The Case
A 37-year-old Filipino male presented to a community emergency department (ED) with acute bilateral flaccid leg paralysis. He had no known medical conditions, took no medications and denied any allergies or substance use. There had been no history of trauma.
On review of systems, he reported several weeks of intermittent leg weakness. He reported that this had mainly affected his proximal leg muscles, noting difficulty when attempting to rise out of a chair. He also described intermittent palpitations, tremor of his fingers, and a 10 pound weight loss over several months. He denied any paresthesia, headache, vision change, bowel change, or bladder change.
On examination he looked well and had stable vital signs with a heart rate of 90 bpm. Cardiac and respiratory examinations were unremarkable. Lower legs demonstrated markedly diminished strength and diminished deep tendon reflexes bilaterally. Sensation to fine touch was intact. He had symmetric and normal upper limb strength, reflexes and sensation to fine touch. A fine tremor was noted in the fingertips. Cranial nerve examination was unremarkable. Head and neck exam demonstrated eyelid retraction and lid lag, as well as a goiter. See Table 1.
Table 1 Laboratory Investigations
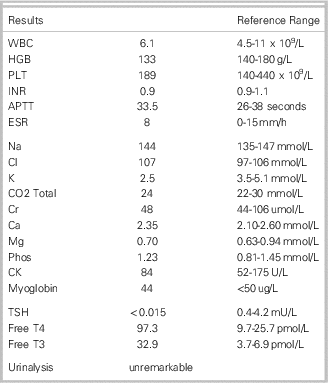
Question 1 – What is the diagnosis?
He was diagnosed with thyrotoxic periodic paralysis
Question 2 – What is the appropriate management acutely?
Under the guidance of the endocrinology service, he was treated with IV potassium supplementation (10 mEq/hr x 8 hours) with complete and rapid resolution of his muscle weakness. He was observed in the ED for 15 hours and then discharged home after regaining normal extremity strength, with a serum potassium of 4.1 mmol/L.
Question 3 – What is the appropriate management on discharge?
Our patient was started on anti-thyroid treatment with methimazole 10 mg po TID, as well as propranolol 20 mg po TID. At his one week follow-up he had experienced no further episodes of weakness, and he had gained 3 pounds. His thyroid function tests had improved (TSH <0.015 mU/L, Free T3 11.1 pmol/L, Free T4 37.1 pmol/L), and his serum potassium was 4.4 mmol/L. No changes were made to his medical management.
Thyrotoxic periodic paralysis
Periodic paralysis is a muscle disease that presents with recurrent episodes of flaccid paralysis. Graves’ disease is the most common underlying etiology, however, any cause of hyperthyroidism can be associated with TPP, including exogenous levothyroxine administration.Reference Pothiwala and Levine 1 , Reference Vijayakumar, Ashwath and Thimmappa 2 TPP most commonly effects Asian and Polynesian populations, with the majority of cases occurring in young males.Reference Pothiwala and Levine 1 - Reference Hsieh, Lyu and Chang 4
Presentation
TPP presents with sudden attacks of painless generalized weakness. Attacks most commonly last several hours, but can persist for days. Patients may have multiple attacks per week, or alternatively be symptom-free for months.Reference Hsieh, Lyu and Chang 4 - Reference Venance, Cannon and Fialho 7 The weakness preferentially affects the girdle muscles of the lower extremities; one typically finds decreased muscle tone, hyporeflexia. Tachycardia can be a sentinel finding.Reference Hsieh, Lyu and Chang 4 , Reference Pompeo, Nepa and Maddestra 5 Rarely, severe arrhythmias and respiratory muscle weakness necessitating mechanical ventilation have been documented.Reference Pompeo, Nepa and Maddestra 5 Potassium levels are variable during these attacks, with reported values as low as 1.1 mmol/L (mean ~2.1 mmol/L). Lower potassium levels are associated with increased severity of clinical weakness.Reference Pompeo, Nepa and Maddestra 5 , Reference Shiang, Cheng and Tsai 8 , Reference Li, Yang and Zhao 9
Attacks most commonly occur after large carbohydrate meals, strenuous physical activity, or stress, however, there may be no apparent precipitant. Though cold exposure is reported as a potential trigger, attacks occur more frequently in the summer.Reference Pothiwala and Levine 1 , Reference Hsieh, Lyu and Chang 4 , Reference Ober 10 , Reference Yu, Tseng and Chuang 11
Pathophysiology
Increased Na-K-ATPase activity seen with hyperthyroidism can drive potassium intracellularly. This is thought to hyperpolarize the muscle membrane, leaving the fibers inexcitable. Indirect adrenergic stimulation also increases Na-K-ATPase activity, the probable explanation for the benefit seen with beta blockers. Insulin and testosterone can also stimulate the ATP activity.Reference Chan, Shinde and Chow 12 - Reference Guerra, Rodriguez del Castillo and Battaner 14 Finally, a loss of function mutation in the potassium channel Kir2.6 is also thought to have a role.Reference Vijayakumar, Ashwath and Thimmappa 2
Management
A differential diagnosis is listed in Table 2.Reference Pothiwala and Levine 1 , Reference Vijayakumar, Ashwath and Thimmappa 2 , Reference Pompeo, Nepa and Maddestra 5 , Reference Shayne and Hart 15 Recommended acute medical treatment includes potassium supplementation.Reference Pothiwala and Levine 1 , Reference Vijayakumar, Ashwath and Thimmappa 2 , Reference Pompeo, Nepa and Maddestra 5 This has been shown to result in more rapid improvement in muscle strength, particularly when given intravenously.Reference Pompeo, Nepa and Maddestra 5 , Reference Shiang, Cheng and Tsai 8 , Reference Cesur, Bayram and Temel 16 The minimal required dose of potassium replacement is unknown, but most sources report administering 10–20 mEq per hour, with total replacement in the acute phase ranging from 40–200 mEq. Cardiac monitoring is important during treatment, as rebound hyperkalemia occurs in over 40% of cases and can potentially be fatal.Reference Vijayakumar, Ashwath and Thimmappa 2 , Reference Pompeo, Nepa and Maddestra 5 , Reference Lu, Hsu and Chiu 17 A nonselective beta blocker like propranolol can reverse muscle weakness. Propranolol can be used in patients who are unresponsive to treatment with potassium, though rebound hyperkalemia can still occur with this treatment strategy. Propranolol 3 mg/kg orally or 1 mg IV repeated Q10 minutes (maximum 3 mg) has been reported to be effective.Reference Shayne and Hart 15 , Reference Lin and Lin 18 - Reference Birkhahn, Gaeta and Melniker 20
Table 2 Differential Diagnosis of Acute Weakness

While potassium replacement is important in the acute phase, management of patients’ hyperthyroidism is also necessary to prevent further attacks. Prior to achieving a euthyroid state, temporizing doses of propranolol ranging from 20–80 mg po Q6–8 h have been shown to reduce the frequency and severity of attacks.Reference Vijayakumar, Ashwath and Thimmappa 2 , Reference Pompeo, Nepa and Maddestra 5 , Reference Shayne and Hart 15 , Reference Conway, Seibel and Eaton 21 Susceptible patients should avoid potential triggers as described earlier.Reference Vijayakumar, Ashwath and Thimmappa 2
Competing interests: None declared.



