Introduction
Continental intraplate igneous rocks may exhibit unusual compositions diverging significantly from typical partial melts generated from volatile-free peridotitic sources, leading to the formation of extreme compositions, such as ultra-alkaline rocks (including nephelinites, leucitites, lamproites) strongly ultrabasic compositions (melilitites, kimberlites), carbonatites and (ultramafic) lamprophyres (Foley et al., Reference Foley2008). Regardless of their rarity, these igneous compositions are very important because their genesis can shed light on how upper mantle anatexis works (Foley et al., Reference Foley, Ezad, Shu and Förster2025).
The unusual features of ultrapotassic lithologies in terms of petrography, mineral compositions, whole-rock geochemistry and isotopic ratios cannot be reconciled with derivation from a classical four-phase lherzolitic mantle, whose melting produces tholeiitic to mildly alkaline basaltic melts, with Na2O + K2O lower than 5–6 wt.% and usually <2 wt.% K2O (Green, Reference Green1970; Frey et al., Reference Frey, Green and Roy1978). For this reason, the petrogenesis of ultrapotassic lithologies is commonly linked to the presence of metasomatic mantle assemblages (Foley et al., Reference Foley2008; Kiseeva et al., Reference Kiseeva, Kamenetsky, Yaxley and Shee2017; Lustrino et al., Reference Lustrino, Ronca, Caracausi, Ventura Bordenca, Agostini and Faraone2020, Reference Lustrino, Pistocchi, Ronca, Innocenzi and Agostini2025; Innocenzi et al., Reference Innocenzi, Ronca, Foley, Agostini and Lustrino2024a, Reference Innocenzi, Ronca, Agostini, Benedetti and Lustrino2024b; Shu et al., Reference Shu, Foley, Ezad, Daczko and Shcheka2024; Foley et al., Reference Foley, Ezad, Shu and Förster2025). Crustal recycling and fluids released by subducting slabs might cause refertilisation events in the form of chromatographic reactions, resulting in the development of mantle reservoirs characterised by variable enrichment in incompatible elements (both major oxides and trace elements) and by isotope signatures that deviate significantly from those typical of peridotitic mantle rocks. The mineralogy of the metasomatic rocks as well as the nature and the physical state of the metasomatic agents are not yet completely understood (Roden and Murthy, Reference Roden and Murthy1985; Kiseeva et al., Reference Kiseeva, Kamenetsky, Yaxley and Shee2017; Förster et al., Reference Förster, Prelević, Schmück, Buhre, Veter, Mertz-Kraus, Foley and Jacob2017; Foley and Ezad, Reference Foley and Ezad2024).
Several petrological investigations have focused on ultramafic xenoliths occasionally entrained in ultrapotassic magmas to constrain their possible mantle sources. They commonly display peculiar mineral assemblages such as clinopyroxenites and glimmerites, examples of which are found along the western branch of the East African Rift (Lloyd et al., Reference Lloyd, Arima and Edgar1985; Link et al., Reference Link, Barifaijo, Tiberindwa and Foley2008; Innocenzi et al., Reference Innocenzi, Ronca, Foley, Agostini and Lustrino2024a). Obviously, being found as xenoliths in ultrapotassic magmas, these lithologies cannot be used as direct constraints to identify the mineralogical and compositional characteristics of the mantle sources of the host rocks. Petrological experiments on mantle rocks other than peridotite are now abundant, though they are mostly confined to anhydrous pyroxenites (Keshav et al., Reference Keshav, Gudfinnsson, Sen and Fei2004; Kogiso and Hirschmann, Reference Kogiso and Hirschmann2001; Borghini et al., Reference Borghini, Fumagalli and Rampone2017), eclogites (Klemme et al., Reference Klemme, Blundy and Wood2002; Spandler et al., Reference Spandler, Yaxley, Green and Rosenthal2008), with the objective of constraining the effects of interaction between metasomatic agents and a peridotitic matrix (Yaxley and Green, Reference Yaxley and Green1998; Förster et al., Reference Förster, Prelević, Schmück, Buhre, Veter, Mertz-Kraus, Foley and Jacob2017, Reference Förster, Buhre, Xu, Prelević, Mertz-Kraus and Foley2019). High-pressure partial melting experiments on hydrous ultramafic rocks such as phlogopite-bearing clinopyroxenite and/or glimmerites (Lloyd et al., Reference Lloyd, Arima and Edgar1985; Sweeney et al., Reference Sweeney, Thompson and Ulmer1993; Funk and Luth, Reference Funk and Luth2013; Foley et al., Reference Foley, Ezad, van der Laan and Pertermann2022; Foley and Ezad, Reference Foley and Ezad2024) result in very variable ranges of glass compositions. Note that experiments carried out at pressures >3 GPa are particularly rare (Foley et al., Reference Foley, Ezad, van der Laan and Pertermann2022; Foley and Ezad, Reference Foley and Ezad2024; Shu et al., Reference Shu, Foley, Ezad, Daczko and Shcheka2024) or have concentrated on delineating subsolidus phases, and not on melt compositions (Konzett, Reference Konzett1997; Konzett et al., Reference Konzett, Sweeney, Thomposon and Ulmer1997). In addition to this relative paucity of experiments at pressures at which many ultrapotassic melts are thought to form, the mineral assemblages used for these melting experiments are typically very similar and less extreme when compared to glimmerites. For example, many starting materials contained amphibole (like in most of the experiments from Foley et al., Reference Foley, Ezad, van der Laan and Pertermann2022 and Foley and Ezad, Reference Foley and Ezad2024) or orthopyroxene (Shu et al., Reference Shu, Foley, Ezad, Daczko and Shcheka2024). Moreover, the effect of accessory phases is commonly overlooked. Only in a few cases have oxides, rutile, titanite and/or apatite been considered, and the effects of different combinations of these minor phases is still poorly constrained (Lloyd, 1985; Funk and Luth, Reference Funk and Luth2013; Foley et al., Reference Foley, Ezad, van der Laan and Pertermann2022; Foley and Ezad, Reference Foley and Ezad2024).
A previous investigation (Innocenzi et al., Reference Innocenzi, Ezad, Ronca, Agostini, Lustrino and Foley2024c), based on near-liquidus experiments (1–2 GPa and 1250–1380°C) on different kamafugite compositions, tried to reconstruct the paragenesis of the melting assemblage of Ugandan, Italian and Brazilian kamafugites, proposing a major role for partial melting of clinopyroxene- and phlogopite-rich paragenesis, with minor olivine and accessory phases (as titanite, apatite and Fe-Ti-oxides).
To verify this hypothesis, in this study we carried out partial melting experiments at 2.7–5 GPa and 1200–1550°C on three hydrous ultramafic rocks, ranging from phlogopite-bearing clinopyroxenite (± olivine) to clinopyroxene-bearing glimmerite. The objective of this experimental study was to characterise the melt compositions generated from these hydrous ultramafic rocks, comparing them with data on natural kamafugites. Two different pressures of 2.7 and 5 GPa have been selected for these experiments as kamafugites crop out in different geodynamic settings, i.e. craton edges, along old mobile belts and on active compressive margins, resulting in variable lithospheric thickness and melting depths. In particular, the estimated mantle sources of the three known kamafugite occurrences range from <90 km to ∼180 km, corresponding to ∼2.7 to 5.0–5.5 GPa (Innocenzi et al., Reference Innocenzi, Ronca, Agostini, Guarino, Foley and Lustrino2025).
Starting materials
The starting materials for this study were chosen on the basis of the results of the near-liquidus experiments (Innocenzi et al., Reference Innocenzi, Ezad, Ronca, Agostini, Lustrino and Foley2024c) carried out on four natural kamafugites from three geographical areas (Toro Ankole Province, western branch of the East African rift in Uganda; Alto Paranaiba Igneous Province in Brazil; and the Cupaello and San Venanzo volcanoes, Intra-Apennine Province in central Italy). The minerals which crystallised in near-liquidus runs allowed Innocenzi et al. (Reference Innocenzi, Ezad, Ronca, Agostini, Lustrino and Foley2024c) to hypothesise their presence in the source of primary kamafugite magma and, therefore, they could represent the main mineralogical components of the melting assemblages.
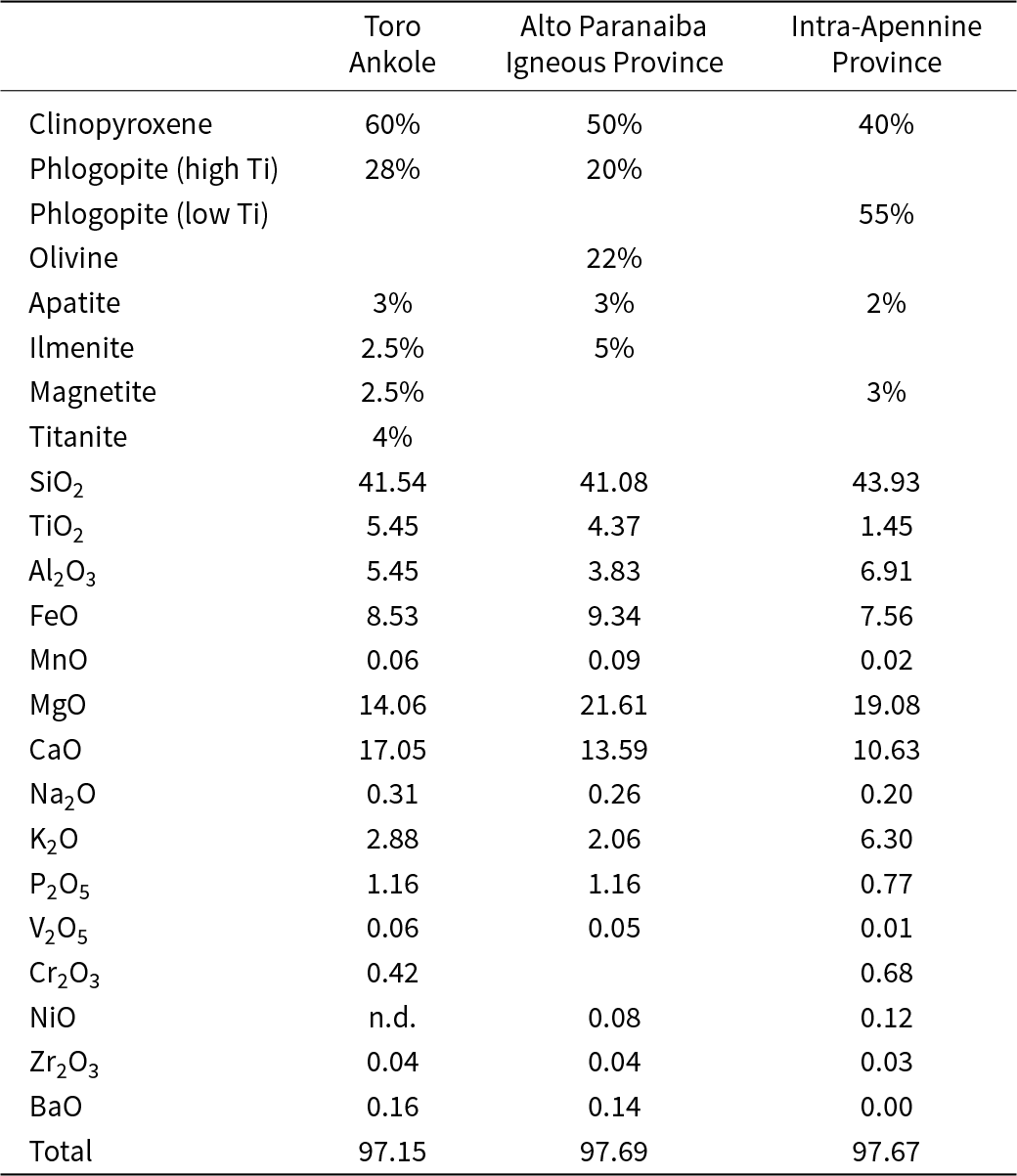
On this basis, we modelled the hypothetical sources for the three volcanic provinces where kamafugite rocks occur: Toro Ankole (TA; Uganda); Alto Paranaiba Igneous Province (APIP; Brazil); and the Intra-Apennine Province (IAP; Italy; Tappe et al., Reference Tappe, Foley and Pearson2003; Innocenzi et al., Reference Innocenzi, Ronca, Agostini, Guarino, Foley and Lustrino2025). The compositions of the starting materials are reported in Table 1. The three assemblages are a phlogopite clinopyroxenite + apatite + ilmenite + magnetite + titanite for TA, an olivine clinopyroxenite + phlogopite + apatite + ilmenite for APIP, and a clinopyroxene glimmerite + apatite + magnetite for IAP. Experiments were not doped with additional H2O or CO2.
Table 1. Modal composition (wt.%) of the starting materials for partial melting experiments (Toro Ankole, Alto Paranaiba Igneous Province and Intra-Apennine Province samples) and relative bulk compositions

For the sake of clarity, it should be noted that the near-liquidus experiments (Innocenzi et al., Reference Innocenzi, Ezad, Ronca, Agostini, Lustrino and Foley2024c) for the two IAP lavas from San Venanzo and Cupaello returned different results. Olivine was observed only in San Venanzo runs and not in Cupaello due to the different bulk composition of the two natural samples (with San Venanzo showing higher MgO than Cupaello; Lustrino et al., Reference Lustrino, Ronca, Caracausi, Ventura Bordenca, Agostini and Faraone2020, Reference Lustrino, Pistocchi, Ronca, Innocenzi and Agostini2025; Innocenzi et al., Reference Innocenzi, Ronca, Agostini, Guarino, Foley and Lustrino2025). Therefore, the reconstruction of the IAP starting material for this study was established using both on the San Venanzo and Cupaello experimental results of Innocenzi et al., (Reference Innocenzi, Ezad, Ronca, Agostini, Lustrino and Foley2024c) and on the partial melting model (Innocenzi et al., Reference Innocenzi, Ronca, Agostini, Guarino, Foley and Lustrino2025) based on mass balance calculations (File Repository 3, https://doi.org/10.5880/fidgeo.2025.025). Olivine is not present in the mixture and phlogopite reaches 55% (Table 1).
Starting materials were prepared by mixing hand-picked minerals from the following natural rocks: Ti-poor phlogopite from a glimmerite xenolith from Kimberley (South Africa); Ti-rich phlogopite, clinopyroxene, titanite and ilmenite from a pyroxene-bearing glimmerite from the western branch of the East African Rift (Uganda); olivine and magnetite from a lherzolite xenolith from Lanzarote (Spain). Apatite comes from Durango (Mexico).
Rock samples were previously disintegrated using a selective fragmentation (SelFrag) instrument at Macquarie University (Sydney, Australia). Mineral compositions were determined by XRD (Panalytical Aeris; Cobalt X-ray source, operating at 40 kV, 15 Ma, with one rotation per second) and by SEM-EDS analyses (Zeiss EVO MA15). These compositions are reported in Table 2.
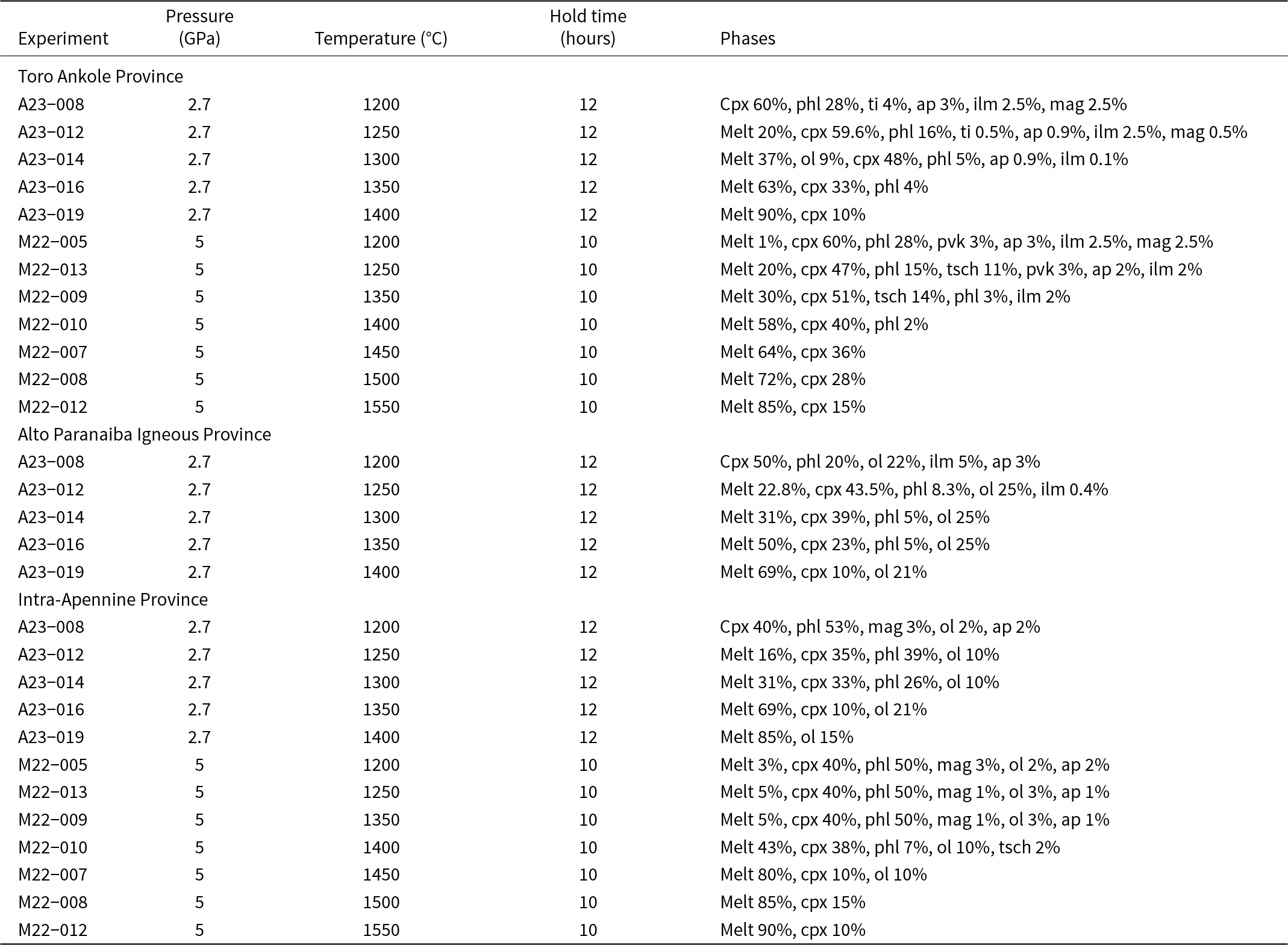
Table 2. Summary of all the partial melting experiments that have been run, with relative temperature, pressure, hold time and resulting assemblage

Abbreviations: cpx – clinopyroxene; phl – phlogopite; ti – titanite; ilm – ilmenite; mag – magnetite; ol – olivine; pvk – perovskite; tsch – tschermakite; ap – apatite
Experimental strategy
A set of five partial melting experiments were run at 2.7 GPa on each of the three starting materials. To constrain better the olivine-free assemblages, further experiments on the TA and IAP source assemblages were performed at 5 GPa. Temperatures ranged from 1200 to 1400°C at intervals of 50°C for the 2.7 GPa experiments, and from 1200 to 1550°C at 50°C intervals for the 5 GPa experiments.
The 2.7 GPa melting experiments were conducted using a GUKO ½ inch rapid quench end-loaded piston cylinder (Ezad et al., Reference Ezad, Shcheka, Buhre, Buhre, Gorojovsky, Shea, Förster and Foley2023), whereas the runs at 5 GPa were performed using a Voggenreiter 1000 ton multi-anvil press with a Walker module at the School of Natural Sciences at Macquarie University, Australia. For the piston cylinder experiments, a natural CaF2-MgO-graphite assembly and B-type thermocouple (Pt30Rh70–Pt6Rh94) were employed. The reaction albite = jadeite + quartz was used for the pressure calibration (Holland, Reference Holland1980) and a friction correction of 20% was applied. For the multi-anvil experiments, an 18/11 Cr-doped MgO assembly with a stepped graphite heater was employed. The temperature was measured using a D-type thermocouple (W97Re3–W75Re25) and monitored with a Eurotherm 350H controller. Pressure calibration followed the reactions of quartz/coesite (2.97 GPa; Bohlen and Boettcher, Reference Bohlen and Boettcher1982) and coesite/stishovite transformation (8.7 GPa; Zhang et al., Reference Zhang, Li, Utsumi and Liebermann1996).
Powders of the three starting materials were loaded in graphite capsules wrapped with a Pt foil (25 μm thick). The graphite capsules were made using a Roland MDX–540 mill. All experiments were first compressed to the desired pressure (2.7 or 5 GPa) and subsequently heated to the target temperature, held for 12 h at 2.7 GPa and 10 h at 5 GPa, quenched to room temperature by cutting power to the graphite and decompressed over several hours (3–16 h). A summary of the run conditions and resulting phases can be found in Table 2.
Analytical methods
The recovered samples were embedded in epoxy resin and polished to a 1 μm diamond finish. Quantitative compositional data of the minerals and glasses, coupled with element-distribution mapping, were acquired using a SEM Zeiss EVO MA15, equipped with an EDS detector (Oxford Ultim Max 100), at Macquarie GeoAnalytical Laboratories (MQGA; Macquarie University). The instrument operates at 20 kV and a working distance of 12 mm. The quality of the quantitative results has been documented by Shu et al. (Reference Shu, Foley, Ezad, Daczko and Shcheka2024), demonstrating good agreement (<2% error) between the EDS measurements and the data obtained from an electron microprobe (JEOL HAX 8530F WDS), at the University of Tasmania.
Mainly due to the low SiO2 and high MgO content of the compositions, the glasses do not always quench, producing glassy patches with several small needle-like microliths. This results in challenges polishing the experimental glasses and locating sufficiently large areas for compositional analysis. This problem is well known in literature (e.g. Shu et al., Reference Shu, Foley, Ezad, Daczko and Shcheka2024), and typically results in glasses with low analytical totals. To ensure that the analyses of experimental glasses that produced low totals were not related to the EDS technique, some analyses were repeated at the Laboratorio di Microanalisi of University of Florence and Istituto di Geoscienze e Georisorse – CNR, using a JEOL JXA-8230 Electronic Microprobe, equipped with five WDS (wave-length dispersive) crystal spectrometers, obtaining similar results (i.e. low major oxide totals; Supplementary material 1).
High quality scanning electron microphotographs were collected using a Teneo FEI–Field Emission Scanning Electron Microscope (FESEM) at MQGA fitted with Bruker XFlash 6-30 EDS detectors, working at 15 kV, with a beam current of 11 nA, a spot size of 1–2 nm and a working distance of 10 mm. FESEM maps are shown in the figures below.
Results
The experimental results are reported separately for each of the three starting materials. Table 2 summarises the experimental conditions and the resulting paragenesis for each run. The abundances of each phase were estimated by mass balance (see Supplementary material).
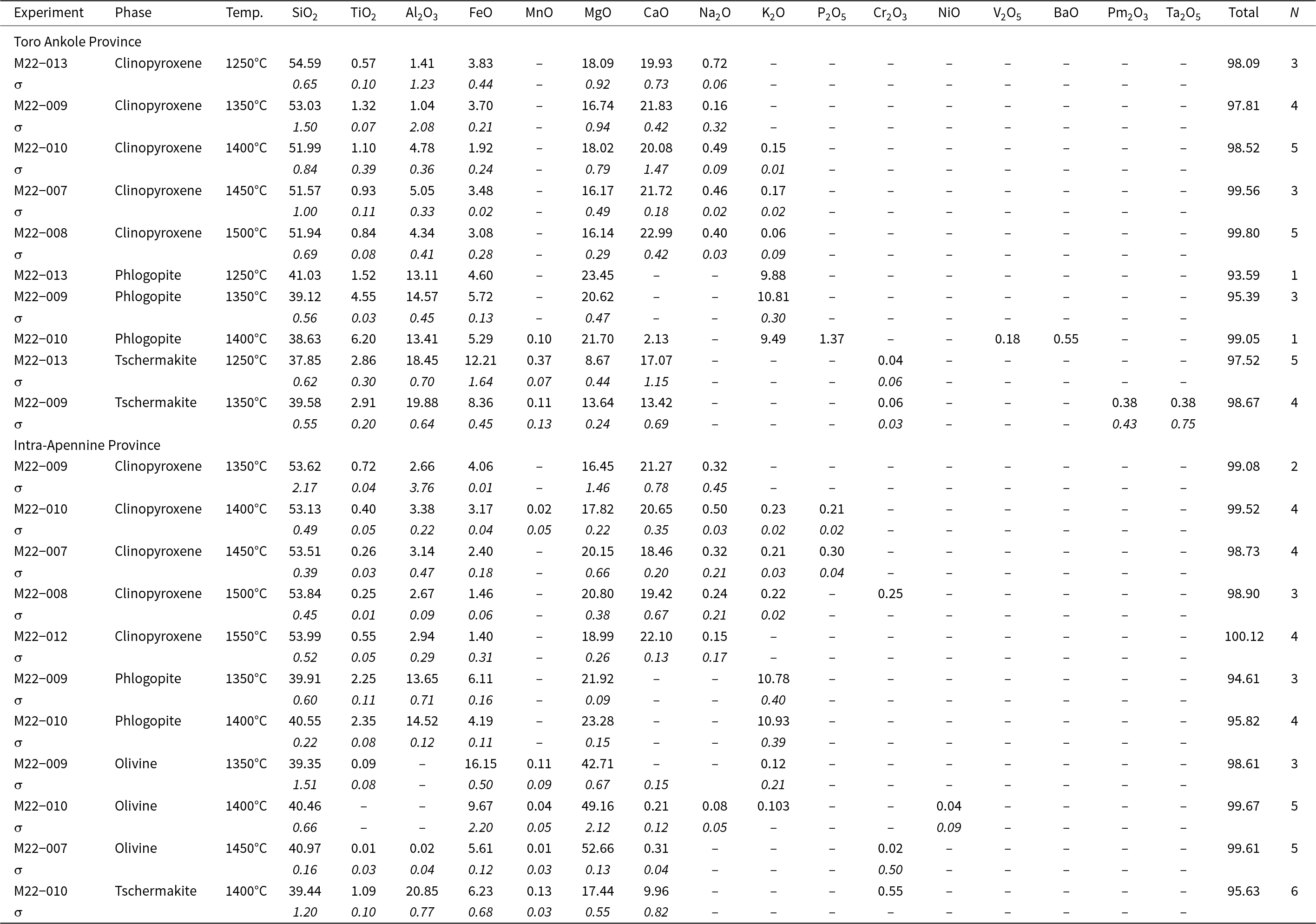
All the glass and mineral data for each experiment are given in the Supplementary tables. Average compositions, together with standard deviation and the number of analyses, for each mineral phase and recovered glasses are reported in Tables 3 and 4 for experiments conducted at 2.7 GPa and in Tables 5 and 6 for experiments at 5 GPa. Major oxides are reported in wt.%, following the journal’s rules, albeit the International System of Units would require mass %.
Experiments on a phlogopite clinopyroxenite + apatite + ilmenite + magnetite + titanite (Toro Ankole Volcanic Province)
Experiments at 2.7 GPa
TA experiments at 1200°C produced the same mineral assemblage as the starting material, indicating sub-solidus conditions (Fig. 1). A measurable amount of melt (∼20 wt.%) appeared at 1250°C, visible as quenched needles (Fig. 2a–c), characterised by very low SiO2 content (29.6 wt.%; Table 3). This glass is anomalously enriched in TiO2 (10.7 wt.%), CaO (14.7 wt.%), K2O (4.3 wt.%) and P2O5 (4.6 wt.%; Table 3 and Supplementary material), reflecting the large contribution of titanite and apatite.

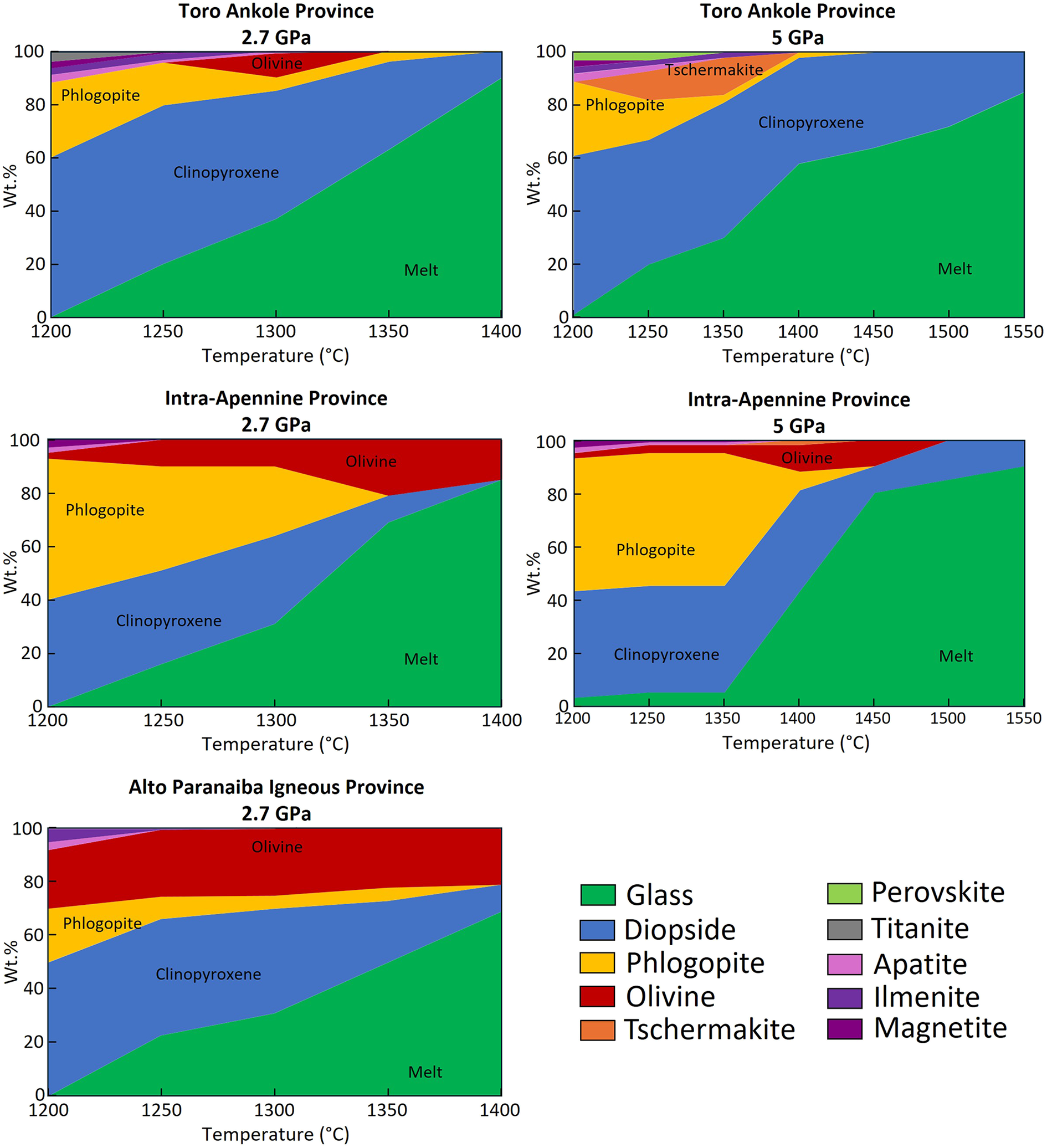
Figure 1. Modal abundances (wt.%) of mineral phases and melt (glass) obtained for each experimental sample (TA, IAP and APIP) at 2.7 and 5 GPa.

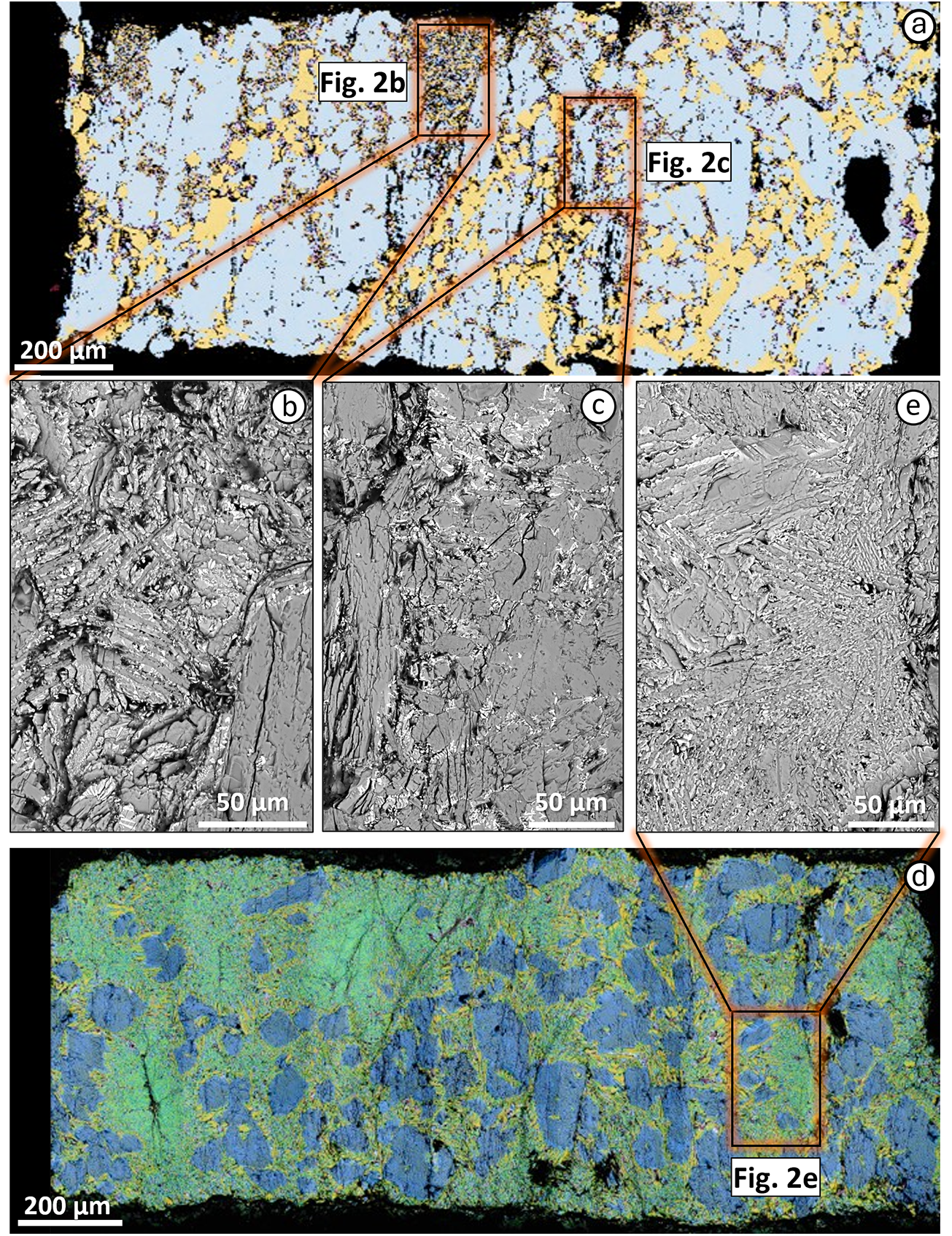
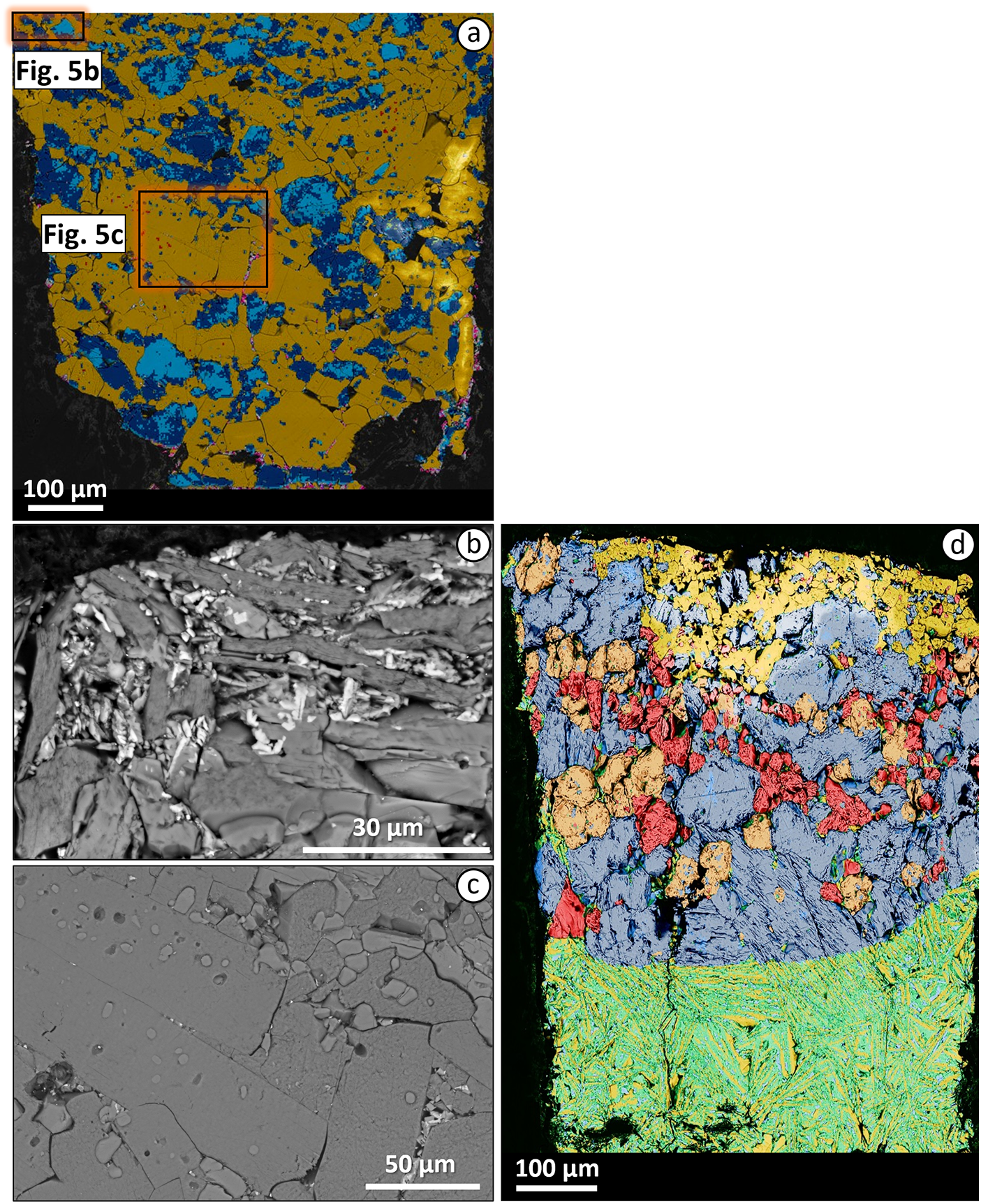
Figure 2. (a) Field-emission-scanning electron microscopy (FESEM) mineral maps for the TA experimental sample A23-012 (2.7 GPa and 1250°C); (b, c) higher-magnification SEM images of quenched needles; (d) FESEM mineral map for the TA experimental sample A23-016 (2.7 GPa and 1350°C); (e) higher-magnification SEM image of the melt pools. Dark blue represents clinopyroxene, light blue – augite, orange – tschermakite, yellow – phlogopite, red – olivine, purple – ilmenite, pink – apatite, light green – perovskite and green – glass.
Table 3. Averaged composition (SEM and EMP analyses, wt.%) of experimental glasses produced at 2.7 GPa for the three starting materials (Toro Ankole, Alto Paranaiba Igneous Province and Intra-Apennine Province). Values for standard deviation (σ) are given in italics. All data are reported in the Supplementary material

N = number of analyses
At 1300°C the degree of partial melting increases to ∼37 wt.% (see map in Supplementary material). Clinopyroxene, phlogopite, apatite and ilmenite are still present. These minerals are joined by peritectic olivine characterised by high Fo (0.83) and slight lime enrichment (CaO = 0.76 wt.%; Table 4), formed as a consequence of the incongruent melting of phlogopite. The glass composition remains strongly ultrabasic (SiO2 = 33.5 wt.%), but its TiO2 and CaO content decrease to 7.3 wt.% and 9.3 wt.%, respectively, while K2O increases to 6.2 wt.% (Table 3, Supplementary material).
Table 4. Averaged composition (SEM and EMP analyses, wt.%) of experimental clinopyroxene, phlogopite and olivine at 2.7 GPa for the three samples (Toro Ankole, Alto Paranaiba Igneous Province and Intra-Apennine Province). Values for standard deviation (σ) are given in italics. All data are reported in the Supplementary material

N = number of analyses; ‘–’ not detected
At 1350°C olivine is no longer present and all the accessory phases (apatite, ilmenite, magnetite and titanite) have melted out (Fig. 1). Only minimal traces of phlogopite (∼4 wt.%) and large amounts of clinopyroxene (33 wt.%) are still found. The area occupied by glass reaches ∼60 wt.% of the experimental charge (Fig. 2d-e). Compared to the 1300°C experiment, the 1350°C glass composition shows a slight enrichment in SiO2 (36.6 wt.%), similar TiO2 (7.1 wt.%), lower K2O (4.1 wt.%) and higher CaO (15.0 wt.%).
The highest-temperature experiment at 1400°C yields a melt fraction of ∼90 wt.%, with clinopyroxene (∼10 wt.%) remaining as the only residual mineral (Table 4). The glass remains ultrabasic (SiO2 = 40.9 wt.%), ultrapotassic (K2O = 3.6 wt.% and K2O/Na2O = 7) and reaches the highest CaO content (16.4 wt.%).
Experiments at 5 GPa
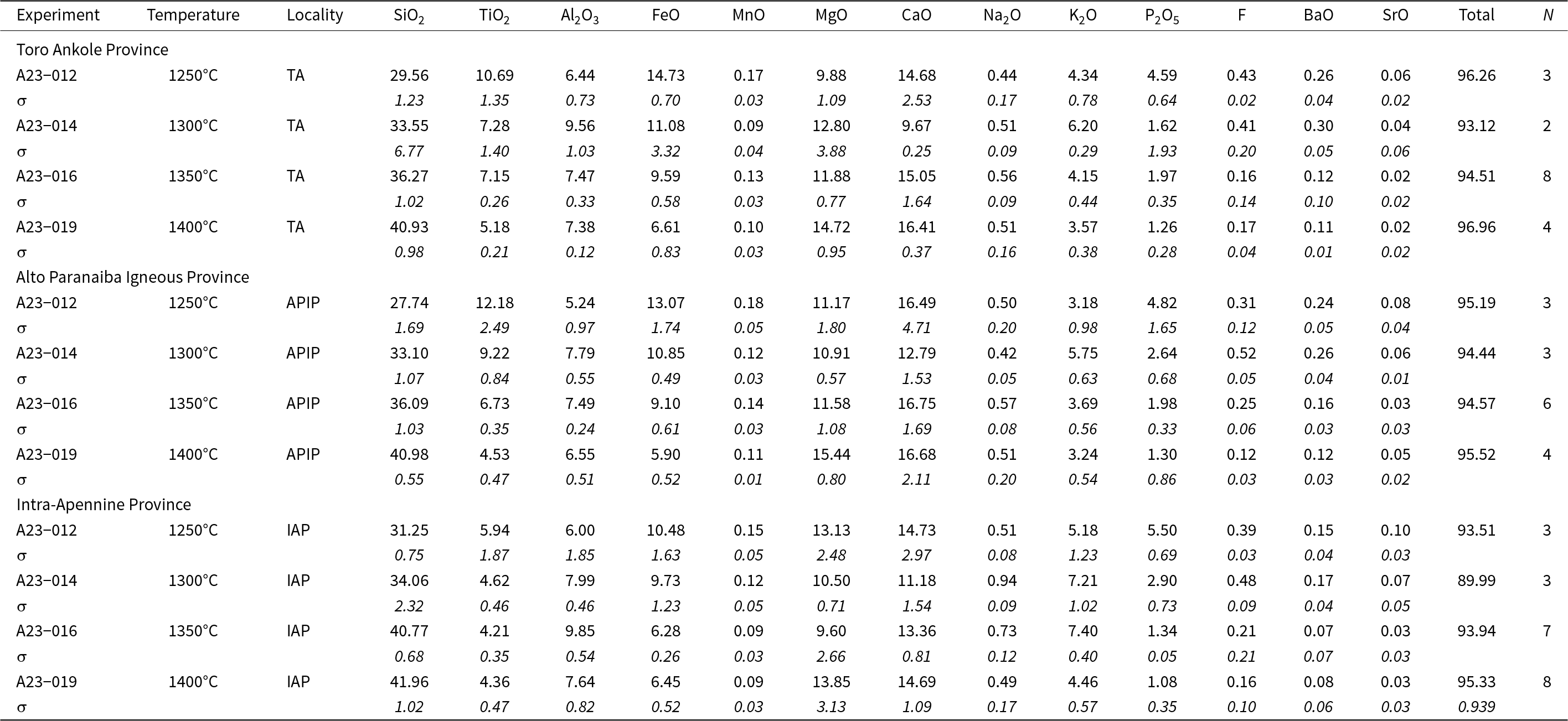
In contrast to the experiment at 2.7 GPa, that at 5 GPa, at 1200°C approaches near-solidus conditions, with ∼1 wt.% of melt (Fig. 3a–c), which was impossible to analyse due to the presence of quenched needles within the small glassy patches (Fig. 1). In addition to perovskite, which is present in place of titanite, the mineral assemblage is the same of the starting material (Fig. 3a).

Figure 3. (a) FESEM mineral maps for TA experimental sample M22-005 (5 GPa and 1200°C); (b, c) higher-magnification SEM images of quenched needles; (d) FESEM mineral maps for TA experimental sample M22-010 (5 GPa and 1400°C). Colours as in Fig. 2.
At 1250°C, the degree of partial melting reaches ∼20 wt.%, with glass concentrated along the capsule border. The mineral paragenesis of this experiment is composed of clinopyroxene, phlogopite, tschermakitic amphibole (Al2O3 = 18.5 wt.%, FeO = 12.2 wt.%, MgO = 8.7 wt.% and CaO = 17.1 wt.%), perovskite, apatite and ilmenite (Table 6). The glass composition is strongly ultrabasic (SiO2 = 26.7 wt.%), with high TiO2, K2O and P2O5 (10.2, 5.5 and 5.2 wt.%, respectively).
At 1350°C, the degree of partial melting remains low (∼30 wt.%), and the melt is mainly concentrated along the grain boundaries (Supplementary material). Clinopyroxene, phlogopite, ilmenite and more abundant tschermakitic amphibole (14 wt.%) are observed in this run (Al2O3 = 19.9 wt.%, FeO = 8.4 wt.%, MgO = 13.6 wt.% and CaO = 13.4 wt.%; Table 6). Experimental glass at 1350°C is strongly ultrabasic (SiO2 = 24.4 wt.%), enriched in TiO2, CaO, K2O and P2O5 (12.2, 11.2, 5.3 and 6.6 wt.%, respectively).
The 1400°C experiment is characterised by only ∼2 wt.% phlogopite, ∼40 wt.% of clinopyroxene and ∼58 wt.% glass (Fig. 3d). The glass is slightly richer in SiO2 (34.9 wt.%), poorer in P2O5 (1.9 wt.%) and similar in terms of TiO2 and K2O contents compared to those analysed in the lower temperature experiments (Table 5).
Table 5. Averaged composition (SEM and EMP analyses, wt.%) of experimental glasses at 5 GPa for the two samples (Toro Ankole and Intra-Apennine Province). Values for standard deviation (σ) are given in italics. All data are reported in the Supplementary material

N = number of analyses; ‘–’ not detected
Experiments at 1450°C, 1500°C and 1550°C contained 64, 72 and 85 wt.% glass, respectively (Fig. 1). Clinopyroxene is the only liquidus mineral phase (Supplementary material). Glasses at 1450 and 1500°C exhibit similar compositions (SiO2 = 36.3 and 37.2 wt.%, TiO2 = 7.2 and 7.3 wt.%, and CaO = 15.8 and 15.7 wt.%). The glass at 1550°C remains ultrabasic (SiO2 = 40.5 wt.%) and ultrapotassic (K2O = 3.0 wt.% and K2O/Na2O = 6), also having high CaO (17.8 wt.%) and TiO2 (5.2 wt.%). No olivine was found in the TA experiments at 5 GPa.
Experiments on a clinopyroxene glimmerite + apatite + magnetite (Intra-Apennine Province)
Experiments at 2.7 GPa
At 1200°C no glass was detected, but the occurrence of incongruent melting of phlogopite is indicated by the presence of ∼2 wt.% olivine (Fig. 1). Increasing the temperature to 1250°C results in ∼15 wt.% melt production (Fig. 4a–b), with the glass showing ultrabasic composition (SiO2 = 31.2 wt.%) and enrichment in K2O (5.2 wt.%), P2O5 (5.5 wt.%) and TiO2 (5.9 wt.%; Table 3). All apatite and magnetite melted out, leaving only phlogopite (∼35 wt.%) and clinopyroxene (∼38 wt.%). Olivine (∼10 wt.%) is also present in the recovered experiment highlighted by chemical and mineralogical mapping (Fig. 4a), but due to its small size no quantitative data could be obtained.

Figure 4. (a) FESEM mineral maps for IAP experimental sample A23-012 (2.7 GPa and 1250°C); (b) higher-magnification SEM images of quenched needles; (c) FESEM mineral maps for IAP experimental sample A23-016 (2.7 GPa and 1350°C); (d, e) higher-magnification SEM image of melt pools and olivine microcrysts. Colours as in Fig. 2.
At 1300°C (Supplementary material), the melt increases to ∼30 wt.%, coexisting with ∼10 wt.% olivine, ∼34 wt.% clinopyroxene and 26 wt.% phlogopite. Olivine is enriched in MgO (Fo86), with 0.38 wt.% CaO (Table 4). The higher proportion of melt results in an increase in SiO2 (34.1 wt.%) and K2O (7.2 wt.%) in the glass, as clinopyroxene joins the melting process and the amount of melting phlogopite increases. On the other hand, CaO decreases (down to 11.2 wt.%) together with P2O5, as the effect of accessory phases dilutes these oxides in the melt composition.
At 1350°C the degree of partial melting reaches ∼70 wt.% and phlogopite is exhausted, while ∼10 wt.% clinopyroxene is still observed (Fig. 4c-d). Olivine (21 wt.%) shows higher MgO (Fo92) and CaO (0.43 wt.%). In the 1400°C experiment, melt and olivine reach a modal abundance of ∼85 wt.% and ∼15 wt.%, respectively. Olivine composition is comparable to that observed in the previous experiment (Fo92). The glasses in the 1400°C experiments show slightly higher SiO2 (42.0 wt.%), TiO2 (4.4 wt.%) and CaO (14.7 wt.%), when compared to the 1350°C glass (40.8 wt.%, 4.2 wt.% and 13.4 wt.%, respectively), but also lower K2O and P2O5, down to 4.5 wt.% and 1.1 wt.%. The evolving glass compositions results from the increasing amount of clinopyroxene contributing to the melt (up to 40 wt.%).
Experiments at 5 GPa
At 1200°C, the extent of melting is low, yielding ∼3 wt.% glass (Fig. 5a–b) and small crystals of olivine (∼2 wt.%) (Fig. 5c). With increasing temperature to 1250 and 1350°C, the degree of partial melting increases only to 5 wt.% (Fig. 1). There is, however, a slightly higher amount of olivine (∼3 wt.%) and intergranular quenched needles may have been lost during polishing. The paragenesis recovered from both experiments is composed of ∼40 wt.% clinopyroxene, ∼50 wt.% phlogopite, ∼1 wt.% magnetite and ∼1 wt.% apatite. Due to the small amount of glassy patches in both the 1250 and 1350°C runs, it was impossible to obtain reliable quantitative compositions.

Figure 5. (a) FESEM mineral maps for IAP experimental sample M22-005 (5 GPa and 1200°C); (b, c) higher-magnification SEM images of quenched needles and olivine microcrysts; (d) FESEM mineral maps for IAP experimental sample M22-010 (5 GPa and 1400°C). Colours as in Fig. 2.
By 1400°C, the melt fraction drastically increases to ∼43 wt.% (Fig. 5d). Olivine reaches 10 wt.% and coexists with ∼2 wt.% tschermakite (Al2O3 = 20.8 wt.%, FeO = 6.2 wt.%, MgO = 17.4 wt.% and CaO = 10.0 wt.%; Table 6), ∼38 wt.% clinopyroxene and ∼7 wt.% phlogopite. The glass is higher in SiO2 (37.9 wt.%) and K2O (8.9 wt.%), coupled with lower TiO2, CaO and P2O5 (Table 6).
Table 6. Averaged composition (SEM and EMP analyses, wt.%) of experimental clinopyroxene, phlogopite, olivine and tschermakite at 5 GPa for the two samples (Toro Ankole and Intra-Apennine Province). Values for standard deviation (σ) are given in italics. All data are reported in the Supplementary material

N = number of analyses; ‘–’ not detected
With increasing temperature, the degree of melting reaches ∼80 wt.% at 1450°C, ∼85 wt.% at 1500°C and 90 wt.% at 1550°C (Fig. 1). The melt compositions show a quite uniform content of SiO2 (42.3 and 42.1 wt.%), TiO2 (2.5 and 2.1 wt.%) and CaO (12.0 and 11.9 wt.%) as the melt approaches the composition of the bulk mixture. With temperature increasing from 1450 to 1550°C, P2O5 and K2O slightly decrease with the increasing melting degree (from 5.9 wt.% to 4.8 wt.% and from 1.2 wt.% to 0.6 wt.%, respectively). In the 1450°C experiment, ∼5 wt.% olivine is still present (see map in Supplementary material), together with ∼10 wt.% clinopyroxene, whereas at 1500 and 1550°C only clinopyroxene (15 and 10 wt.%, respectively) still persists (Table 6).
As in the 2.7 GPa experiments, olivine compositions show an increase in MgO and a decrease in FeO (Fo82 at 1350°C, to Fo94 at 1450°C). The full set of data is reported in Table 6.
Experiments on an olivine clinopyroxenite + phlogopite + apatite + ilmenite (Alto Paranaiba Igneous Province)
Experiments at 2.7 GPa
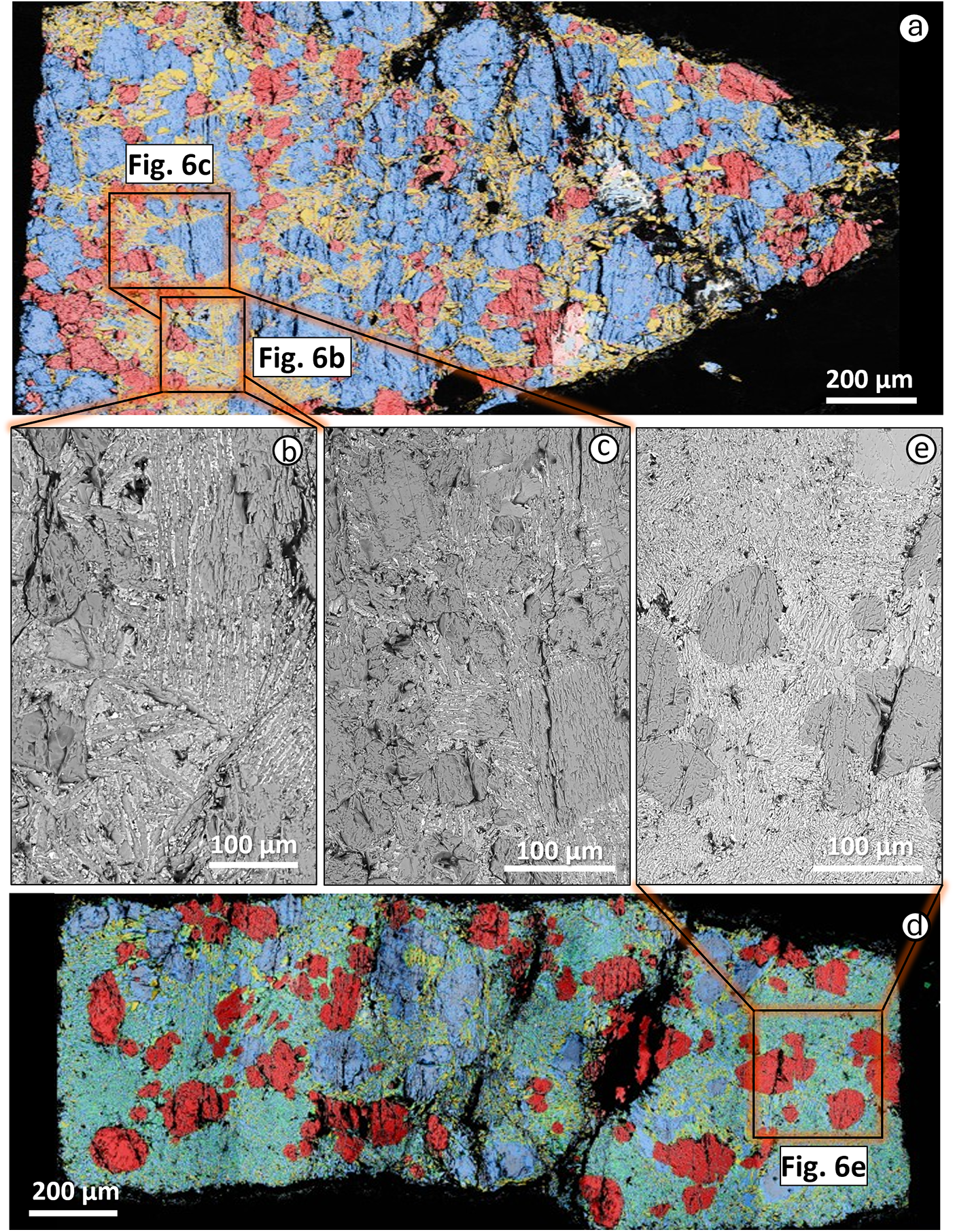
The APIP experiments have been conducted only at 2.7 GPa (Table 2). Due to the composition of the starting materials and the lower modal abundance of phlogopite, this sample showed the lowest degree of melting at any given temperature. At 1200°C the sample was subsolidus, whereas ∼23 wt.% melt appears at 1250°C (Fig. 1). In the 1250°C experiment, apatite melted out, while ∼43 wt.% clinopyroxene, ∼8 wt.% phlogopite, ∼25 wt.% olivine and <1 wt.% ilmenite were still observed. The initial melt exhibits a strongly ultrabasic composition (SiO2 = 27.7 wt.%), roughly overlapping with TA and IAP runs (Table 3 and Harker diagram, see Supplementary material), but TiO2, MgO and CaO are much higher (12.2 wt.%, 11.2 wt.% and 16.5 wt.%, respectively). At 1300°C, the melt fraction increases up to ∼31 wt.% (Supplementary material), showing a slightly higher SiO2 content than that in the TA run at the same temperature (33.1 wt.%), but similar TiO2 (9.2 wt.%), CaO (12.8 wt.%), K2O (5.7 wt.%) and P2O5 (2.6 wt.%). Accessory phases (apatite and ilmenite) melted out, leaving only ∼23 wt.% clinopyroxene, ∼25 wt.% olivine and ∼5 wt.% phlogopite. Olivine content slightly increased due to the incongruent melting of phlogopite. At 1350°C, the melt fraction reaches ∼50 wt.%, with phlogopite, olivine and clinopyroxene still abundant in the residuum (Table 4; Fig. 6a-c). The 1400°C experiment contains ∼69 wt.% melt (Fig. 6d-e), joined by ∼21 wt.% olivine and ∼10 wt.% clinopyroxene. The increasing modal amount of clinopyroxene participating to the partial melting, at same percentage of phlogopite and accessory phases (apatite and magnetite), resulted in an increasing SiO2 (from 36.1 to 41.0 wt.%) and MgO in the glass (from 11.6 to 15.4 wt.%). For the same reason, the FeO, K2O and P2O5 contents decrease (from 9.1 to 5.9 wt.%, from 3.7 to 3.2 wt.% and from 2.0 to 1.3 wt.%, respectively; Table 3).

Figure 6. (a) FESEM mineral maps for APIP experimental sample A23-014 (2.7 GPa and 1300°C); (b, c) higher-magnification SEM images of quenched needles; (d) FESEM mineral maps for APIP experimental sample A23-019 (2.7 GPa and 1400°C); (e) higher-magnification SEM images of melt pools. Colours as in Fig. 2.
In all the runs, olivine shows almost uniform composition (Table 4), although a trend of slightly increasing MgO and decreasing FeO (from Fo84 at 1250°C to Fo92 at 1400°C) can be observed. CaO remains quite uniform (0.2–0.4 wt.% in the 1250°C, 0.4 wt.% in the 1300°C, 0.1–0.7 wt.% in the 1350°C and 0.3–0.6 wt.% in the 1400°C).
Discussion
High-pressure and high-temperature experiments conducted in this study highlight that partial melting of phlogopite clinopyroxenites and clinopyroxene-bearing glimmerites produces SiO2-poor and CaO- and K2O-rich compositions overlapping those of natural kamafugites. Furthermore, the results indicate that accessory phases can significantly influence the geochemical composition of the resulting melt, in particular TiO2 or P2O5 contents, which are very dependent on the amount of titanite, ilmenite and apatite.
Comparison of natural and experimental compositions
In the following sections, experimental melt compositions are compared to natural kamafugites (references in Supplementary material), which represent volcanic rocks with ultrabasic to basic and potassic to ultrapotassic compositions, characterised by high CaO and variable incompatible trace-element enrichment, with the common feature of having primary kalsilite as a groundmass phase (Innocenzi et al., Reference Innocenzi, Ronca, Agostini, Guarino, Foley and Lustrino2025). Rocks classified as kamafugites in literature show a wide compositional spread, even when belonging to the same volcanic area or district. The large geochemical variability of kamafugites is probably related to heterogeneous lithospheric mantle sources (Innocenzi et al., Reference Innocenzi, Ronca, Agostini, Guarino, Foley and Lustrino2025).
Natural kamafugites display a wide range of Mg# (from 57 to 93) as well as a large variation in major oxide contents. In addition to Cupaello lavas, which are interpreted to be evolved samples (Lustrino et al., Reference Lustrino, Pistocchi, Ronca, Innocenzi and Agostini2025), little evidence of fractionation has been found in other worldwide kamafugites (Melluso et al., Reference Melluso, Lustrino, Ruberti, Brotzu, De Barros Gomes, Morbidelli, Morra, Svisero and d’Amelio2008; Rosenthal et al., Reference Rosenthal, Foley, Pearson, Nowell and Tappe2009; Guarino et al., Reference Guarino, Wu, Lustrino, Melluso, Brotzu, de Barros Gomes, Ruberti, Tassinari and Svisero2013; Lustrino et al., Reference Lustrino, Ronca, Caracausi, Ventura Bordenca, Agostini and Faraone2020; Innocenzi et al., Reference Innocenzi, Ronca, Foley, Agostini and Lustrino2024a, Reference Innocenzi, Ronca, Agostini, Benedetti and Lustrino2024b, Reference Innocenzi, Ronca, Agostini, Guarino, Foley and Lustrino2025).
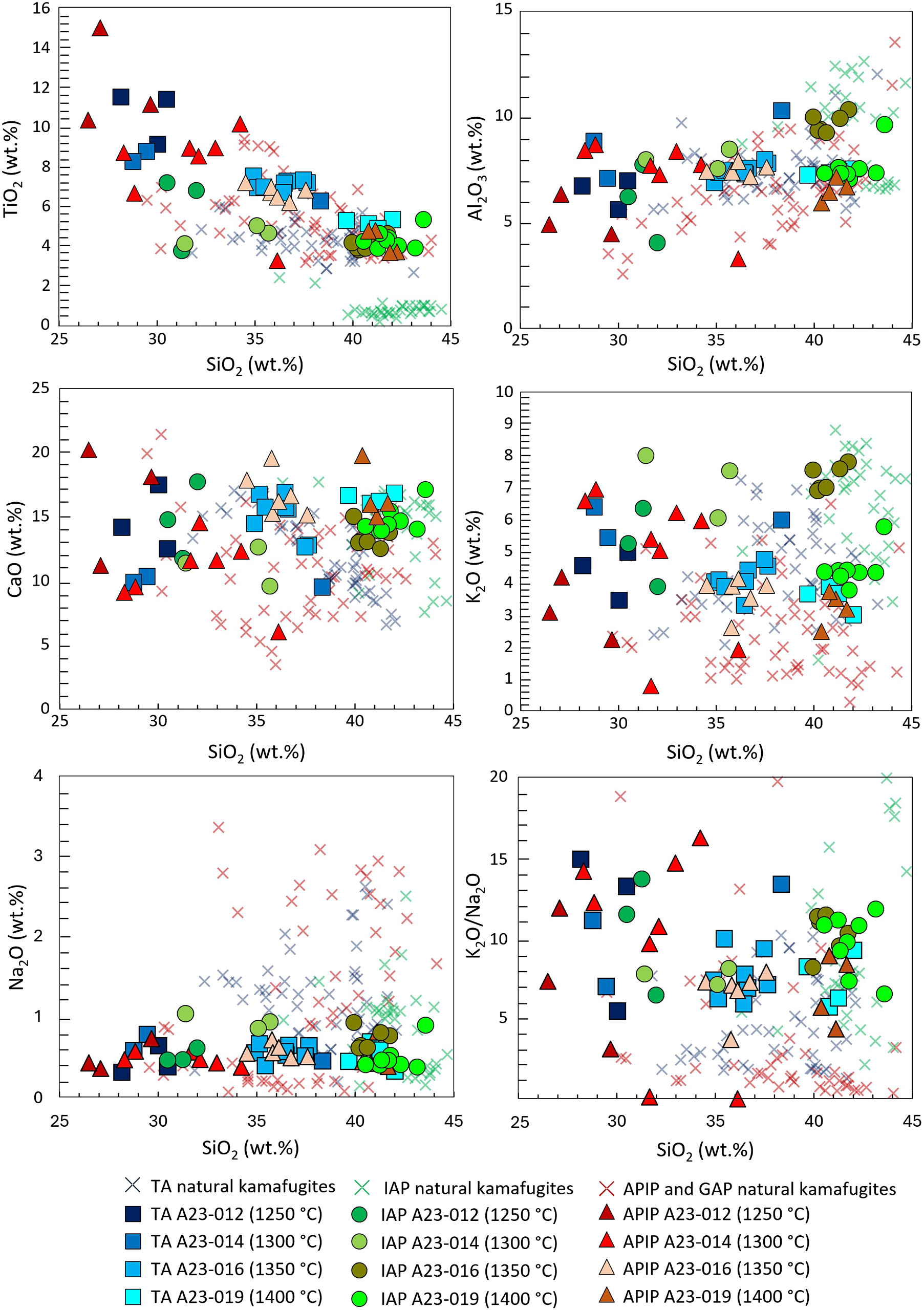
Natural and synthetic compositions are plotted in Harker diagrams for selected major oxides vs. SiO2 in Fig. 7 (experiments carried out at 2.7 GPa) and Fig. 8 (experiments at 5 GPa). Further diagrams are presented in the Supplementary material. Here, clear trends in experimental glasses with increasing melting degree are outlined. The melts formed at the lowest temperatures (1250–1350°C) have low SiO2, very high TiO2 and P2O5, probably due to the partial or complete melting of the accessory phases (titanite, apatite and Fe-Ti-oxides in TA, apatite and ilmenite in APIP, and apatite and magnetite in IAP), whereas only low amounts of the major phases contribute to the melt. These low-degree melts do not match the compositions of kamafugites or of any other natural melts. At higher temperatures (1350–1400°C) and consequently larger degrees of partial melting (50–90 wt.%) the experimental melts resemble the natural kamafugites for most of the major oxides (Figs 7 and 8). However, the FeOtot contents of experimental melts for all three provinces are slightly lower than natural counterparts, possibly indicating minor FeO loss during the experiments (FeOtot in ∼90% of the experiments is lower than 1.2 wt.%).

Figure 7. Harker diagram for major oxides (TiO2, Al2O3, CaO, K2O, Na2O) and K2O/Na2O vs. silica (wt.%) for TA, IAP and APIP experimental glasses at 2.7 GPa. Kamafugite compositions from the literature are also plotted for comparative purposes (references in Supplementary material).

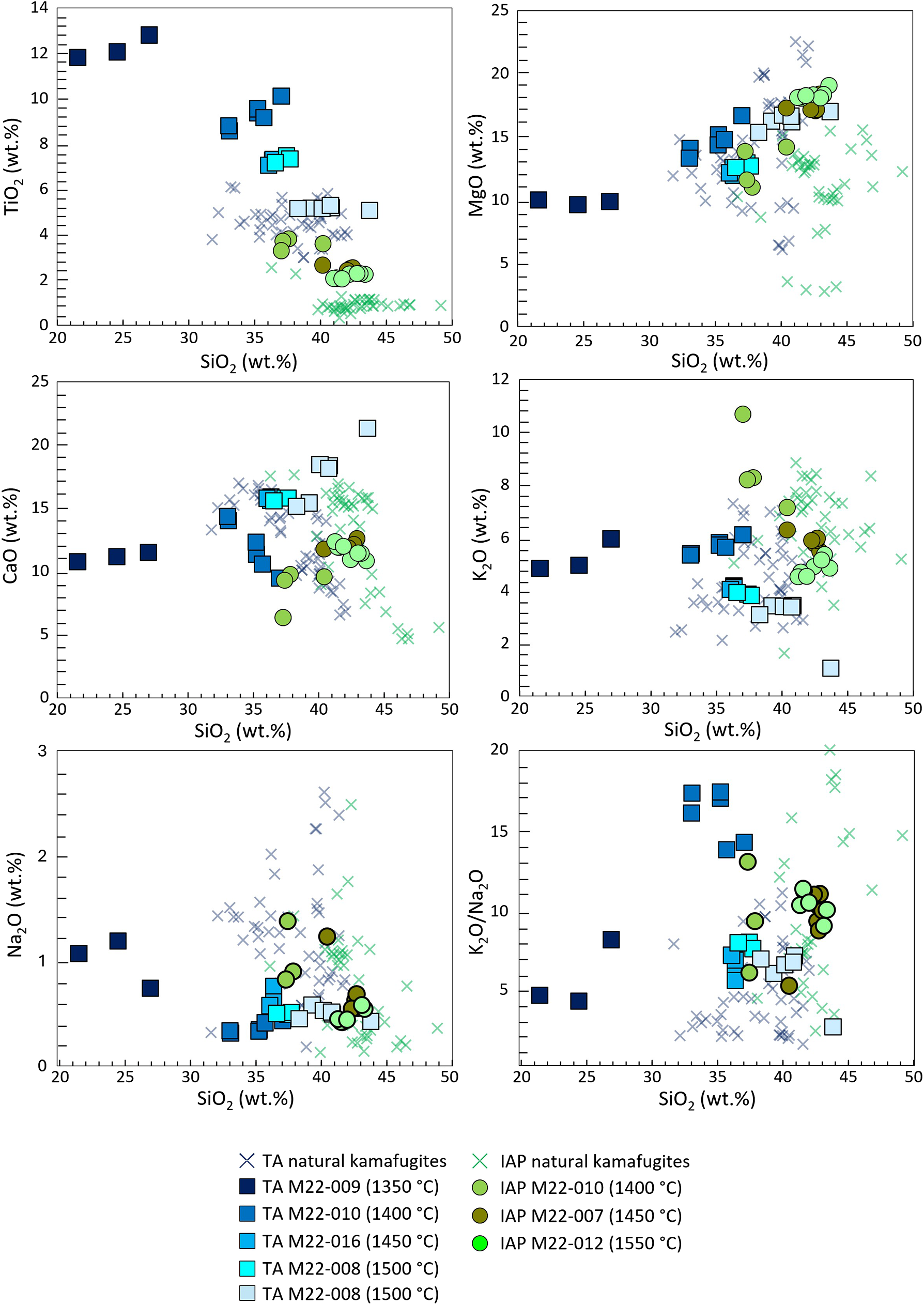
Figure 8. Harker diagram for major oxides (TiO2, MgO, CaO, K2O, Na2O) and K2O/Na2O vs. silica (wt.%) for TA and IAP experimental glasses at 5 GPa. TA and IAP kamafugite compositions from the literature are also plotted for comparison, references as in Fig. 7.
Toro Ankole kamafugites show variable compositional features together with a large spectrum of igneous products in general. Indeed, kamafugitic compositions are replaced by carbonatitic tuffs moving from South to North of the region (e.g. Eby et al., Reference Eby, Lloyd and Woolley2009; Zartner, Reference Zartner2010). Moreover, different lithologies (i.e. kamafugites, leucitites, nephelinites and olivine-bearing melilitites) with many compositional similarities have been identified in the TA districts (e.g. Rosenthal et al., Reference Rosenthal, Foley, Pearson, Nowell and Tappe2009; Muravyeva et al., Reference Muravyeva, Belyatsky, Senis and Ivanov2014). The minor differences recorded among the samples are considered to be derived from a significantly metasomatised lithospheric mantle characterised by significant heterogeneity, also at short lateral scales. Different abundances of clinopyroxene, phlogopite and carbonate in the source may explain all the rock compositions recorded in TA (Innocenzi et al., Reference Innocenzi, Ronca, Foley, Agostini and Lustrino2024a). Furthermore, variable degrees of melting and of interaction with the surrounding mantle matrix might also contribute to explain the observed geochemical diversity (Rosenthal et al., Reference Rosenthal, Foley, Pearson, Nowell and Tappe2009; Muravyeva et al., Reference Muravyeva, Belyatsky, Senis and Ivanov2014; Pitcavage et al., Reference Pitcavage, Furman, Nelson, Kalegga and Barifaijo2021). Involvement of mantle peridotite in the melting process would cause a dilution of the melt, resulting in less extreme composition (e.g. lower alkali contents). The type of interaction is affected considerably by the Mg# of the melt and the eventual equilibrium condition with the surrounding mantle (e.g. Foley et al., Reference Foley, Ezad, Shu and Förster2025).
TA melts produced at 2.7 GPa and 1350–1400°C (60 and 90 wt.% melt, respectively) show good overlap with natural kamafugites for almost all the oxides (SiO2, Al2O3, MgO, K2O and P2O5). TiO2 and CaO differ only slightly, with higher contents in the synthetic glasses than for the natural rocks (see Innocenzi et al., Reference Innocenzi, Ronca, Foley, Agostini and Lustrino2024a and references therein). Na2O (0.39–0.78 wt.%) is displaced toward the lower end of the field of natural samples (0.18–3.60 wt.%; Innocenzi et al., Reference Innocenzi, Ronca, Foley, Agostini and Lustrino2024a and reference therein; Fig. 7). At 5 GPa, experimental melts at 1450, 1500 and 1550°C (64, 72 and 85 wt.% melt, respectively; Fig. 1) approach natural compositions. SiO2, Al2O3, MgO and K2O match well, whereas CaO, Na2O, and P2O5 only partially overlap (Fig. 8). TiO2 contents in all analysed glasses is too high, and only at 1550°C is there an overlap with the upper end of the natural kamafugite field (5.1–5.3 wt.% in synthetic glass vs. 3.0–6.1 wt.% in natural Ugandan samples).
Brazilian kamafugites, from both the APIP and Goiás Alkaline Provinces (GAP, SE Brazil) are plotted together to give a better representation of compositions of the whole region and to allow a better comparison with other natural kamafugites. As for the other two cases, the differences among the different Brazilian kamafugites can be partially ascribed to the effects of diffuse alteration events, such as analcimisation (Sgarbi and Gaspar, Reference Sgarbi and Gaspar2002; Brod et al., Reference Brod, Barbosa, Junqueira-Brod, Gaspar, Diniz-Pinto, Sgarbi, Petrinovic, Comin-Chiaramonti and Gomes2005; Melluso et al., Reference Melluso, Lustrino, Ruberti, Brotzu, De Barros Gomes, Morbidelli, Morra, Svisero and d’Amelio2008; Guarino et al., Reference Guarino, Wu, Lustrino, Melluso, Brotzu, de Barros Gomes, Ruberti, Tassinari and Svisero2013). APIP experimental glass obtained at 2.7 GPa and 1350 to 1400°C (50 and 70 wt.% melt, respectively) show good overlap with natural kamafugites for almost all the major oxides (e.g. SiO2, Al2O3, MgO, K2O and P2O5). TiO2 and CaO differ only differ, with larger contents in the synthetic glasses than for the natural rocks (Innocenzi et al., Reference Innocenzi, Ronca, Agostini, Guarino, Foley and Lustrino2025 and references therein). Na2O (0.38–0.81 wt.%) is displaced toward the lower end of the field of natural samples (0.05–4.45 wt.%; Innocenzi et al., Reference Innocenzi, Ronca, Agostini, Guarino, Foley and Lustrino2025 and reference therein; Fig. 7).
As regards IAP, only literature data from San Venanzo and Cupaello lavas with SiO2 contents of >38.1 wt.% are reported, as this province also hosts many pyroclastic products containing variable amounts of secondary carbonate-bearing lithologies (Lustrino et al., Reference Lustrino, Luciani and Stagno2019, Reference Lustrino, Ronca, Caracausi, Ventura Bordenca, Agostini and Faraone2020, Reference Lustrino, Pistocchi, Ronca, Innocenzi and Agostini2025; Innocenzi et al., Reference Innocenzi, Ronca, Agostini, Benedetti and Lustrino2024b and references therein). Though they are spatially and temporally very close, the eruptive centres of San Venanzo and Cupaello are characterised by distinct geochemical (i.e. San Venanzo kamafugites have lower TiO2 and higher MgO–Al2O3 than Cupaello) and mineral paragenesis (i.e. San Venanzo lavas are olivine-rich clinopyroxene-poor, whereas Cupaello lavas are almost completely olivine-free and clinopyroxene-rich; Lustrino et al., Reference Lustrino, Ronca, Caracausi, Ventura Bordenca, Agostini and Faraone2020, Reference Lustrino, Pistocchi, Ronca, Innocenzi and Agostini2025). Recently, Lustrino et al. (Reference Lustrino, Pistocchi, Ronca, Innocenzi and Agostini2025) attributed these differences to a prolonged fractional crystallisation history (olivine + phlogopite + melilite + spinel + kalsilite) starting from a less evolved magma similar in composition to San Venanzo primitive kamafugite (sample SAV6 of Lustrino et al., Reference Lustrino, Ronca, Caracausi, Ventura Bordenca, Agostini and Faraone2020). Regardless of the non-primitive composition, Innocenzi et al. (Reference Innocenzi, Ezad, Ronca, Agostini, Lustrino and Foley2024c) performed near-liquidus experiments on a Cupaello sample in order to evaluate other possibilities. It is plausible that the lithospheric mantle beneath central Italy could be extremely heterogeneous and laterally variable, involving slightly different metasomatic assemblages (i.e. variable concentrations of metasomatic phlogopite and clinopyroxene). This, coupled with different degrees of partial melting could be an alternative explanation of the several differences between San Venanzo and Cupaello. As the matter is still debated, both San Venanzo and Cupaello data are reported on the Harker diagrams (Figs 7 and 8), thus resulting in the wide field of IAP kamafugites.
Only the 2.7 GPa experiments carried out at 1350 and 1400°C partially overlap the natural IAP rocks. The most remarkable difference occurs for TiO2, which is very low in San Venanzo and Cupaello volcanic rocks (<1.3 wt.%), compared to the experimental glasses (>3.8 wt.%). MgO shows slightly lower contents (6.4–12.5 wt.%) in the experimental melts than in the natural San Venanzo samples (7.6–15.5 wt.%; Supplementary material). P2O5 in the IAP experimental glasses are too high (1.3–1.4 wt.%) with respect to the natural San Venanzo composition (0.32–1.44 wt.%), whereas they overlap for the Cupaello samples (∼1.2 wt.%). These characteristics for Cupaello may be interpreted as a primary feature of the mantle source, but they may also result from the fractionation of an apatite-free kalsilitolite cumulate, to allow for the increase of P2O5 in the residual melt (e.g. Lustrino et al., Reference Lustrino, Pistocchi, Ronca, Innocenzi and Agostini2025). A fractionation history for Cupaello lavas is also supported by the poor overlap of IAP experimental glasses with the natural rocks, with the exception of SiO2, Al2O3 and P2O5 at 1400°C.
At 5 GPa, experimental glasses for IAP and TA at 1450, 1500 and 1550°C (80 to 90 wt.% melt; Fig. 1) approach the natural compositions. For IAP, the agreement is weaker than for TA, as most major oxides do not fall in the natural rock fields (e.g. higher TiO2, lower Al2O3 and CaO). This may be caused by the high degree of partial melting, which would dilute Al2O3 and K2O, the contents of which are lower than the natural examples, causing an increase in MgO and CaO from the clinopyroxene, which is the last mineral phase to completely melt out. For IAP, at 1400°C (∼40 wt.% melt) the overlap with natural compositions improves for MgO and K2O, but the experimental melt composition is still influenced significantly by the undiluted contribution from the accessory phases. Also the crystallisation of tschermakite would affect the composition of the resulting glasses. A degree of partial melting between 40 and 80 wt.% could probably shift the compositions of melts towards natural compositions.
Further consideration of source parageneses
Past melting experiments on hydrous mineral-rich non-peridotitic ultramafic compositions (such as phlogopite-bearing clinopyroxenites) show solidi consistently lower than for peridotite, even when compared with hydrous peridotite compositions (Kogiso et al., Reference Kogiso, Hirschmann and Pertermann2004; Lambart et al., Reference Lambart, Laporte, Provost and Schiano2012; Ezad et al., Reference Ezad, Blanks, Foley, Holwell, Bennett and Fiorentini2024; Foley et al., Reference Foley, Ezad, Shu and Förster2025). The composition of hydrous non-peridotitic ultramafic rocks is sensitive to the modal abundances of phases entering the melt, both as concerns major oxides (Foley et al., Reference Foley, Ezad, van der Laan and Pertermann2022) and trace elements (Foley and Ezad, Reference Foley and Ezad2024). The differences between natural kamafugites and the experimental glasses reported in this study may thus be related to different modal proportions of the minerals in the mantle sources and/or on the variable compositions of each phase.
The experimental melts are always characterised by higher TiO2 than natural kamafugites. For TA and APIP, this is probably due to the phlogopite used in the experiments, which probably has a greater TiO2 content than the phlogopite in the source of these two regions. Alternatively, it may be explained by a smaller amount of titanite and/or ilmenite (∼1–2%) in the melting assemblage. For IAP, this may be caused either by an excessive TiO2 content of clinopyroxene (1.58 wt.%) or by a proportion of magnetite (9.40 wt.%) in the starting material which is too large. Alternatively, considering the subduction-modified composition of IAP rocks (Lustrino et al., Reference Lustrino, Duggen and Rosenberg2011, Reference Lustrino, Chiarabba, Carminati, Foulger, Hamilton, Jurdy, Stein, Howard and Stein2022), the low TiO2 content could simply be a mantle source characteristic.
The other main differences are in Na2O and in K2O/Na2O ratio, which tend to be higher and lower, respectively, in the natural rocks than in the experimental melts, especially those at 2.7 GPa (Figs 7 and 8). This issue related to Na2O has previously been recognised for TA kamafugites (Lloyd et al., Reference Lloyd, Arima and Edgar1985) but remains unsolved. It is unlikely to be related to the presence of amphibole in the melting assemblage. Indeed, near-liquidus experiments (e.g. Edgar et al., Reference Edgar, Green and Hibberson1976; Innocenzi et al., Reference Innocenzi, Ezad, Ronca, Agostini, Lustrino and Foley2024c) seem to exclude the stability of amphibole in kamafugite mantle sources, and no amphibole has been found in the abundant nodules embedded in the Ugandan kamafugites (Link et al., Reference Link, Barifaijo, Tiberindwa and Foley2008). In a set of partial melting experiments on phlogopite-bearing clinopyroxenite nodules, Lloyd et al., (Reference Lloyd, Arima and Edgar1985) used a clinopyroxene with greater Na2O (∼1.15 wt.%) than that used in the present study (0.51 wt.%) obtaining glass with more Na2O (up to 1.95 wt.%). This is much more coherent with the TA natural composition, which hosts clinopyroxenes with 0.18–2.61 wt.% Na2O. Finally, <1% of apatite in the TA melting assemblage would guarantee a good match between natural and experimental compositions.
For APIP, the difference in Na2O content is even greater. Experiments conducted at near-liquidus conditions (1350°C; 2 GPa) and at lower temperatures (1000°C; 2 GPa; Innocenzi et al., Reference Innocenzi, Ezad, Ronca, Agostini, Lustrino and Foley2024c) resulted in crystallisation of clinopyroxene with a Na2O content ranging from 0.72 (sub-liquidus run) to 2.05 wt.% (near-liquidus run). The much lower Na2O content of the clinopyroxene used in the present study (0.51 wt.%) can easily account for the divergence between the experimental glasses and the natural samples. Guarino et al. (Reference Guarino, Bonazzi, Nimis, Azzone, Cariddi and Zanetti2024) analysed diopsidic clinopyroxene from xenocrysts and lherzolite xenoliths in APIP kimberlites with Na2O in the 1.02–1.72 wt.% range. It follows that relatively Na2O–rich clinopyroxene can be present in APIP lithospheric mantle, but unfortunately, mineralogical constraints for the APIP kamafugite mantle source from natural rocks are more restricted than for TA.
The good match for MgO between natural TA kamafugites and experimental glasses may support the hypothesis that olivine is not involved in the genesis of Ugandan kamafugites (Supplementary material). Indeed, olivine is not needed to explain the geochemical features of the lavas, and the nodules almost completely lack this mineral (Link et al., Reference Link, Barifaijo, Tiberindwa and Foley2008; Innocenzi et al., Reference Innocenzi, Ronca, Foley, Agostini and Lustrino2022, Reference Innocenzi, Ronca, Foley, Agostini and Lustrino2024a). The lower melting temperature of the metasomatic veins than surrounding peridotite, coupled with the high mobility of the melts would have caused a rapid upwelling to the surface limiting interaction with the surrounding peridotite mantle (Foley et al., Reference Foley, Musselwhite and van der Laan1999; Foley and Ezad; Reference Foley and Ezad2024). Interestingly, the MgO content of APIP experimental glasses (10.3–16.5 wt.%), where olivine was included in the starting materials, are very similar to that recorded for TA (10.6–16.0 wt.%) at the same conditions, where no olivine was used in the starting material. This is probably because olivine gives little contribution to the melt at low melting degrees. In order to obtain more MgO in the experimental glass, even higher melting degrees should be reached.
The IAP experimental glasses have less MgO content than Italian kamafugites, possibly due to the peritectic crystallisation of olivine formed through incongruent melting of phlogopite, which would decrease MgO in the glass (Prelević et al., Reference Prelević, Förster, Buhre, Gülmez, Grützner, Wang and Foley2024; Foley et al., Reference Foley, Ezad, Shu and Förster2025). Alternatively, we can interpret the lower MgO in the IAP experimental glasses to the actual presence of olivine in San Venanzo mantle source, which was not used in the starting material of experiments. The presence of small amounts of highly magnesian olivine participating in the melts might result in a better overlap with the natural melt compositions. It is also important to highlight that previous literature studies (Lustrino et al., Reference Lustrino, Ronca, Caracausi, Ventura Bordenca, Agostini and Faraone2020) suggested a limited digestion of country rocks (mainly carbonates) by an ultrabasic magma in San Venanzo, a process that could have contributed to the discrepancy observed between natural and synthetic compositions. The melting assemblage in San Venanzo must also have contained <1 wt.% of apatite to ensure a better overlap between natural and experimental values, as supposed for the TA case.
The presence of olivine in the melting assemblages suggested for APIP and IAP might be related to the reaction with the surrounding mantle, either after the partial melting of the hydrous metasomes or during the percolation of the metasomatic fluids (Foley, Reference Foley1992; Mallik et al., Reference Mallik, Lambart and Chin2021; Shu et al., Reference Shu, Foley, Ezad, Daczko and Shcheka2024; Innocenzi et al., Reference Innocenzi, Ezad, Ronca, Agostini, Lustrino and Foley2024c; Prelević et al., Reference Prelević, Förster, Buhre, Gülmez, Grützner, Wang and Foley2024). Another crucial point could be represented by the composition of the surrounding mantle, i.e. ultra-depleted or fertile assemblages, which reacts differently with the percolating melts/liquids (e.g. Cooper et al., Reference Cooper, Scott, Brenna, Palmer, le Roux, Cooper, Reid and Stirling2024).
Conclusions
Partial melting experiments have been carried out on assemblages ranging from phlogopite clinopyroxenite (± olivine) to clinopyroxene glimmerite at 1250–1550°C and at 2.7–5 GPa. The glasses produced are characterised by extremely variable compositions, influenced significantly by the degree of melting and by the relative contributions of the mineral phases to the melt. Relatively low-degree partial melting results in extreme compositions (e.g. very low SiO2, very high TiO2 and P2O5), due to the participation of titanite, apatite and Fe-Ti-oxides in TA, apatite and ilmenite in APIP, and apatite and magnetite in IAP in the melting assemblage. In these cases, the experimental glasses do not mimic any natural silicate igneous rocks, but they probably represent good candidates for metasomatic melts infiltrating within the lithospheric mantle matrix (Fitzpayne et al., Reference Fitzpayne, Giuliani, Maas, Hergt, Janney and Phillips2019; Foley et al., Reference Foley, Ezad, van der Laan and Pertermann2022). Metasomatic products in mantle xenoliths commonly contain high proportions of Ti- and Fe-oxide minerals (Harte et al., Reference Harte, Winterbrun, Gurney, Menzies and Hawkesworth1987). The volatile-rich nature of these melts will also affect their physical and chemical properties resulting in lower density and greater mobility compared to anhydrous SiO2-rich compositions (Russell et al., Reference Russell, Hess and Dingwell2022; Schäfer and Foley, Reference Schafer and Foley2002; Prelević et al., Reference Prelević, Förster, Buhre, Gülmez, Grützner, Wang and Foley2024). With increasing temperature and amount of melt produced, glass compositions approach those of natural magmas, the main characteristics of which are low silica, high alkali (especially K2O) and high lime. The results of partial melting experiments suggest that high degrees of partial melting (>50 wt.%) of clinopyroxene ± phlogopite assemblages found in the lithospheric mantle can lead to the formation of ultrapotassic and/or ultracalcic compositions. This confirms the hypothesis of Lloyd et al. (Reference Lloyd, Arima and Edgar1985), according to which the Toro Ankole ultrapotassic rocks could be derived from hydrous ultramafic sources.
We observe a partial overlap between the compositions of experimental glasses and natural kamafugites. In particular, a good match for some major oxides (especially SiO2, MgO, K2O and P2O5) with experiments at 1350 and 1400°C is observed for Brazilian and Ugandan lavas. This could illustrate the importance of clinopyroxene as the dominant phase (50 and 60 wt.% for Brazilian and Ugandan lavas, respectively) contributing to melt composition, coupled with phlogopite and other accessory phases, such as apatite and ilmenite. As the modal abundance of phlogopite in the starting materials of the TA experiments was greater than for APIP, the K2O content of the experimental glass was consequently higher, together with Al2O3. Experimental results also support the presence of olivine in the APIP source, even if only partially contributing to the MgO budget. In contrast, the composition of the experimental glasses produced from an olivine-free phlogopite-pyroxenite assemblage, are in reasonable agreement with those of the natural kamafugites from the Toro Ankole volcanic Province.
The experimental glasses obtained from the clinopyroxene glimmerite assemblage roughly match the lava compositions from San Venanzo, with the suggestion of the presence of olivine in the melting assemblage in addition to a slightly smaller amount of magnetite and apatite. Experimental results confirm the importance of phlogopite in the source of Italian kamafugites, contributing to their very large K2O content, and of clinopyroxene, responsible for the high CaO content. The possible interaction between San Venanzo magmas and sedimentary limestones (Lustrino et al., Reference Lustrino, Ronca, Caracausi, Ventura Bordenca, Agostini and Faraone2020) may further complicate the Italian framework, as well as the presence of carbonates in the sources, a feature not investigated in the present study.
The presence of accessory phases in the mineral assemblage of mantle sources is fundamental to explain many of the geochemical features of the natural rock samples. For example, apatite (<3 wt.%) controls the P2O5 content of the whole-rock, whereas titanite (<4 wt.%) and ilmenite (<5 wt.%) are required to reach the high TiO2 content of the Ugandan and Brazilian kamafugites (3.0–9.5 wt.%).
This indicates that the large variety of compositions of the natural lavas might be related to different degrees of melting of clinopyroxenite (± olivine) variably enriched in phlogopite and some accessory minerals, such as apatite and TiO2-bearing phases. Different abundances of the main (clinopyroxene and phlogopite) and accessory (e.g. olivine) phases in the mantle sources are probably related to laterally heterogeneous lithospheric mantles. This, together with variable degrees of melting and interaction with the surrounding peridotitic matrix, with or without additional crustal interaction at shallow depth, would be able to give rise to almost all the primitive kamafugite magma compositions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1180/mgm.2025.10160.
Acknowledgements
The present work is a distillation of the PhD thesis of the first author at Sapienza University of Rome. The authors thank Domenico Mannetta for his help during the polishing process. FI thanks Dr Sean Murray for his help in the Macquarie GeoAnalytical Laboratories. FI also thanks Professors Lorenzo Fedele (University of Naples Federico II, Italy) and Michele Mattioli (University of Urbino Carlo Bo, Italy) for their help in improving the readability of an early version of this manuscript in the form of a PhD thesis manuscript.
Financial statement
It was financially supported by funds Ateneo Sapienza 2022 and 2024 to ML, Ateneo Sapienza 2022 to SR and Ateneo Sapienza 2023 to Silvio Mollo, by IGG–CNR P-CT0049 to SA, by “Avvio alla Ricerca” grant 2022 to FI and by Australian Research Council grant FL180100134 to SFF.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.