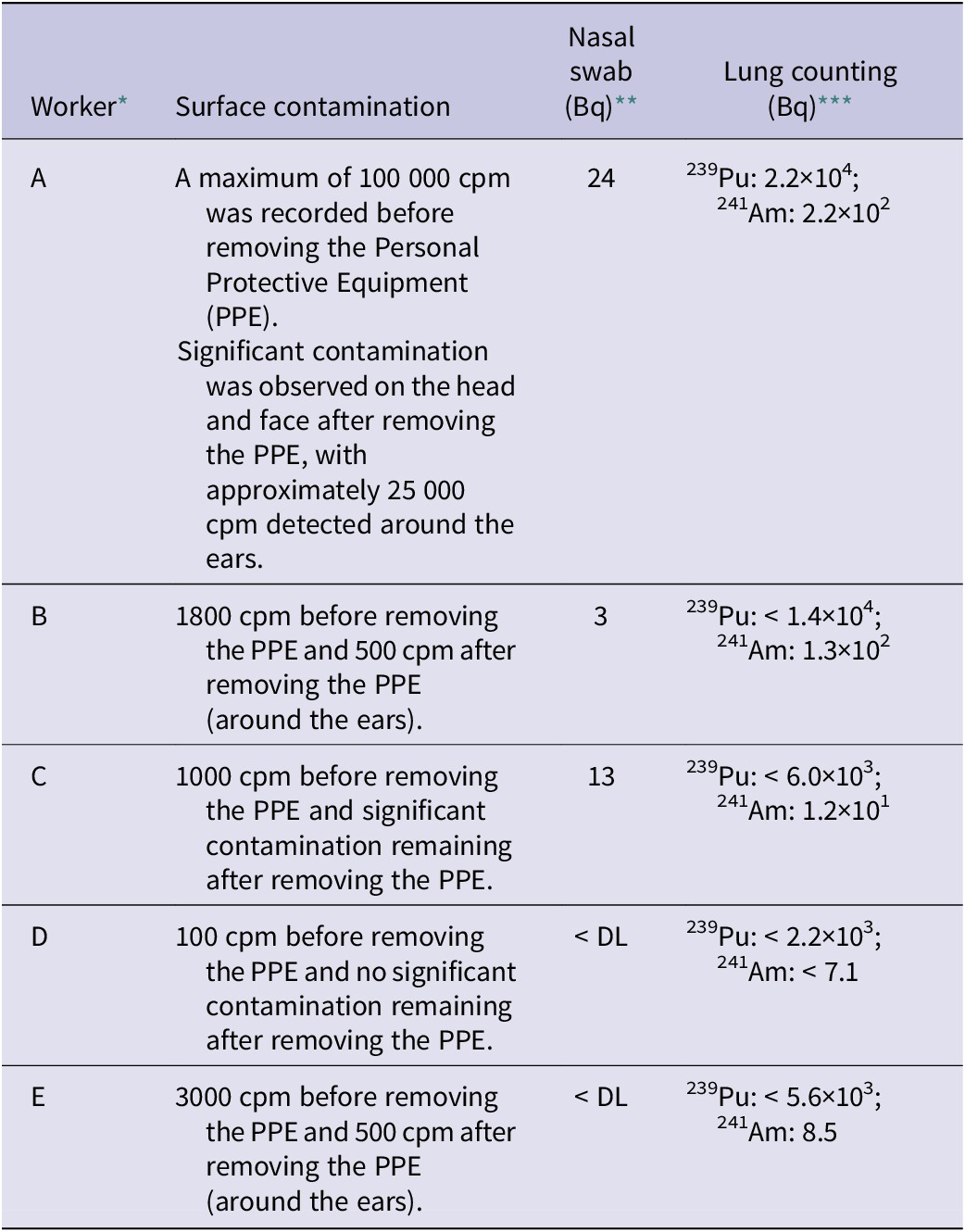
Subsequently, the 5 workers were transferred to the NFCEL, another JAEA base, where lung counting was conducted, followed by intravenous administration of Ca-DTPA. The treatment began at around 10:05 p.m., approximately 11 hours after the accident. While nebulized inhalation of chelating agents is an alternative administration method, intravenous injection was chosen in this case for its more reliable drug delivery.Reference Tominaga, Shimomura and Tanosaki3 Table 1 summarizes the results of body contamination measurements, nasal smears, and lung counting for the 5 workers. Worker A, who exhibited the highest radioactivity in nasal smears, was the primary operator standing in front of the hood during the procedure. Lung counting initially detected 2.2 × 104 Bq of 239 Pu and 2.2 × 102 Bq of 241Am in Worker A; however, this was later determined to be due to surface contamination on the body rather than internal contamination.
Table 1. Results of surface contamination, nasal swabs, and lung counting at JAEA

* The indexes of workers differ from those in the JAEA report (Ref. 3).
** DL: Detection limit (0.57 Bq per a pair of 2 nasal swab samples taken from the left and right nostrils).
*** <A indicates that the value is below the detection limit A. The detection limit of lung counting varies depending on the physique of the individual being tested.
The following morning, the 5 workers were transported to QST, where a second dose of Ca-DTPA was administered in the evening. Body contamination inspections at QST confirmed the presence of residual contamination in trace amounts.Reference Tatsuzaki, Tominaga and Kim2 Subsequent lung counting at QST detected no characteristic X-ray peaks of Pu isotopes from any workers; however, gamma-ray peaks at 59.5 keV (emission yield: 35.9%) of 241Am were observed in 2 workers (A and C). For bioassay analysis, QST handled urine samples, while fecal samples were processed by JAEA. This arrangement was made because, at the time, QST lacked sufficient experience and capacity for fecal sample analysis.
The Ca/Zn-DTPA treatment was conducted in cycles of 5 consecutive daily intravenous doses, with rest periods in between, and was repeated multiple times. No side effects from DTPA were observed in this case. However, to prevent excessive urinary excretion of zinc, the treatment combined Ca-DTPA, which has a stronger chelating effect, with Zn-DTPA, which has fewer side effects. Additionally, due to proper explanations of test results and counseling provided by JAEA’s industrial physicians, the psychological impact on the workers was minimal.
Worker A, who received the highest internal dose, underwent a total of 11 treatment cycles by the end of December 2017. During the treatment period, the workers were hospitalized at QST, where urine sampling and analysis, as well as in vivo counting, were conducted as needed.
Subsequent investigations by JAEA determined that the contents of the storage container consisted of previously used samples for X-ray analysis, specifically plutonium-containing powders solidified with an epoxy resin adhesive. The possible chemical composition included oxides and carbides. However, JAEA did not disclose the isotopic composition of Pu/Am isotopes due to nuclear security considerations. The primary cause of the accident was identified as gas generation from the radiolytic degradation of the epoxy resin by alpha radiation, which increased the internal pressure within the plastic bag. Additionally, based on the contamination found on the half-face masks worn by the workers, it was inferred that radioactive material adhered to their faces and potentially entered the masks due to reduced mask seal integrity during the rupture of the container contents, as well as during activities like conversation or sweating.4
Methods
In Vivo Counting
Following JAEA’s report that 239Pu and 241Am had been detected through their lung counting, QST conducted lung monitoring to confirm these findings. Both JAEA and QST employed lung monitors equipped with High-Purity Germanium (HPGe) detectors and iron shielding chambers with a thickness of 20 cm. However, the number and size of detectors differed.
QST’s lung monitor consisted of 2 detector units positioned above the left and right sides of the chest, with each unit containing 2 HPGe detectors. The effective crystal area of the entrance window of each detector was 3800 mm2. Details of this lung monitor are described elsewhere.Reference Naito, Tamakuma and Mihei5 JAEA’s lung monitor, by contrast, used 1 HPGe detector (5,000 mm2) on each side of the chest.
QST’s lung monitor was calibrated using a JAERI phantom6 and a set of 241Am lung-shaped sources. To correct the counting efficiency of the lung monitor depending on the difference in chest wall thickness among the 5 individuals, overlay chest plates attached to the phantom (20% fat composition) were used. The CWT of each subject was estimated using an empirical formula based on height (H, cm) and weight (W, kg):
![]() $ CWT=-0.25+6.504\bullet \left(W/H\right) $
.Reference Kanai and Kurihara7 Later ultrasound measurements revealed that this formula overestimated the CWT by an average of 6 mm. However, since the effect on the counting efficiency for 59.5 keV gamma rays from 241Am was negligible, the estimated chest wall thicknesses were used without modification. The detection limit for 241Am with the lung monitor was approximately 10 Bq for a 30-minute measurement, assuming a CWT range of 20-30 mm.
$ CWT=-0.25+6.504\bullet \left(W/H\right) $
.Reference Kanai and Kurihara7 Later ultrasound measurements revealed that this formula overestimated the CWT by an average of 6 mm. However, since the effect on the counting efficiency for 59.5 keV gamma rays from 241Am was negligible, the estimated chest wall thicknesses were used without modification. The detection limit for 241Am with the lung monitor was approximately 10 Bq for a 30-minute measurement, assuming a CWT range of 20-30 mm.
During the hospitalization of the 5 workers at QST, liver counting was also performed several times using a separate HPGe detector (6500 mm2) in an iron-shielded room located in another building. This measurement targeted Pu isotopes and 241Am uptake in the liver. The calibration of this system was conducted using an LLNL phantom,6 with a detection limit of approximately 10 Bq for 241Am over 30 minutes of measurement.
In Vitro Bioassay
The urinary bioassay by QST targeted Pu isotopes and 241Am. The process involved organic decomposition, iron co-precipitation, elemental separation using chromatographic resin, and radionuclide identification and quantification via alpha spectrometry. The detection limits for 238Pu, 239/240Pu, and 241Am were approximately 1 mBq per sample.Reference Yang, Ohno and Kim8 Regarding uranium, based on information from JAEA that Pu and uranium were mixed in the storage container, QST analyzed the first urine samples collected from the workers using an Inductively Coupled Plasma Mass Spectrometry (ICP-MS). However, no uranium was detected (detection limit: ~1 mBq/sample), so no further analysis was conducted. After June 8, 24-hour urine samples were collected. On the day of the accident and the following day, urine samples were collected both before and after the initial Ca-DTPA administration by JAEA.
JAEA’s fecal bioassay focused on early feces samples collected during the first 5 or 6 days post-intake. After pretreatment, Pu and Am were chemically separated and analyzed via alpha spectrometry for radionuclide identification and quantification. For fecal samples in which 241Am was detected through gamma-ray measurements with a germanium detector post-pretreatment, those results were adopted as the 241Am analysis values. For 241Pu, portions of the pretreated solution were measured using liquid scintillation counting. Detection limits were 4 mBq/sample for Pu isotopes (excluding 241Pu) and 241Am, and 0.4 Bq/sample for 241Pu.
Due to nuclear security considerations and JAEA’s policies, this paper presents only the results for 239/240Pu (which cannot be separated via alpha spectrometry) and 241Am from the bioassay analyses. In this paper hereafter, unless otherwise specified, 239/240Pu will be referred to simply as 239Pu (as the dose coefficients for 239Pu and 240Pu are nearly identical).
Dose Assessment
In this case, the committed effective dose (CED) for workers, as defined in ICRP Publication 60,9 was evaluated in accordance with Japan’s current regulations. The exposure pathway was assumed to be inhalation via the nasal cavity, based on the detection of alpha radioactivity in nasal smear samples from the workers. The biokinetic models used to derive the dose coefficients for inhalation of Pu and Am (Sv per Bq intake) consisted of the Human Respiratory Tract Model,10 the Gastrointestinal Tract Model,11 and the Systemic Model.12 Japanese regulations adopt dose coefficient data from ICRP Publication 6813 for workers, specifically the values for inhalation intake with an aerodynamic median activity diameter (AMAD) of 5 μm.
The approach to internal dose assessment was partially based on the IDEAS guidelines,Reference Doerfel, Andrasi and Bailey14 using personal monitoring data to estimate the particle size and absorption type that affect the evaluation of inhalation doses. Because the particle size and chemical form of the inhaled materials were unknown at the time of the dose assessment, we assumed the default parameters (AMAD and Absorption Type), as described in the Result section. The calculation and analysis of internal exposure doses were conducted using IMBA Professional PlusReference Birchall, Puncher and Marsh15 and an original code derived from REIDAC.Reference Kurihara, Hato and Kanai16
Results
In Vivo Counting
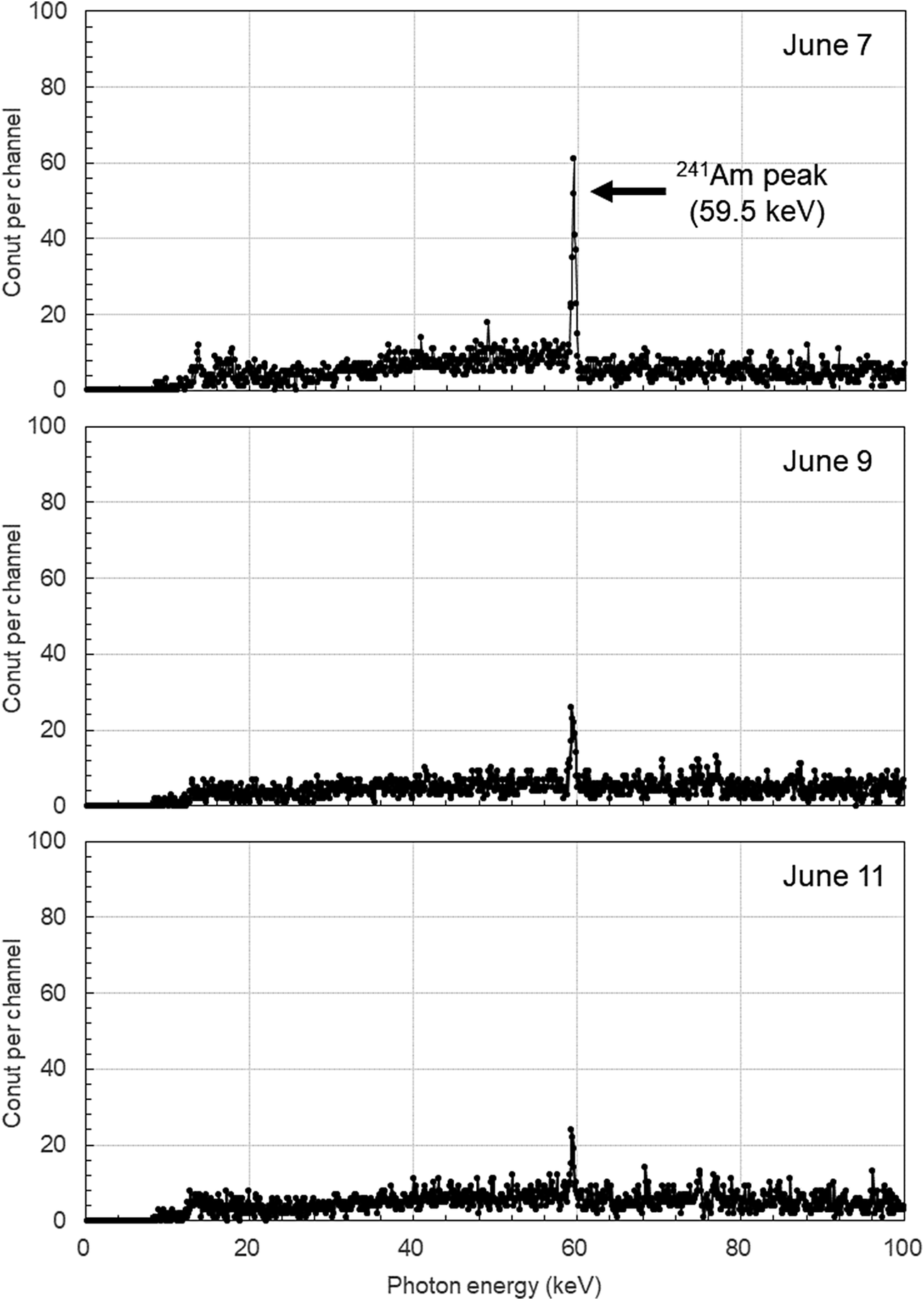
The first lung counting conducted at QST on June 7 detected the 59.5 keV gamma-ray peak of 241Am in workers A and C. However, the characteristic X-ray peaks of Pu isotopes (17.0 keV and 20.3 keV) were not observed. For the other workers, neither 241Am nor Pu was detected. The 241Am lung contents for workers A and C were estimated to be approximately 50 Bq and 10 Bq, respectively.
In the lung counting conducted on June 9, the lung content of worker A had decreased to approximately 20 Bq, while that of worker C was below the detection limit. Subsequent lung counting for workers A and C showed barely detectable levels of 241Am. Figure 1 displays the pulse height spectra of the lung counter for Worker A.

Figure 1. Pulse height spectra from the lung counting of worker A on June 7 (top), 9 (middle), and 11 (bottom). Measurement time: 30 minutes. Each channel in the spectra corresponds to approximately 1 keV. Although the spectrum was recorded up to approximately 400 keV, only the range up to 100 keV is displayed.
Liver measurements were conducted several times starting 1 month after the accident, based on the prediction that 241Am would accumulate in the liver over time. However, 241Am was not detected in any of the workers.
In Vitro Bioassay
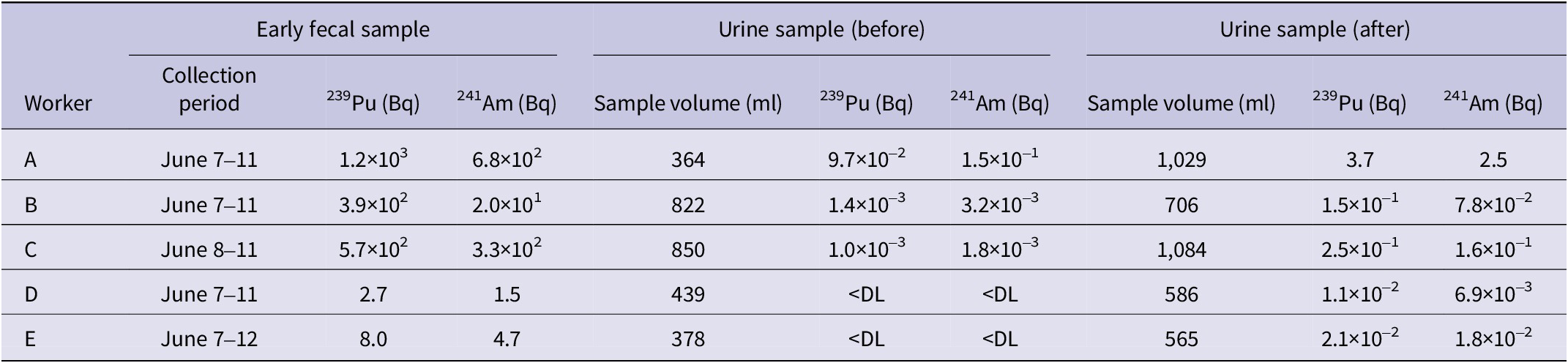
Table 2 summarizes the bioassay data, including early fecal excretion and urine samples collected before and after the initial administration of Ca-DTPA. In the early fecal excretion, trace amounts of 239Pu and 241Am were detected even in workers D and E, whose nasal smear samples showed no detectable contamination. A simple comparison of the urinary excretion values before and after the initial Ca-DTPA administration reveals a 40-250-fold increase for 239Pu and a 20-90-fold increase for 241Am following the treatment.
Table 2. Results of bioassay for early fecal samples and urine samples collected before and after the initial Ca-DTPA administration

Particle Size of Inhaled Aerosols
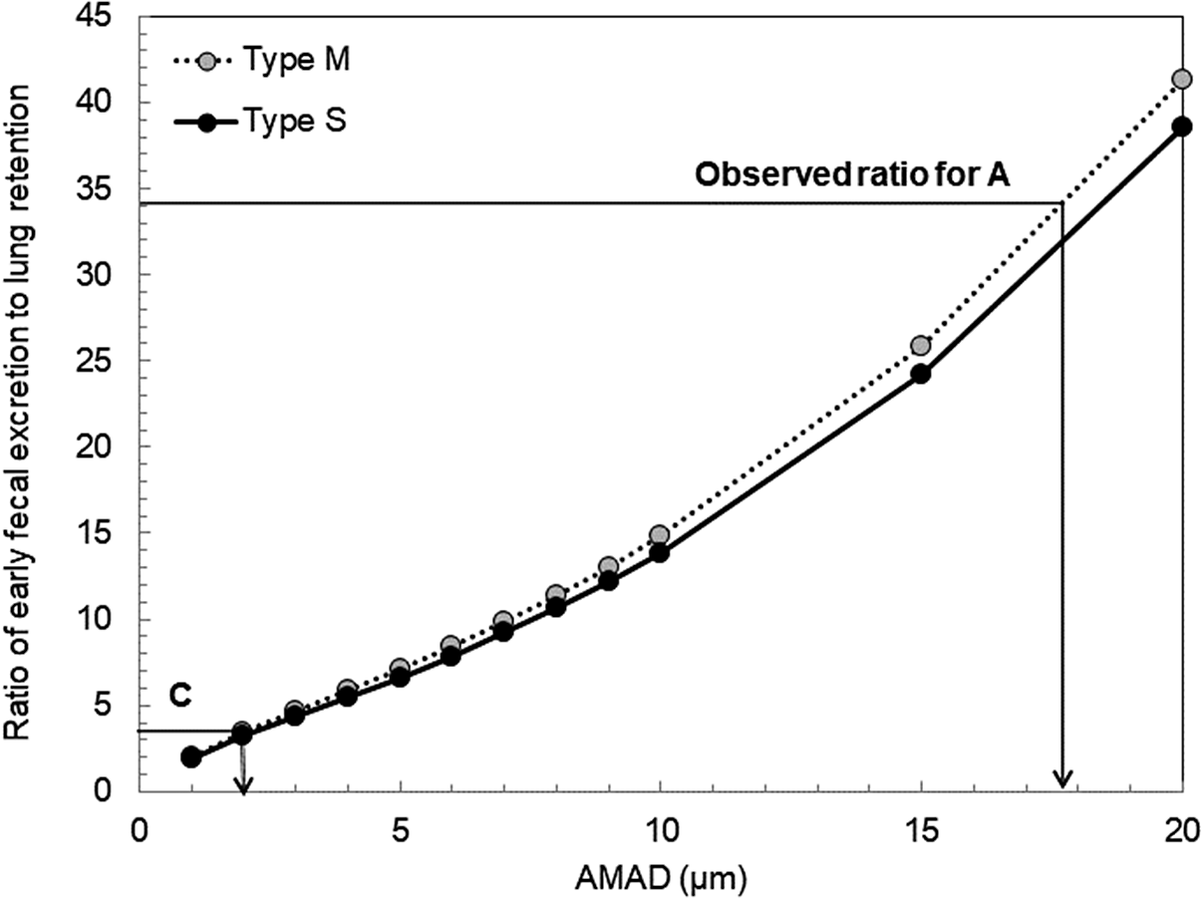
The particle size of aerosols inhaled by the workers was evaluated using the method described in the IDEAS Guidelines,Reference Doerfel, Andrasi and Bailey14 which utilizes the ratio of early fecal excretion to lung retention that varies depending on the particle size. This method was applied to workers A and C, in whom 241Am was detected by lung counting.
Figure 2 shows the early fecal excretion rate (total excretion over 5 days post-intake) to lung retention rate ratio as a function of AMAD for 241Am. The observed ratios from the individual monitoring of workers A and C are also shown in the figure. Here, the deposition rates in various regions of the respiratory tract, including the lungs, were calculated assuming a particle density of 3.0 g/cm,3 a shape factor of 1.5, and a geometric standard deviation of 2.5 for a log-normal particle size distribution.13

Figure 2. Ratio of early fecal excretion to lung retention as a function of the AMAD of inhaled particles.
The lung contents for workers A and C were set at 20 Bq (measured on June 9) and 10 Bq (measured on June 7), respectively. Although ICRP Publications 6813 and 7817 specify the default absorption type as Type M (Moderate) for all 241Am compounds, the lung retention/early fecal excretion ratios for Type S (Slow) were also calculated. The results showed no significant difference between Types M and S. Based on Figure 2, the particle sizes inhaled by workers A and C were estimated to be over 10 μm and several micrometers, respectively. However, we set the AMADs for workers A and C at 5 μm and 1 μm, respectively (see Table 3). These values align with the default settings used in ICRP Publication 68, resulting in conservative dose estimations, as discussed in the Discussion section.
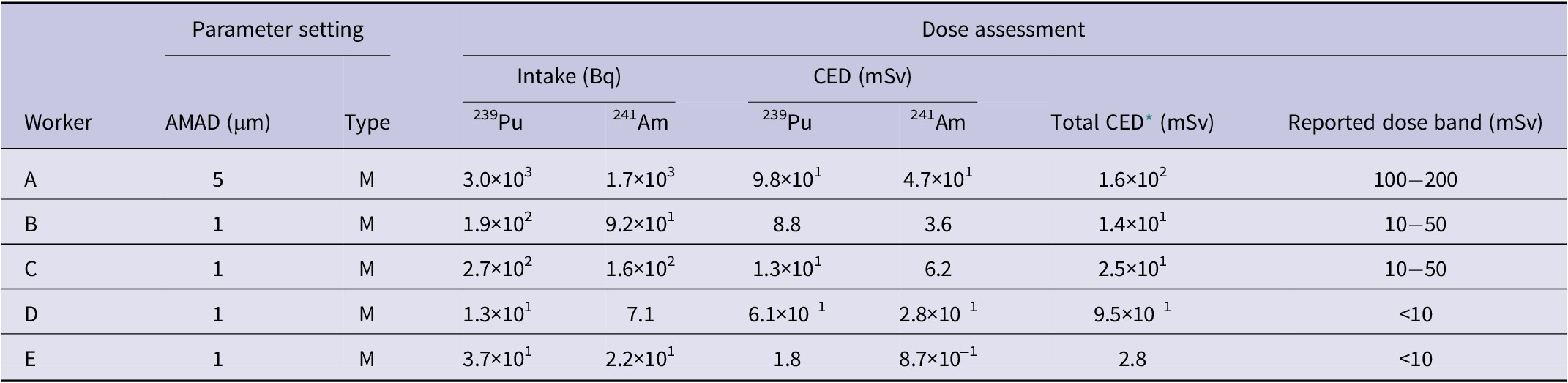
Table 3. Results of internal dose assessment for five workers and parameter setting

* Including doses from other Pu isotopes.
It should be noted that the early fecal excretion to lung retention ratios shown in Figure 2 remain nearly unchanged when lung retention values from the first day post-intake are used, as the difference in lung retention between the first and third days (for both Types M and S) is approximately 5%.
Absorption Type
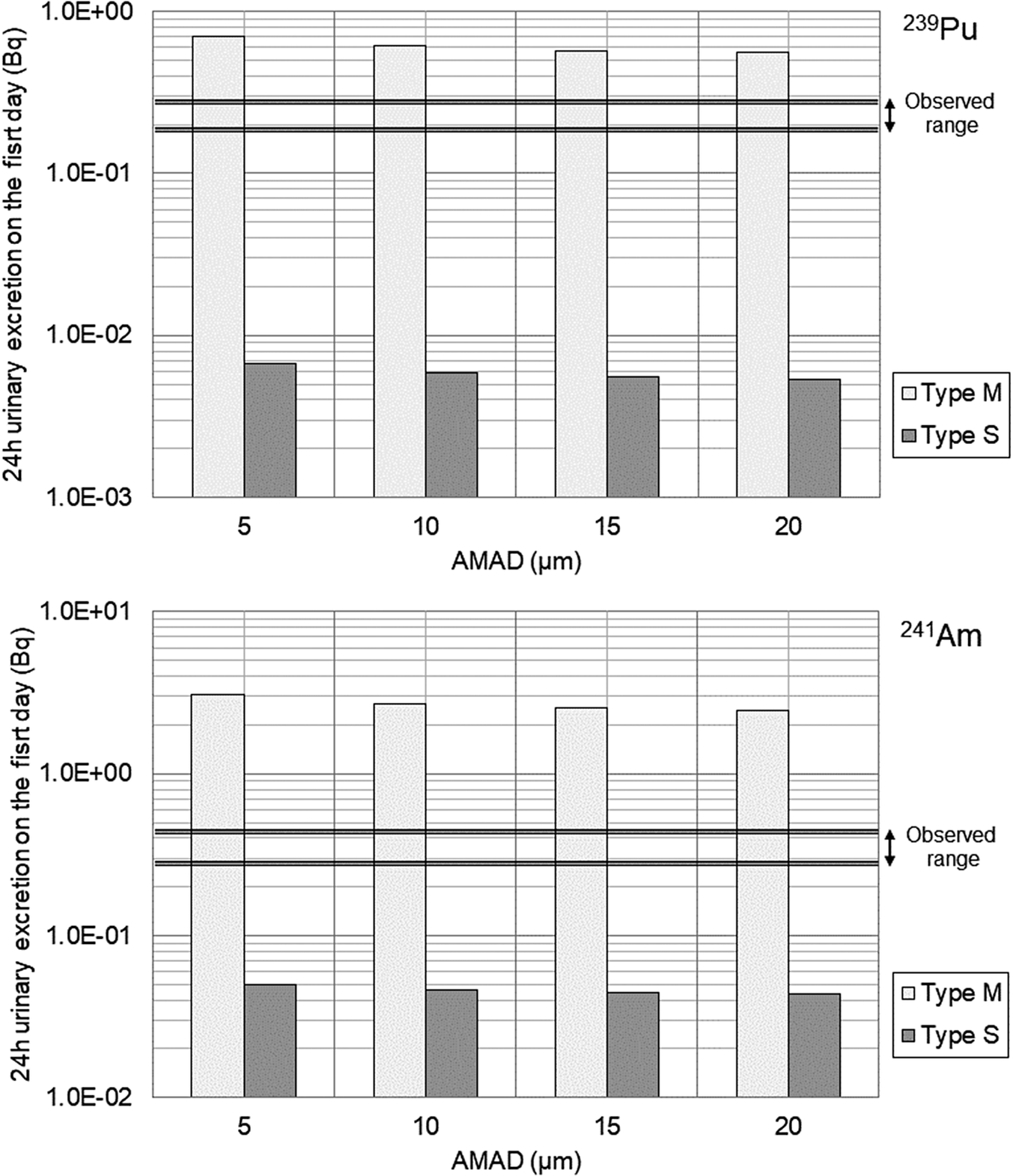
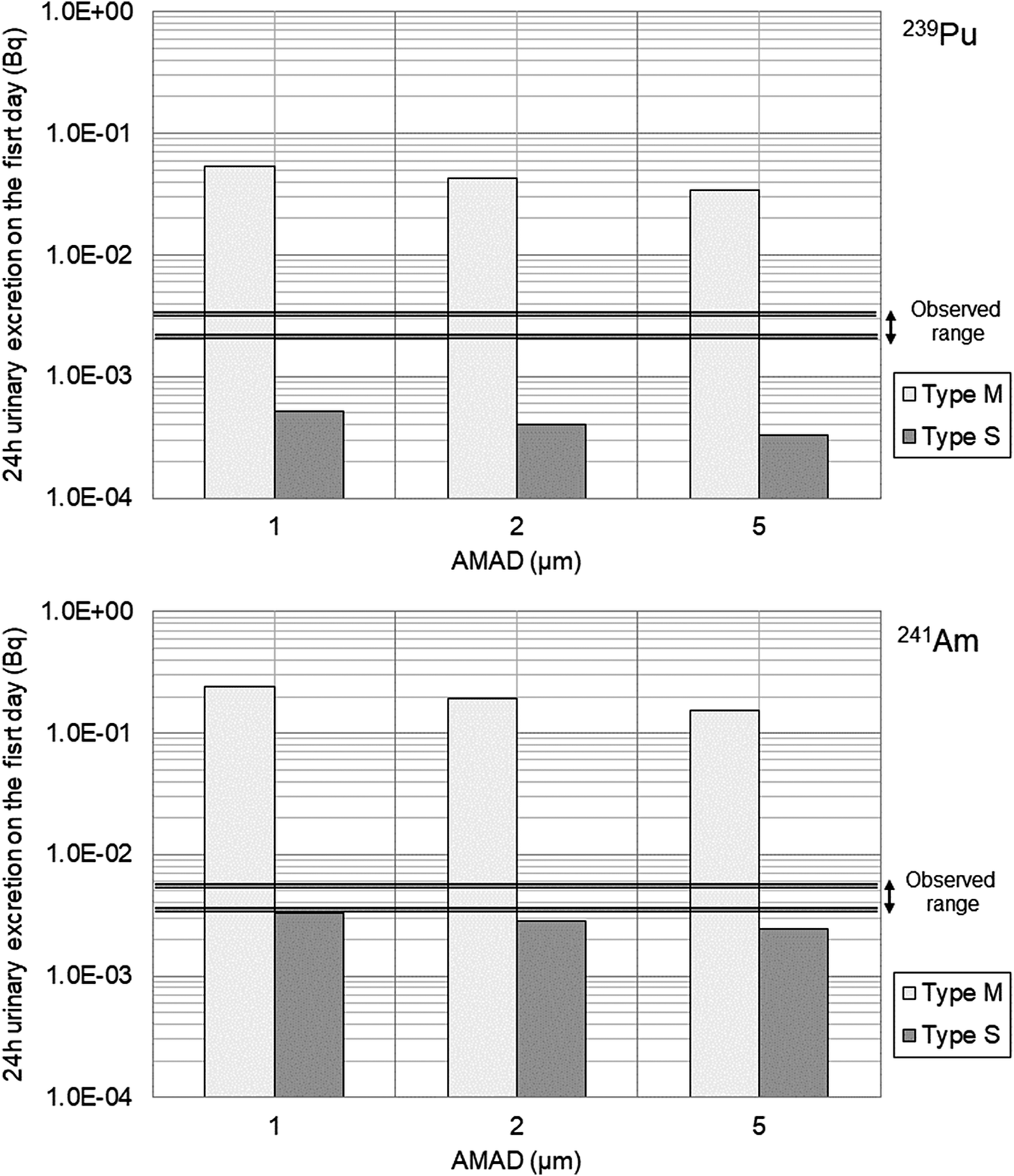
Differences in the absorption type of inhaled aerosols within the respiratory tract significantly affect urinary excretion. Using the intake amounts estimated from the initial fecal excretion data (by varying particle size), the 24-hour urinary excretion on the first day of intake was calculated for 239Pu and 241Am. The results for Worker A and Worker C are shown in Figure 3 and Figure 4, respectively.

Figure 3. Predicted 24-hour urinary excretion on the first day following intake in comparison to the observed range for Worker A (upper panel for 239Pu, lower panel for 241Am).

Figure 4. Predicted 24-hour urinary excretion on the first day following intake in comparison to the observed range for Worker C (upper panel for 239Pu, lower panel for 241Am).
The figures also display bands representing 2-3 times the analytical values of the first urine samples collected after the accident before the first Ca-DTPA administration (Table 2) for comparison. ICRP Publication 6813 specifies Type S for oxides of Pu and Type M as the default for other forms.
The results suggest that the absorption type for the inhaled aerosols of both workers A and C lies between Type M and Type S. However, the results for Worker A were closer to Type M, whereas those for Worker C appeared to exhibit a higher proportion of Type S properties than those for Worker A.
Dose Assessment
Based on the above results, conservative dose estimates for the particle size and absorption type of the inhaled particles were determined, as discussed later. Using these parameters, the intake amounts and CEDs for the 5 workers were calculated from the early fecal excretion data, as shown in Table 3. The CED for Worker A, who had the highest internal exposure, was estimated to be 160 mSv, which is below the 250 mSv (or 250 mGy) threshold used to derive the Clinical Decision Guide (CDG) intake values.18 This dose level was established based on considerations of both stochastic and deterministic radiation effects. The dose assessment results, published by QST on July 10, 2017, were presented as a broad dose range to account and accommodate for potential refinements in future assessments, while also respecting the privacy of individual workers. As mentioned in the introduction, this dose assessment did not consider the dose reduction effects of Ca/Zn-DTPA treatment.
Prolonged Urinary Bioassay
To confirm the therapeutic effects of Ca/Zn-DTPA treatment, analyses of urine samples collected before and during the treatment period were conducted. For Worker A, who had the highest internal dose, more than 10 treatments were carried out, resulting in the collection of approximately 100 urine samples in total.
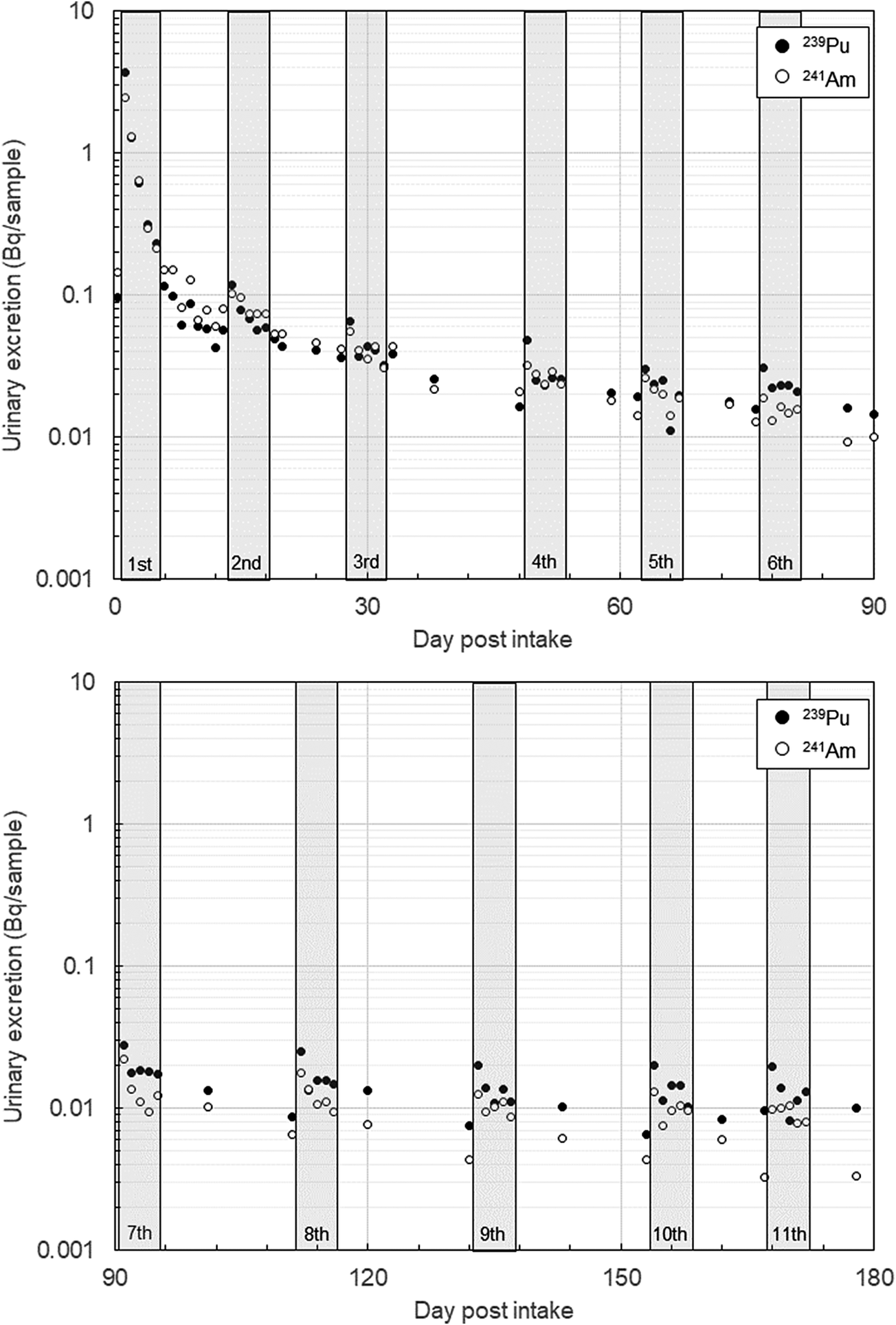
The urinary excretion of 239Pu and 241Am for Worker A is shown in Figure 5. Each plot in the figure represents the activity of individual urine samples collected over 180 days after the accident. During this period, 11 treatment sessions were conducted. Except for the first 3 samples, all were 24-hour urine samples.

Figure 5. Results of Worker A’s urinary bioassay. Each plot corresponds to the analytical value of an individual urine sample. All samples except the first 3 are 24-hour urine samples. The collection periods for the first 3 samples were approximately 11 hours, 20 hours, and 17 hours, respectively. The gray bands in the figure indicate the treatment periods. Refer to the text for details.
Worker A’s treatment regimen included 5 consecutive days of Ca-DTPA administration for the first and second treatments, 5 consecutive days of Zn-DTPA administration for the fifth treatment, and a combination of Ca-DTPA on the first day followed by Zn-DTPA for the next 4 days for all other treatments.
A common trend observed during the treatment periods was that urinary excretion peaked on the day following the first administration of the treatment cycle. However, except for the initial treatment, the effect of Ca/Zn-DTPA in promoting urinary excretion appeared lower. The total urinary excretion over the 180 days, including intervals when urine samples were not collected, was estimated to be approximately 10 Bq for each of 239Pu and 241Am.
Discussion
This case marked the first instance in Japan where Ca/Zn-DTPA treatment was employed as a medical intervention for an internal contamination accident involving Pu and Am. The efficacy of Ca/Zn-DTPA treatment, specifically its dose-reduction effects, varies depending on the physicochemical properties of Pu/Am and the circumstances of the intake route.Reference Grappin, Bérard and Ménétrier19, 20 It is empirically recognized that earlier treatment yields better results. However, when Ca/Zn-DTPA is administered intravenously, as in this case, it is anticipated that the treatment would be less effective for inhalation intake of insoluble compounds. In this case, the initial administration of Ca-DTPA occurred approximately half a day after the inhalation of Pu/Am. Changes in urinary excretion levels between before and after the administration are shown in Table 2. A simple comparison of these data reveals an increase in urinary excretion levels by tens of times, with the increase being more pronounced for 239Pu than for 241Am. Empirical evidence suggests that DTPA administration can lead to approximately an enhanced factor of 50 in urinary excretion of Pu.17 Similarly, during the initial treatment period in this case, comparable results were observed. However, as seen in the urinary bioassay results for Worker A, subsequent treatments did not produce such a significant increase. This result suggests that the absorption rate of Pu/Am from the respiratory tract into the bloodstream rapidly decreased over time, which is consistent with the characteristics of the absorption types described earlier. The Human Respiratory Tract Model represents a 2-phase absorption process—Rapid Dissolution and Slow Dissolution—where the slower absorption phase dominates for both Type M and Type S. The faster absorption phase, with a biological half-life of 10 minutes, accounts for 10% in Type M and 0.1% in Type S.10
In this case, the dose assessment was conducted based on the results of fecal bioassays previously performed at JAEA.Reference Kurihara, Momose and Tasaki21 However, the particle size of inhaled particles, which was derived from the early fecal excretion to lung retention ratio, and the absorption type, which was based on urinary samples collected before the initial administration of Ca-DTPA, were also used. Among individual monitoring methods for inhalation intake of Pu/Am, fecal bioassay is the most sensitive. Nevertheless, it should be noted that dose assessments based on fecal bioassays are highly influenced by particle size. For example, in the dose assessment for Worker A, when the assumed particle size (5 μm AMAD) was changed to 10 μm, the CED decreased from 160 mSv to 100 mSv (with the absorption type remaining as M). On the other hand, the lung retention-to-initial fecal excretion ratio calculated using the updated human respiratory tract model, which includes a particle transport pathway from the ET1 region to the ET2 region,22 tends to decrease as fecal excretion increases. This suggests that the particle size of the inhaled aerosols was smaller than the results shown in Figure 2.Reference Tani, Ishigure and Kim23
Regarding particle size, Takasaki et al.Reference Takasaki, Yasumune and Yamaguchi24 conducted autoradiography analyses of smear filters (14 samples) and a dust filter (1 sample) collected from the accident site. They evaluated the AMAD of Pu particles attached to each sample. Assuming that the Pu particles were composed of plutonium dioxide (density: 11.5 g/cm3), the AMAD was determined to range from 5.6-14.4 μm for the smear filters and 3.9 μm for the dust filter. When assuming plutonium nitrate (density: 2.9 g/cm3), the AMAD values were slightly smaller. The dust filter was collected from a location separated by 1 glovebox from the hood where the Pu storage container was handled. It is likely that larger particles which scattered outside the hood settled closer to the source due to gravitational deposition. Indeed, Pu particles exceeding 10 μm were detected on smear filters collected from the floor directly in front of the hood. Although these findings may not directly represent the aerosols inhaled by workers, they provide valuable information for dose assessment.
In the dose assessment for the 5 workers, a conservative approach was adopted by assuming a particle size of 5 μm for Worker A and 1 μm for the other workers. This assumption is reasonable, given that Worker A was working closest to the hood and likely inhaled larger particles compared to the other workers. However, from the perspective of consistency with the biokinetic model, the assumption of a 5 μm particle size for Worker A may be somewhat underestimated. With a particle size of 5 μm, the intake amount calculated from the early fecal excretion of 241Am (680 Bq) would be 1700 Bq, resulting in an estimated lung retention of approximately 100 Bq (a retention rate of 5.5%) 3 days post-intake. This value is higher than that from Worker A’s lung measurement data (~20 Bq). In contrast, assuming a particle size of 10 μm, the lung retention calculated under the same conditions would be approximately 50 Bq, which aligns more closely with the uncertainty range (scattering factor: 2.3) of the lung measurement for low-energy photons.Reference Doerfel, Andrasi and Bailey14 For the other workers, the 1 μm assumption was used because it results in a higher dose coefficient compared to 5 μm, as listed in ICRP Publication 68, making it appropriate for conservative dose evaluation.
For determining the absorption type, as described in the Results section, the analysis of pre-Ca-DTPA urine samples from Workers A and C was used as a reference. The urinary excretion results were intermediate between absorption Types M and S, and for the purpose of dose assessment, the more conservative Type M was selected. While this depends on particle size, the dose coefficients for 239Pu and 241Am for Type S are approximately 30% of those for Type M. Furthermore, according to the analysis of storage container samples by JAEA, the dispersed material included resin-solidified samples used for X-ray analysis, and the nuclear material was confirmed to consist of oxides and carbides in terms of chemical form. It should be noted that this information was not incorporated into the dose assessment presented in this paper, as the analysis results were obtained after the accident. However, the absorption type estimated from our bioassay data appeared to align with the classification specified in ICRP Publication 68 for the chemical forms identified. Based on the above considerations, we conclude that the internal dose assessment for the workers was conservatively performed within a reasonable range. Although there remains significant room for more realistic evaluation, including simulations using a biokinetic model that incorporates chelation effects,Reference Tani, Ishigure and Kim25 the individual monitoring data obtained in this case would be valuable for researchers and technicians responsible for internal dose assessments.
Conclusion
This paper provides a detailed description and discussion of the dose assessment conducted for Japan’s first case of actinide internal exposure requiring medical intervention, which occurred on June 6, 2017. The dose assessments for the 5 workers involved in the accident were confirmed to have been conservatively performed within a reasonable framework. The maximum dose based on these evaluations was 160 mSv; however, applying more realistic assumptions regarding particle size and absorption type suggests that the dose might be lower. Further research is required to clarify the dose reduction effects of Ca/Zn-DTPA treatment.
Author contribution
E.K. and O.K. primarily analyzed the data and drafted the manuscript. T.T., H.T., and M.A. were involved in the medical treatment of this case and the application for research ethics approval. K.Y. acquired the data, while K. T. supported data analysis. C.T. and T.M. assisted with dose assessment. All authors reviewed and approved the manuscript.
Acknowledgments
The authors would like to express their sincere gratitude to the 5 workers involved in this accident for their proactive cooperation in providing informed consent and participating in individual monitoring. We would also like to extend our heartfelt thanks to all the members of QST and JAEA who contributed to the response to this accident.
Competing interests
The authors have no conflict of interest, financial or otherwise.
Ethical standard
This work was approved by the research ethics committee of QST (research protocol numbers:17-018,18-006 and18-007).