INTRODUCTION
Campylobacter jejuni is a bacterial illness that is transmitted by the faecal–oral route. Symptoms typically occur within 2–5 days following exposure (range 1–10 days) and include diarrhoea, which may be bloody, abdominal pain, fever, nausea, vomiting and malaise. Symptoms typically resolve spontaneously within 7 days but extended illness and relapses may occur. Complications following infection can include Guillain–Barré syndrome and reactive arthritis [Reference Braam and Heymann1].
Campylobacter bacteria are most commonly found in poultry and cattle but may also be found in domestic animals, swine, sheep, rodents, and birds [Reference Blaser, Taylor and Feldman2, Reference Abulreesh, Paget and Goulder3]. People can become infected through contact with infected animals or animal waste and through ingestion of the organism in contaminated food and water [Reference Chowdhury4]. A relatively low dose of bacteria is required to cause illness [Reference Black5, Reference Robinson6]. Outbreaks have been reported in association with undercooked poultry, unpasteurized milk and contaminated water supplies [7–Reference Pearson12]. However, campylobacteriosis cases are typically sporadic, with the source of the Campylobacter exposure frequently remaining unidentified. Additional data are needed to better define environmental reservoirs and transmission routes.
On 16 June 2007, 787 racers participated in a 67 km cross-country mountain bike race held in a small community in British Columbia (BC) in muddy conditions. In the subsequent days, the race organizer was made aware of comments on a race-related web forum regarding illness in race participants. This prompted contact with the local public health unit which received 13 laboratory reports of C. jejuni infection in racers on 25 June 2007. This paper reports the findings of the ensuing outbreak investigation.
METHODS
Epidemiological investigation
In-depth interviews and a visual assessment of the environment were conducted to understand relevant exposures during the event that may have predisposed race participants to infection. Interviews were conducted with 15 ill individuals, as well as volunteers, race organizers, community members, a park ranger and waste disposal services. The visual assessment included sections of the race course and the surrounding area. In addition, the race-related web forum was reviewed to gain information about the race course, mountain biking and hypotheses proposed by racers. Information from the interviews conducted and from the web forum discussion assisted with questionnaire development.
A retrospective cohort study was conducted among race participants between 29 June and 10 July 2007. Clinical cases were defined as occurring in race participants who had ⩾3 loose stools in a 24-h period between 17 and 26 June 2007. Laboratory-confirmed cases were defined as occurring in race participants with a stool specimen positive for C. jejuni. Individuals were defined as well (non-cases in race participants) if they did not meet the clinical or laboratory-confirmed case definitions. Participants who reported diarrhoea but did not specify the date of onset or had an onset prior to 17 June, or did not specify the number of stools in a 24-h period were excluded from the analysis.
The questionnaire included questions about clinical symptoms, laboratory testing and potential sources of exposure including food and water distributed at stations along the race course, food served at a barbeque following the race, natural water sources and mud along the trail.
An email with a link to an online questionnaire was sent to all race participants. The online questionnaire was created using Ultimate Survey Enterprise.NET (version 3.0.7) software (Prezza Technologies Inc., USA). Data were stored in an SQL (Structured Query Language) database. Univariate analysis was performed using SPSS version 13 (SPSS Inc., USA). Multivariate logistic regression analysis was conducted using Stata version 9 (Stata Corp., USA). Variables were selected for the model if they were significant based on the univariate analysis and/or had relevance due to biological and/or environmental plausibility.
Routine surveillance for Campylobacter in BC includes reporting of laboratory-diagnosed cases through the integrated public health information system (iPHIS) that is accessible by local health jurisdictions and provincial authorities. These data were reviewed to identify additional cases associated with the mountain bike race.
Microbiological investigation
Initial samples of creek water accessible from the course and bottled drinking water served during the race were collected and tested soon after the 16 June race. Additional environmental sampling was conducted during 5–7 July following preliminary data analysis. This included the collection of mud, muddy water and animal faecal samples from the race course and from the yards of individuals with horses and chickens near the race course. The data analysis revealed being splashed in the face or mouth, and/or swallowing muddy water in certain areas of the race course was associated with illness. These locations were all found in the latter half of the race and so the majority of samples were taken from this area (Fig. 1). Samples were also restricted to areas that were still wet or moist.

Fig. 1. Mountain bike race course and location of environmental and animal faecal samples collected. Most sampling sites were in the lower section of the race course as this area was where riders stated they had the greatest exposure to mud. Racers were asked if they could recall being splashed in the face/mouth and/or swallowing muddy water within the different sections of the race (e.g. between points 1 and 2, 2 and 3, etc.). The highlighted areas were significantly associated with illness.
All environmental samples were stored at 4°C until they were tested at the British Columbia Centre for Disease Control (BCCDC) Laboratory Services for total coliform and generic E. coli counts using an enzyme substrate method (Colilert quanti-tray) to determine the most probable number (MPN) [Reference Eaton13]. Samples were tested for Campylobacter using selective media and differentiation plates. Animal faecal samples were shipped on ice to the BC Animal Health Centre where they were tested for Campylobacter. Following enrichment procedures using Bolton broth, subculturing was performed on Campy-Line agar and any suspect Campylobacter colonies were subcultured on Columbia blood agar as previously described [Reference Line14]. Any colonies present would have been Gram stained. All curved Gram-negative vibroid cells recovered would have been identified to the species-level.
Human stool samples were tested using selective media and differentiation plates. The hippurate hydrolysis test was used for preliminary identification to species-level. Direct sequencing of a polymerase chain reaction (PCR) amplified region of the genome and comparison to a chaperonin 60 sequence database (cpnDB) was performed at the BCCDC to confirm the Campylobacter species [Reference Hill15]. Multi-locus sequence typing (MLST) was performed on 14 C. jejuni isolates from race participants using a previously reported method [Reference Dingle16, Reference Dingle17]. An ABI 3130xl Genetic Analyzer (Applied Biosystems, USA) was used for DNA sequencing and DNAStar software (DNAStar Inc., USA) for sequence assembly. The allele numbers, sequence type (ST) and the ST clonal complexes to which the isolates clustered were determined using the C. jejuni MLST database [18].
RESULTS
Epidemiological investigation
In-depth interviews and case-finding activities revealed that all initial cases occurred in racers. There were no clinical or laboratory-confirmed cases in race volunteers, spectators or other members of the community.
Based on routine surveillance, 32 C. jejuni laboratory-confirmed cases were identified and known to be associated with the bike race. However, of the racers who completed the questionnaire, 25 identified themselves as having laboratory-confirmed C. jejuni. Only 14 clinical specimens were sent to the provincial laboratory (BCCDC) for MLST analysis.
Food and water stations were set up at three locations along the race course as well as at the finish line (Fig. 1). Foods served included fruit, bagels and granola bars. Drinking water was sourced from a large bottled-water company and was served in paper cups to racers. A hose connected to the municipal water supply was available at the finish line to wash faces, bodies and bikes. A meal was served at the end of the race for racers and volunteers. Interviews revealed that most racers had significant mud exposure.
Race participants originated from the race host community, other parts of BC and Canada and internationally. Participants were accommodated in private homes, in various hotels and at local camp grounds prior to the race. Besides the race and one common meal, they shared no other meals or activities.
The race course is commonly used for other cycling events, dog walking and horseback riding. Wildlife in the area includes bears, black-tailed deer, cougars, coyote, weasels, rabbits, rodents, snakes, toads, snails, and a variety of birds. A small number of chicken were located at a residence about 1 km from the race course. There was a permanent outhouse structure and four portable toilets located at the finish line, six other portable toilets and a septic tank were also located on and near the trail, respectively. Numerous permanent bathrooms with septic tanks and drain fields were located at a park through which the trail ran. Additionally, septic tanks and fields were located in the residential areas near the trail. There were no obvious sources of environmental contamination such as malfunctioning septic tanks/fields and the local waste disposal company had no reports of leakage/overflow of portable toilets or septic tanks in the area. There was also no evidence of manure being spread in the area.
There were 787 individuals who started the race. The survey was completed by 549 (70%) of these individuals. Of these people, 12 did not meet the clinical, laboratory-confirmed or well (non-case) case definitions and 537 (98%) were included; 312 were defined as well and 225 racers were defined as ill, of which 25 indicated they had laboratory confirmation of Campylobacter infection. The attack rate based on those included in the study was 42%. The peak number of cases occurred on 18 June, when 81 (36%) of the ill individuals (clinical and laboratory-confirmed cases) reported symptom onset (Fig. 2). Only two individuals reported symptom onset after 23 June. Abdominal cramps and fever were the most commonly reported symptoms in ill individuals who, by definition, all had diarrhoea (Table 1).
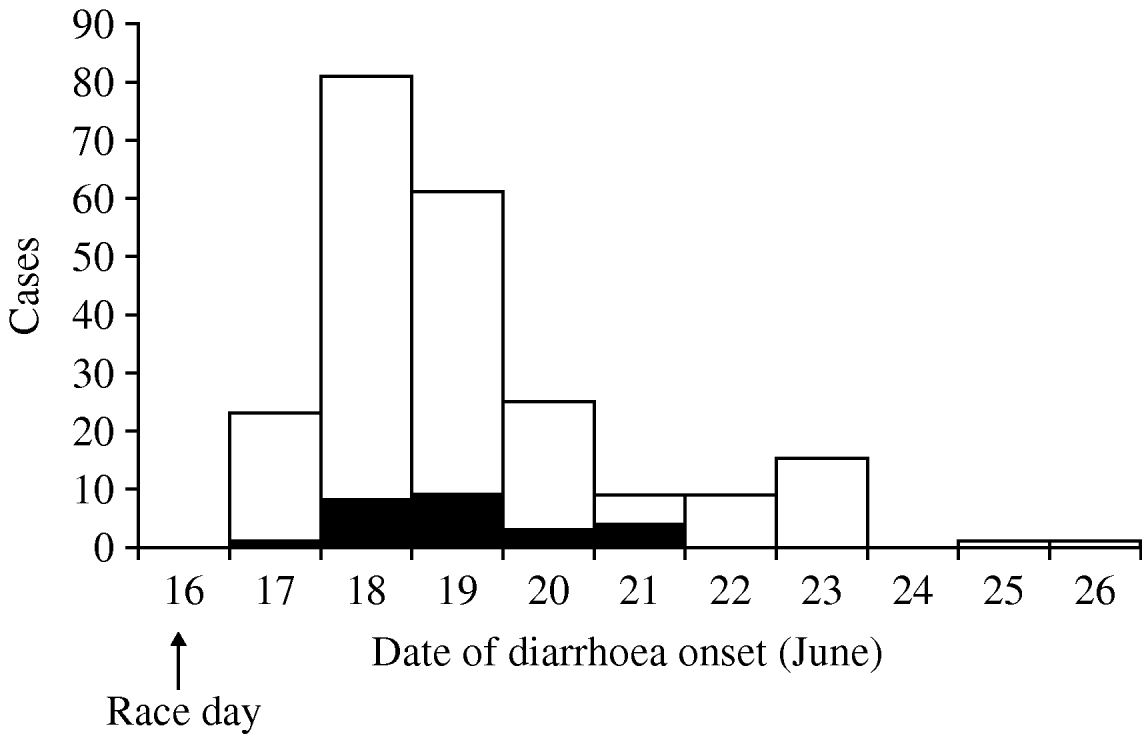
Fig. 2. Date of onset of symptoms of campylobacteriosis cases associated with a mountain bike race (n=225). ▪, Confirmed case; □, clinical case.
Table 1. Frequency of clinical symptoms reported by illFootnote * racers (n=225)
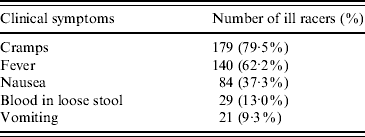
* All racers must have had ⩾3 loose stools in a 24-h period to be defined as ill.
A univariate analysis of all of the food items distributed at stations along the course and eating at the race-related barbecue revealed that food was not associated with illness (Table 2). Drinking cups of water from official stations was associated with illness [relative risk (RR) 2·04, 95% confidence interval (CI) 1·44–2·88]. However, refilling water bottles/personal hydration packs at these same stations was not. Additionally, there were 25 individuals who did not drink cups of water and/or refill water bottles/personal hydration packs at official stations during the race but who became ill.
Table 2. Univariate analysis of food, water and mud exposures following a campylobacteriosis outbreak associated with a bike race
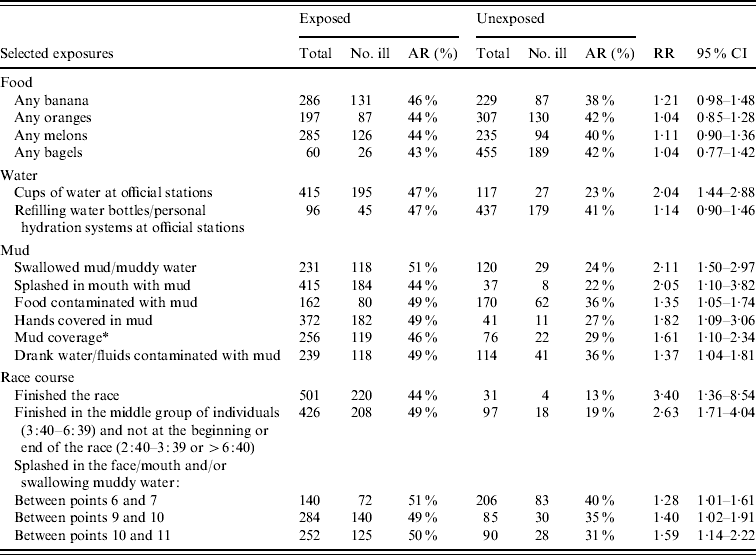
AR, Attack rate; RR, relative risk; CI, confidence interval.
* Scale of 1–5 where 5 is the muddiest (1–3=No; 4=not included; 5=Yes).
Swallowing muddy water (RR 2·11, 95% CI 1·50–2·97), being splashed in the mouth with mud (RR 2·05, 95% CI 1·10–3·82), eating food with mud on it (RR 1·35, 95% CI 1·05–1·74), and having muddy hands while eating (RR 1·82, 95% CI 1·09–3·06) were all significantly associated with illness (Table 2). Individuals were asked to rate their mud coverage from 1 to 5 with 5 being the muddiest. Those who scored themselves 5 were more likely to have become ill than those who scored themselves 1–3 (RR 1·61, 95% CI 1·10–2·34). Drinking water/liquids contaminated with mud (RR 1·37, 95% CI 1·04–1·81) was also significantly associated with illness.
Based on the questionnaire information as well as additional data on laboratory-confirmed cases identified through routine surveillance we knew the case status of 523 individuals who completed the race. The majority of individuals (n=426) finished with race times (in hours:minutes) in the middle of the distribution of participants (finish times of 3:40–6:39). Racers who finished in this time period were more likely (RR 2·63, 95% CI 1·71–4·04) to be ill than those who finished early (2:40–3:39) or were among the last riders to complete the race (>6:40) (n=97).
There were 31 individuals who did not complete the entire race and only four (13%) of these individuals became ill. Individuals who completed the race were more likely to become ill (RR 3·40, 95% CI 1·36–8·54). Recalling being splashed in the face/mouth and/or swallowing muddy water in certain sections of the race course was also associated with illness (Table 2). These locations were all found in the latter half of the race (Fig. 1).
We hypothesized that the ingestion of mud may have occurred via two different delivery mechanisms. Racers may have ingested mud after being splashed directly in the mouth or face. Alternatively, cups of water may have served as a vehicle for the ingestion of mud from racers' hands and faces. These hypotheses were examined in a multivariate model (Table 3) where adjustments for potential confounders were taken into consideration. The association between mud ingestion and illness remained significant [odds ratio (OR) 4·08, 95% CI 2·03–8·21, P<0·001] but the association between drinking cups of water and illness became insignificant (OR 2·71, 95% CI 0·82–8·95, P=0·10).
Table 3. Multivariate logistic regression analysis for direct and indirect ingestion of mud
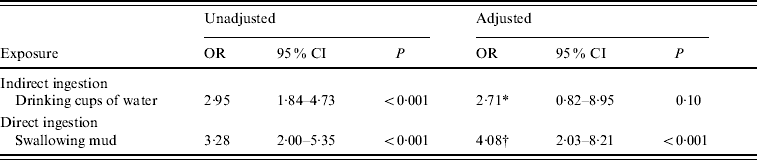
OR, Odds ratio; CI, confidence interval.
* Adjusted for age, gender, mud on hands, mud coverage and interaction term between drinking mud and cups of water.
† Adjusted for age, gender, and interaction term between being splashed in mouth and time in race pack.
Stratified data analysis was conducted to test for effect modification of mud ingestion by drinking water from cups; however, an effect modification was not seen. Recalling swallowing mud (unadjusted) (OR 3·28, 95% CI 2·00–5·35) was significantly associated with illness. When stratifying by drinking cups of water, for those who drank cups of water recalling swallowing mud was significantly associated with illness (OR 3·12, 95% CI 1·81–5·39) but it also remained significant for those who did not drink cups of water (OR 3·56, 95% CI 1·07–11·92).
Microbiological investigation
All drinking-water samples and environmental water samples tested negative for Campylobacter (n=7) as did all mud/muddy water (n=14) and animal faecal (n=6) samples. Most mud samples (12/14; 86%) tested positive for E. coli. All mud samples were positive for faecal coliforms with counts ranging from 855 to >24192/100 ml with seven having counts ⩾24192/100 ml.
MLST analysis revealed that the 14 clinical isolates tested presented identical patterns suggesting that they were clonal, belonging to ST538, which clusters within the ST45 clonal complex. This ST had not previously been seen in tested clinical C. jejuni isolates in BC.
DISCUSSION
We have described a C. jejuni outbreak associated with a mountain bike race, where the investigation points towards contaminated mud as the likely source of infection.
We used multiple mud exposure indicators in our questionnaire to reduce the risk of recall bias and elucidate ways of possible consumption. Even though the strength of the relationship with illness varied between indicators, the fact that all indicators were significantly associated with illness strengthened the argument that mud could be the source.
The collective evidence from the environmental, microbiological and multivariate analysis supports the conclusion that mud, and not drinking water, was the likely source of contamination. We show that drinking cups of water was only significantly associated with illness as a result of the role it played in mud ingestion. Racers reported inserting muddy fingers inside the water cups and photographs from race day depict racers whose faces were completely covered in mud, explaining how contamination of drinking water could occur. We tested for effect modification of mud ingestion by drinking water from cups but no effect was seen. This is not surprising as there were a number of different vehicles apart from drinking cups of water, such as direct splashing and mud on food items, which would facilitate mud ingestion. Additional data, including the bottled water testing negative for Campylobacter, the refilling of water bottles/hydration packs at official stations not being associated with illness and 25 ill individuals indicating they did not drink any water from official stations, provide further support for the conclusion that water was not the source of contamination. Racers who finished during the middle time period, when the majority of individuals completed the race, were more likely to become ill than those who finished early or late. This may be the result of increased splashing and mud coverage from other racers and therefore increased ingestion of mud at the contaminated site.
The epidemiological data point towards mud as the likely source of contamination. However, it is possible that local media reports of the preliminary findings from the investigation suggesting contaminated mud as the possible source and racers' discussion as to the potential source(s) for the outbreak on a race-related web forum may have biased racers' responses. We also cannot exclude the possibility that recall bias may have occurred. If there was a systematic difference in recalling mud exposure between the ill and well individuals then the risk estimates may be distorted. However, as explained above, many mud exposure variables were used to get at mud ingestion and all variables, not just the one about direct ingestion, were significantly associated with illness.
Risk estimates may not have been as high as might have been expected because we were not able to pinpoint a specific area of potential contamination to enquire about. Even if this was possible, recalling mud consumption weeks after the mountain bike race is challenging. Race day conditions were extremely muddy and the hands and faces of racers were covered in mud. A relatively low dose of Campylobacter is required for infection so the amount consumed may not need to have been great [Reference Black5, Reference Robinson6].
Since completing the entire race, compared to only part of it, and mud ingestion in the latter portion of the race were associated with illness, it is likely that the contamination occurred in that area of the course. Enhanced environmental and faecal sampling from this area of the race course did not yield any samples positive for Campylobacter.
However, samples were taken several weeks after the exposure, and recovery of Campylobacter from the environment is known to be difficult [Reference Ridley19]. Contamination of the mud also probably occurred at a particular spot on the course that may not have been sampled.
The presence of E. coli and high coliform counts does provide evidence that the course contained significant faecal contamination. However, it should be noted that some studies have shown no correlation between indicator bacteria and Campylobacter isolation [Reference Carter20, Reference Kemp21].
The MLST data for clinical isolates revealed that a single, rare strain of C. jejuni caused the outbreak. Two ST538 strains have previously been isolated from human cases in Canada and Australia and one in a horse with gastroenteritis in the UK [18, Reference Mickan22]. Based on a search in the pubMLST database this was the only entry of an equine-derived ST strain (ID: 1676 isolate AVH2) [18]. In some studies, C. jejuni was rarely isolated from horses [Reference Karenlampi23–Reference Prescott and Bruin-Mosch25] while Khalil et al. reported 100% of 17 horses sampled in Pakistan and Sweden carried Campylobacter [Reference Khalil26]. Horses are a potential source of contamination of the race trail as many are housed near the beginning/end of the race course and horses use parts of the trail. Strains that belong to STs which cluster within the ST45 clonal group have also been isolated from cats, dogs, chickens and humans [Reference Kakoyiannis, Winter and Marshall27].
There have been a number of campylobacteriosis outbreaks associated with bike races/tours that have not been published in indexed journals [Reference Skowronski28–31]. In 1993, there were two mountain bike race events in BC, Canada associated with C. jejuni infections. Although contamination of the water system by cow manure was considered a possible source of that outbreak, water samples tested negative [Reference Skowronski28]. Outbreaks of C. jejuni also occurred following bike races in Norway in 1997 and 1999. Both races took place in rainy conditions during a time of year when the fields near the race course were fertilized with cow manure. Laboratory confirmation of Campylobacter from a source was not possible but authors suggested splashed water ingested by riders was the likely source of the outbreaks [Reference Kapperud29]. Another outbreak of C. jejuni infection was associated with a bike tour in Virginia, USA in 2003 but the source of exposure could not be found [Reference Norton30]. Last, in Powys, Wales in 2008 a campylobacteriosis outbreak occurred in association with a mountain bike race on a course that was very muddy and contaminated with sheep faeces. The report indicates that ingestion of mud was the most statistically significant risk factor [31].
Mud and water in the environment can become contaminated with Campylobacter containing faecal matter from wildlife and/or humans. Ingestion of this mud or water can lead to illness. When there is excessive splashing of muddy water as occurs in mountain biking/cycling events in wet conditions, there is the obvious risk of mud ingestion. This outbreak and outbreaks associated with bike races at three of the four locations described above occurred in wet/muddy conditions. Therefore, racers and organizers should be educated on the potential risks of ingesting mud/muddy water from the race course. Recommendations were given to race organizers, including closing the trail to domestic animals prior to the race and reviewing the trail for any obvious signs of environmental contamination such as the use of manure or other excessive amounts of animal waste and septic tank spillage. In addition, clean running water should be available at stations to allow racers to clean mud off of hands and faces prior to eating and drinking. An alternate form of water delivery, such as bottles of water instead of paper cups, which are less easily contaminated, should also be considered. We also recommended that racers use front and rear fenders to reduce splashing of mud up onto their and other riders' faces, respectively. In 2008, the race occurred in dry conditions and no campylobacteriosis cases were reported. A follow-up assessment on the cohort to determine if there were any serious or long-term sequelae was not conducted.
Exposures to Campylobacter, related to outdoor recreational activities that occur in specific climatic conditions, may be a frequent occurrence that is not well documented. It is important to be aware of the possibility of Campylobacter contamination in the environment. A better understanding of this may provide clues to the elusive reservoir. This investigation highlights the need for increased research in environmental factors/sources for Campylobacter proliferation and highlights the fact that environmental conditions can play a role in Campylobacter outbreaks.
ACKNOWLEDGEMENTS
We acknowledge the following individuals for their contributions: Jim Lang, Gerry Woods and Len Clarkson for their assistance in navigating the bike trail and in sample collection. Sunny Mak from the BCCDC who designed the map (Fig. 1) and integrated all relevant information. Naomi Dove for assistance during the investigation and identification of ill racers and race times. Sara Forsting for assistance in developing and posting the online questionnaire. We also thank Sean Bryne and his laboratory staff at the BC Animal Health Centre for testing of faecal samples.
DECLARATION OF INTEREST
None.






