Introduction
Background
Malaria during pregnancy is a significant source of concern in public health because of the negative repercussions it can have, not only on the mother but also on the developing foetus [Reference Adam, Ibrahim and Elhardello1]. According to the World Malaria Report by World Health Organization (WHO), there were 241 million cases of malaria in the year 2020 in 85 malaria endemic countries, an increase from the 227 million cases in 2019 [Reference Aleem and Bhutta2]). Concurrently, around 33.8 million pregnancies occurred during the same duration, with 34 percent of women accounting to 11.6 million being exposed to malaria infection during pregnancy [Reference Aleem and Bhutta2]).
According to literature, there are two types of malaria that can occur during pregnancy: placental malaria (PM) and gestational malaria (GM), both of which are diagnosed by demonstrating the presence of Plasmodium spp. in the placenta or the mother’s peripheral blood using a thick blood smear (TBS), polymerase chain reaction (PCR), or rapid diagnostic tests [Reference Almaw3]. Simple, quick, and more convenient, rapid diagnostic techniques have great potential in malaria detection. They may be of great utility as helpful instruments in the global delivery of health services by improving overall diagnosis of malaria infections. However, the testing procedure must be improved further to overcome the shortcomings of the present implementation. In spite of its drawbacks, such as time and expense, PCR remains the gold standard for identification of malaria parasites [Reference Balduzzi, Rücker and Schwarzer4].
Several unfavourable effects have been reported to occur after parasite sequestration, including maternal anaemia, foetal growth restriction, abortion or stillbirth, premature delivery, and low birthweight (LBW) [Reference Cardona-Arias and Carmona-Fonseca5]. Malaria contributes to up to 26% of cases of severe anaemia during pregnancy in endemic regions, and it is responsible for between 0.5 and 23% of all maternal fatalities caused by malaria [Reference De Beaudrap6]. In sub-Saharan Africa, malaria during pregnancy is responsible for up to 20% of LBW, or 35% of all avoidable LBW [Reference Dellicour7, Reference Desai8]. Successful malaria preventive measures during pregnancy have been shown to reduce perinatal death by 27% [Reference Dellicour7].
In malaria-endemic regions, pregnancy and the disease have been shown to worsen each other, especially for first-time mothers and individuals who were previously resistant to malaria. Though it has been previously reported that multigravida bear the heaviest burden of malaria in pregnancy both in terms of prevalence and outcome, it is now widely acknowledged that women with greater gravidities, even in areas of low transmission, are also susceptible [Reference Dellicour7].
About 125 million pregnant women worldwide are at risk of contracting malaria caused by either Plasmodium falciparum or Plasmodium vivax each year [Reference Dosoo9]. While Plasmodium falciparum malaria is responsible for most of the malaria-related morbidity, Plasmodium vivax may also play a crucial role in certain regions of South America and Southeast Asia [Reference Falade10]. A systematic review of sub-Saharan Africa concluded that the prevalence of Plasmodium falciparum was (22.1%, 95% CI: 17.1–27.2 %), followed by Plasmodium vivax 3% (95%CI: 0–5%), Plasmodium malariae 0.8% (95%CI: 0.3–0.13%), and Plasmodium ovale 0.2% (95%CI: −0.01–0.5) [Reference Furuya-Kanamori, Barendregt and Doi11]. Similarly, another meta-analysis has shown a significant incidence of malaria in pregnancy in Colombia, which emphasizes the urgent need for researchers, research funding organizations, government agencies, and health authorities to pay more attention to its research and intervention [Reference Guyatt and Snow12].
Based on the significant burden of malaria on the pregnancy outcomes and the health of pregnant women, marked variation in the available evidence is recorded due to diagnostic technique variability, heterogeneity in the enormity of disease, low sample size in some studies, lack of solid meta-analysis of relevant literature, and a substantial lack of understanding on the prevalence of malaria associated in pregnancy, which highlights the significance of a systematic review to quantify the prevalence of disease and understand the underpinnings pertaining to the causality and the burden of outcomes associated. Thus, the current review aims to assess the overall prevalence of malaria in pregnancy along with time-specific burden, that is, during antenatal visits and during delivery and to deduce the specie-specific and regional prevalence of infection. Secondarily, the review also aims to estimate the proportions of adverse pregnancy outcomes and its association with the presence of malarial infection.
Methods
Study design
Using the guidelines provided by ‘Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)’, a systematic review was conducted. Comprising of a 27-component checklist, the PRISMA guidelines aids in producing a transparent and coherent review which can be easily understood and interpretated globally [Reference Guyatt and Snow13].
Data source and searches
To find relevant articles, a thorough and systematic search was conducted on 31 July 2023 across two electronic databases (including PubMed and CENTRAL) using precise and accurate search strategies. Publications from the year 2000 to 2023 were searched using database specific strategies. To ensure completeness and entirety, manual searches were also conducted in addition to cross-referencing of source articles to avoid missing out any important source of evidence.
Search strategies
Based on the MeSH terminologies specific to the objectives and aims of the study, the following search strategy was developed to retrieve studies from databases.
(“Malaria”[Mesh] OR “Malaria, Vivax”[Mesh] OR “Malaria, Falciparum”[Mesh] OR “P. vivax malaria” OR “P. falciparum” OR “maternal malarial” OR “congenital malaria” OR “foetal malaria” OR “malaria in pregnancy” OR “malaria in pregnant”) AND ("Pregnancy"[Mesh:NoExp] OR pregnancy OR pregnant OR “malaria in pregnancy” OR pregnant women OR pregnant woman) AND (parasite densities OR diagnostic test* OR diagnostic* OR endemicity OR Intermittent Preventive Treatment OR IPT OR Intermittent Preventive Therapy OR Insecticide Treated Nets OR drug therapies)
Eligibility criteria
All the studies quantifying the burden of malaria in pregnancy along with the impact of Plasmodium falciparum and vivax on maternal and child adverse outcomes were taken into consideration. The studies considered eligible were those that were published after the year 2000, were in English language, and catered human subjects only.
The exclusion criteria involved: (1) Clinical trials in which the randomization was done on a predefined criterion; (2) Cohort studies in which the exposure of interest was malaria cases; (3) Case control studies in which the cases were malaria patients as this would not enumerate the burden; (4) Study designs including case reports, case series, commentaries, editorials, narrative reviews, and systematic reviews; (5) Studies using data from previous publications of the author.
To avoid double-counting/the same data being pooled more than once, data reported from different studies, such as those by the same authors, were checked to ensure patient cohorts were non-overlapping.
Study selection and data extraction
Articles retrieved from the databases were screened by two independent reviewers at a title and abstract level. Articles not immediately ruled out as irrelevant were then reviewed in a similar manner on a full-text basis. Where the relevance of an article was deemed ambiguous, or reviewer decisions conflicted, consensus was reached amongst the authors. Data were then extracted from each included article by a reviewer.
Extracted parameters included author names, publication year, location of study, diagnostic test used for malaria, malaria case count, strain of organism involved, time point in pregnancy at which diagnosis was made, sample size, and calculated prevalence. Additionally, where reported, data were extracted on complications and adverse outcomes for the pregnant women and their foetuses/offspring, for both test-positive and test-negative pregnant women. These data were used to perform secondary analyses to evaluate the association between malaria and maternal and infant morbidity.
Some studies reported adjusted odds ratios but not dichotomized data. Due to the non-uniformity in the method by which these odds ratios were computed, pooling them was deemed invalid and they were not extracted for meta-analysis.
Studies using multiple diagnostic modalities
Certain included studies tested the same subjects at the time time point for malaria using multiple diagnostic tools. Based on the evidence, a hierarchy of selection was determined to prefer PCR data, followed by microscopy, and then rapid diagnostic tests [Reference Guyatt and Snow13, Reference Kaforau14]. In this manner, the most reliable data for a cohort at a given time point were pooled in the analysis without double or triple counting.
Studies reporting prevalence of multiple strains or at multiple time points
Some included studies did not explicitly state an overall prevalence of malaria but reported prevalence in a strain-wise fashion. In these cases, it was evaluated if the reported patient positive for different strains of malaria were non-overlapping groups. Where this condition was met, the groups were combined, and the overall prevalence was calculated and utilized in the analysis.
Similarly, some studies reported prevalence data for a cohort during ANC and then again during delivery. Given that these estimates were taken at distinct points in time, they were considered separate datapoints and pooled in overall estimates of prevalence.
Peripheral and placental malaria
Where studies clearly reported overall prevalence data, the data were extracted and analysed simply. However, some studies reported results having tested participants for both peripheral and placental malaria. In such cases, data on peripheral infection were pooled and analysed and placental infection data were only used if that on peripheral infection was not reported.
Data analysis
The proportions of pregnant women who tested positive for malaria using any diagnostic technique were tabulated. Similarly, the proportions of pregnant women with adverse pregnancy outcomes were also recorded for both test-positive and test-negative women.
Along with confidence intervals of 95%, the following quantitative assessments of malaria were deduced:
-
1. Overall prevalence of malaria in pregnancy irrespective of the diagnostic test used, period of pregnancy and organism involved.
-
2. Prevalence of infection during antenatal care and at delivery.
-
3. Regional disparities of malaria proportions according to UNICEF regions.
-
4. Association of malaria with adverse pregnancy outcomes.
Due to heterogeneity caused by experimental differences between the included articles, all reported results were computed using a random-effects model meta-analysis. Point estimates and 95% confidence intervals are reported, while heterogeneity was evaluated using the Tau-squared and I-squared metrics, which represent the variance of the distribution of estimates reported by included studies and the percentage of that variation not attributable to sampling error, respectively. Forest plots were constructed for each outcome of interest highlighting the effect measure, confidence interval, sample size, and its associated weightage. Both pooled estimates and sub-groups estimates were illustrated using effective plots.
Publication biases were assessed using DOI plots and LFK index [Reference Kaforau14]. The sensitivity analysis was conducted through the leave-one-out method. This method recalculates the effect sizes and heterogeneity by removing one study each time [Reference Kattenberg15]. Additionally, meta regression analyses were conducted to evaluate differences in proportions within subgroups of region, species, and diagnostic test.
R-Studio version 2022.07.1 was used to carry out the meta-analysis using the package ‘meta’ (version 6.1.0) [Reference Lawn16], and a p-value of less than 0.05 was taken as benchmark of significance.
Quality assessment
Each study included in the systematic review underwent a quality assessment to evaluate the research methodology employed in each study to ensure the reliability and validity of its findings. The Joanna Briggs Institute (JBI) critical appraisal tools, widely acknowledged and reliable for quality assessment, were used to investigate each study [Reference Mabrouk17]. It covers variations of study designs including analytical cross-sectional analysis, case–control, and cohort studies which were used to report the quality of studies in this systematic review. This tool aims to understand the extent to which the study has considered the potential bias in its design and implementation. An overview of the results has been provided in the tables.
Results
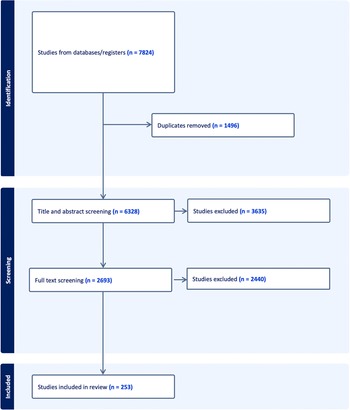
Figure 1 below depicts the selection process of the studies included in the review. Initially, 7824 studies were retrieved out of which only 253 qualified for the final inclusion.
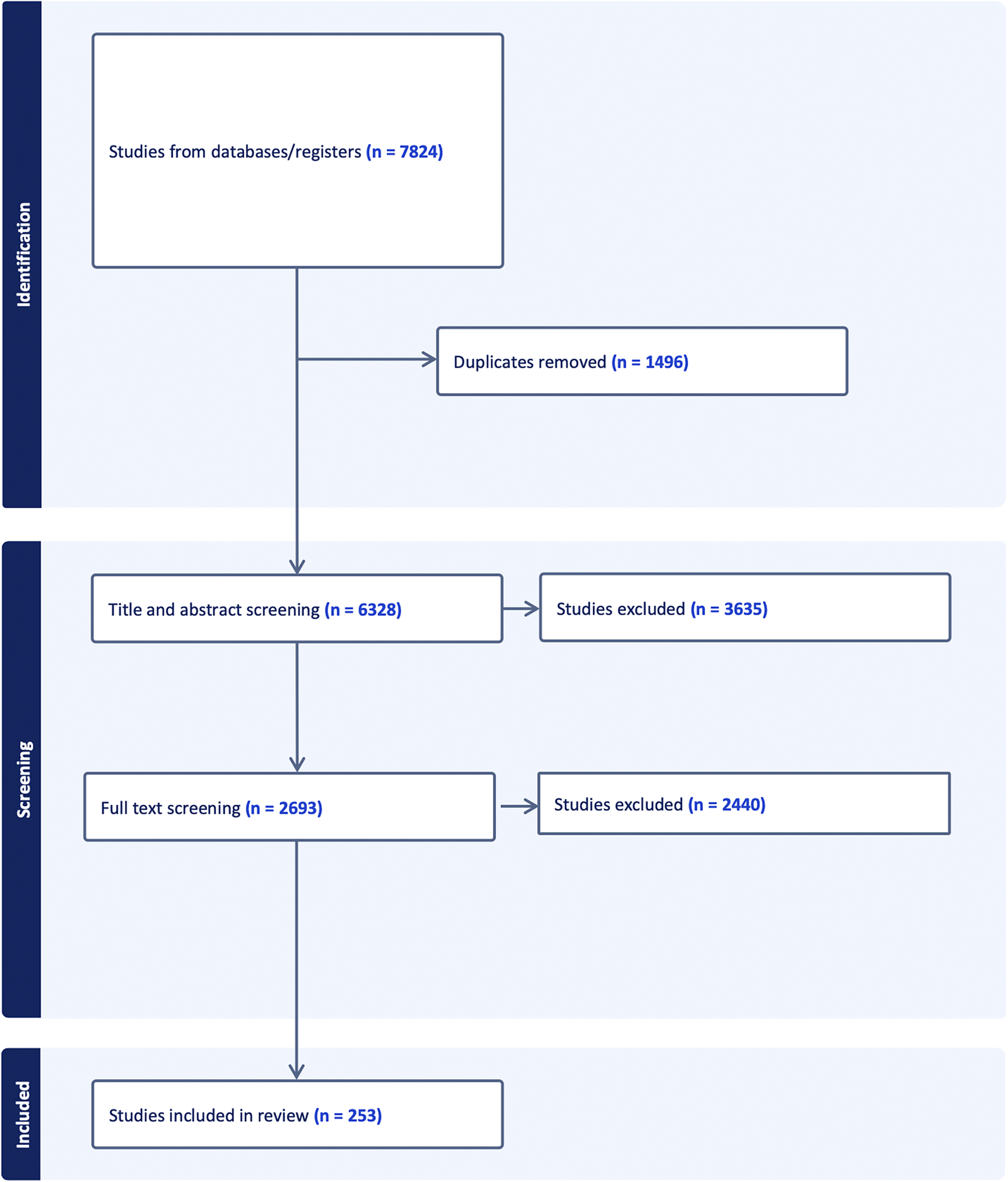
Figure 1. PRISMA diagram of included studies.
The characteristics of the included studies including the author and the year, title, study design, region, sample size, point of pregnancy at which the data were recorded, and diagnostic test used are summarized in Table 1 below.
Table 1. Characteristics of included studies

Prevalence trends
Supplementary Figure 2 shows overall trends of prevalence of malaria in an ascending order of years, estimated from 253 studies. As evident, the proportions have remained relatively persistent with the passing years and no significant reduction has been observed from the year 2000 to year 2023.
According to the pooled estimates, the prevalence of malaria was 18.95% (95% CI: 16.95–21.11, n=375,792) based on random-effects model. Similarly, when bifurcated on the time of reporting, the prevalence of malaria during antenatal visits was 20.09% (95% CI: 17.43–23.06, n =282,169, studies = 182) and during delivery was 17.32% (95% CI: 14.47–20.61, n = 93,623, studies = 121) using the same random-effects model. The heterogeneity was deduced using I-squared test, which was reported to be 99% in each model. Sensitivity analysis showed no change in the heterogeneity (Supplementary Appendix Figure 1a). The DOI plot was symmetrical indicating no publication bias (Supplementary Appendix Figure 1b).
Specie-specific prevalence
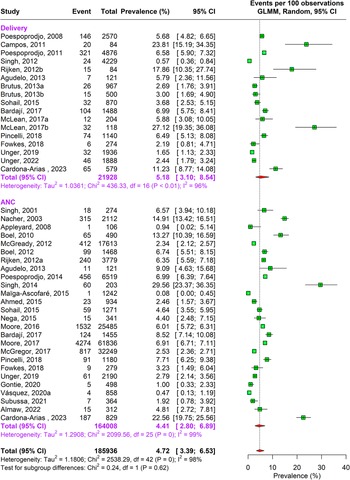
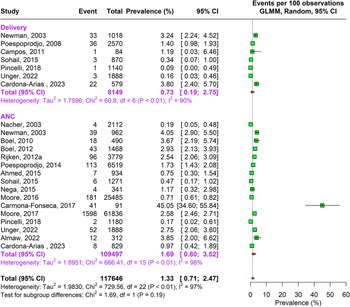
During the antenatal period, the prevalence of malaria caused by Plasmodium falciparum alone was 17.76% (95% CI: 15.04–20.85, n = 269,537, studies = 166) using random-effects model. This was followed by Plasmodium vivax caused infections accounting to 4.41% (95% CI: 2.80–6.89, n = 164,008, studies = 26) prevalence. In about 1.69% (95% CI: 0.80–3.52, n = 109,497, studies = 16) pregnant women, traces of both Plasmodium falciparum and vivax species were found as shown in Supplementary Figure 3a and Figures 2 and 3.
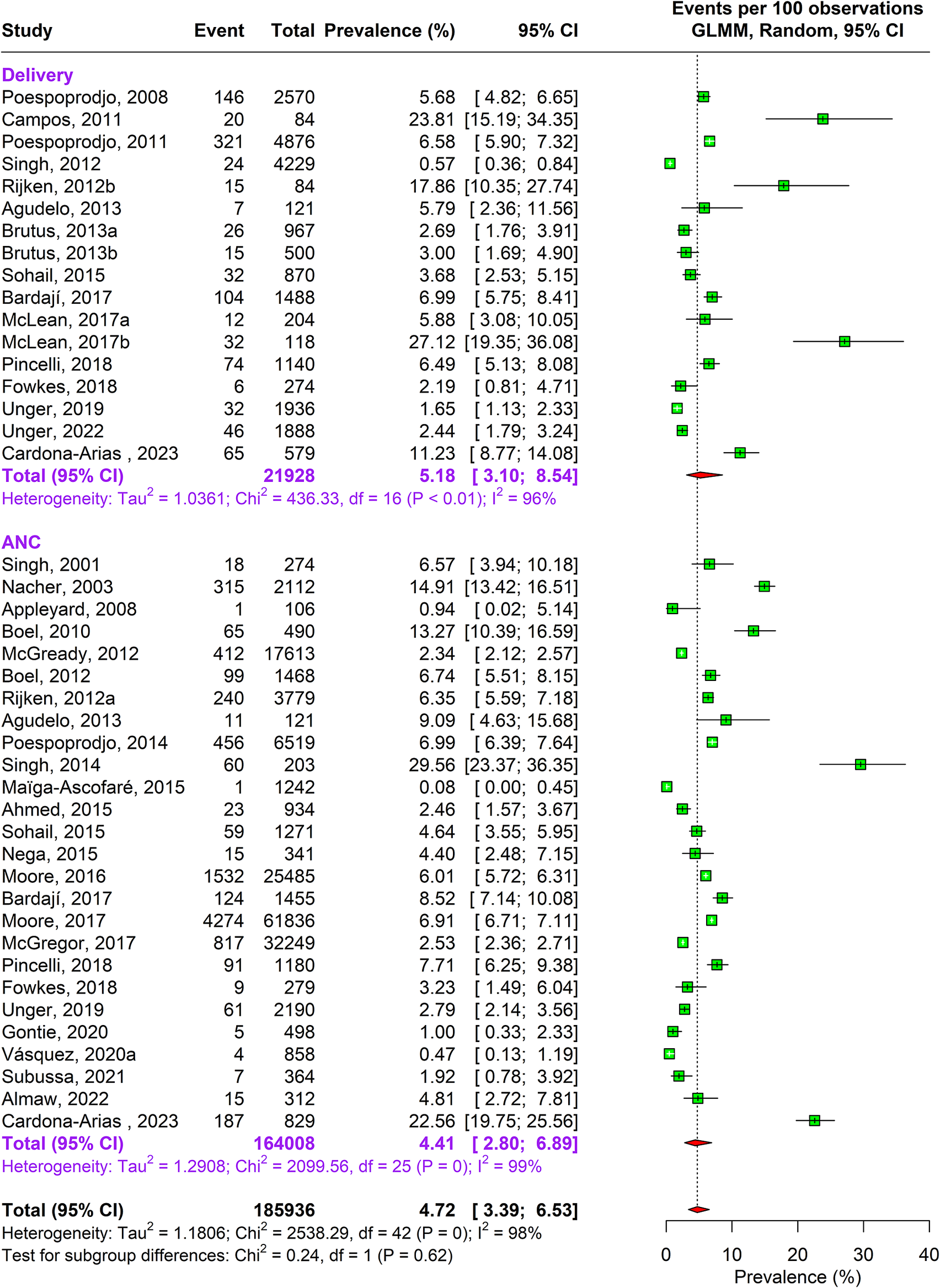
Figure 2. Forest Plot depicting Plasmodium vivax pooled estimates of prevalence of malaria with 95% CIs.
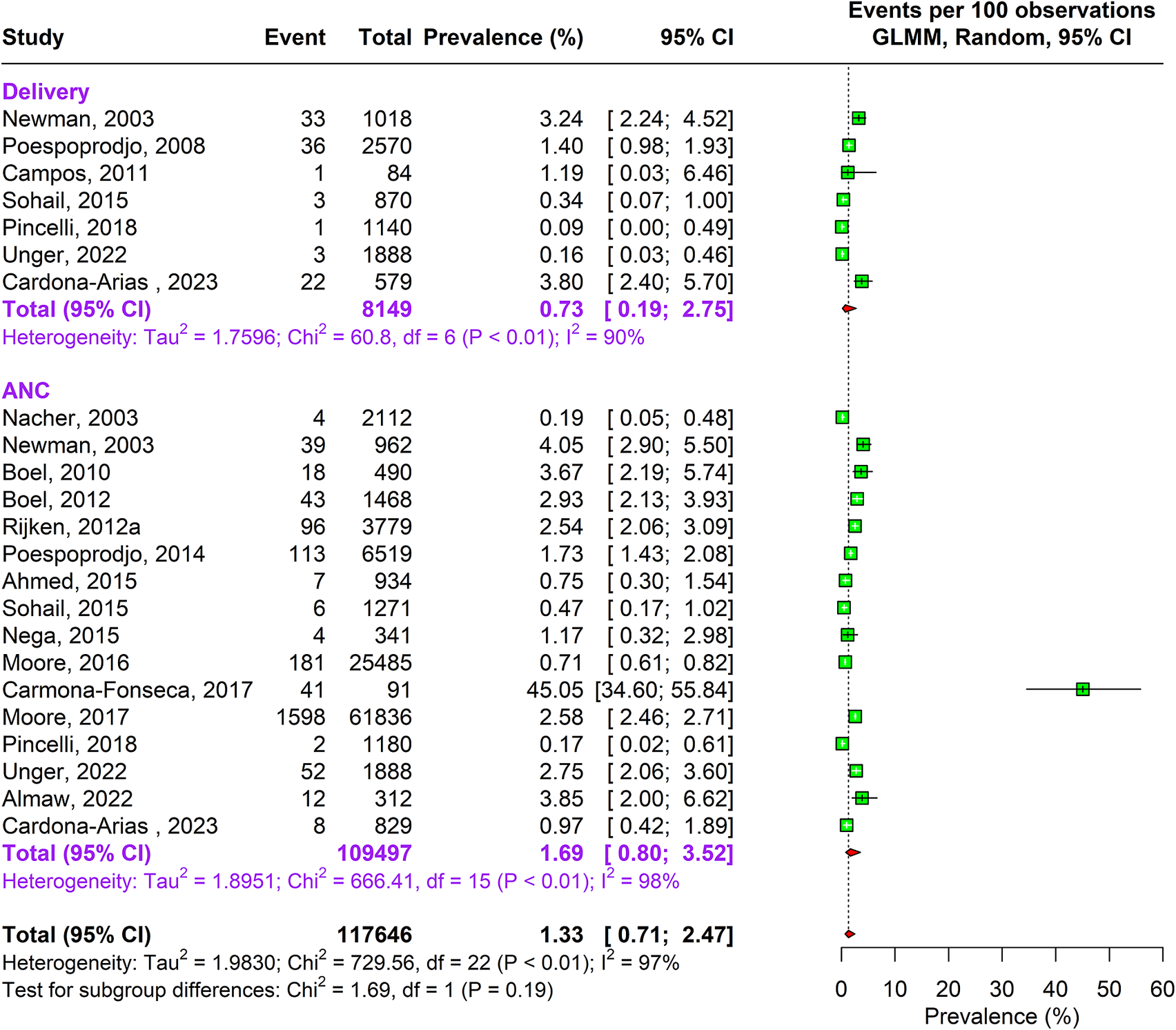
Figure 3. Forest Plot depicting Plasmodium falciparum and vivax pooled estimates of prevalence of malaria with 95% CIs.
A similar pattern of infection was observed during delivery. Approximately 16.55% (95% CI: 13.57–20.04, n= 73,417, studies = 113) pregnant women were infected by Plasmodium falciparum and 5.18% (95% CI: 3.10–8.54, n= 21,928 studies = 17) by Plasmodium vivax, and 0.73% (95% CI: 0.19–2.75, n = 8149, studies = 7) were infected by both Plasmodium falciparum and vivax. The sensitivity analysis showed no change in heterogeneity (Supplementary Appendix Figure 3a–c). The DOI plots showed no asymmetry for Plasmodium falciparum but for Plasmodium vivax alone and combined vivax and falciparum thus concluding positive publication bias (Supplementary Appendix Figure 2a–c).
Regional distribution of malarial infection
The meta-analysis revealed that the highest proportion of malarial infection during ANC was observed in Africa approximating 21.50% (95% CI: 18.52–24.81, n = 110,012, studies = 143). This was followed East Asia and Pacific region accounting to 17.28% (95% CI: 9.29–29.86, n = 157,986, studies = 18). The lowest prevalence was observed in South Asia 8.66% (95% CI: 3.06–22.17, n = 8,513, studies = 9) followed by Latin America and Caribbean region 14.20% (95% CI: 6.31–28.91, n = 3,929, studies = 7) as shown in Supplementary Figure 4a. Sensitivity analysis revealed no significant difference. A symmetrical DOI plot was also indicative of no publication bias (Supplementary Appendix Figures 4a and 5a).
A similar random-effects meta-analysis at the time of delivery revealed that the prevalence of malaria in Africa was 20.41% (95% CI: 17.04–24.24, n = 46,925, studies = 95), in East Asia in Pacific Region was 16.33% (95% CI: 8.46–29.19, n = 22,214, studies =12), in Latin America and Caribbean region was 5.28% (95% CI: 2.68–10.12, n = 4,834, studies = 7), and in South Asia was 4.14% (95% CI: 1.52–10.80, n = 19,071, studies = 6) as shown in Supplementary Figure 4b. Sensitivity analysis revealed no significant difference. On the other hand, DOI for delivery showed minor asymmetry favouring positive publication bias (Supplementary Appendix Figure 5b).
Associations with prevalence of malaria
Adverse pregnancy outcomes have shown mild-to-moderate associations with the prevalence of malarial infection in pregnancy.
Anaemia
A statistically significant association was observed between anaemia and malaria presence in 62 studies as shown in Figure 4. The odds of having anaemia were 2.40 times (95% CI: 1.87–3.06) in malaria-positive women as compared to malaria-negative women. The heterogeneity of the studies as calculated with I-squared value was 86%. Sensitivity analysis revealed that the effect size of meta-analysis was deviating significantly due to two studies; hence, they were excluded (Supplementary Appendix Figure 6). The DOI plot showed minor asymmetry thus depicting minimal publication bias (Supplementary Appendix Figure 7).
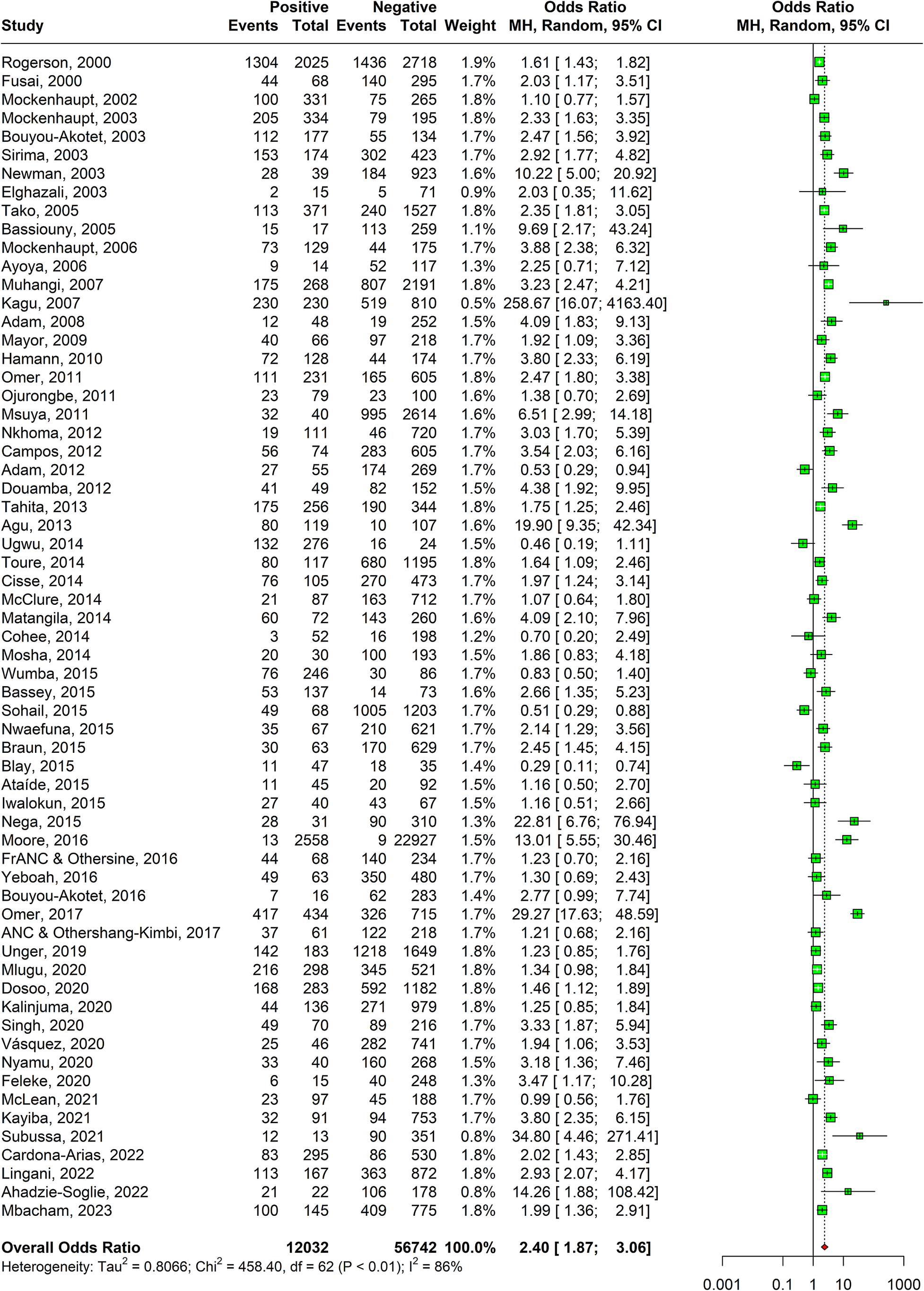
Figure 4. Forest plot confirming association of malaria in pregnancy and anaemia.
Low birthweight
A significant association of low birthweight of the babies and malaria-positive women was also observed after pooling estimates from 42 studies as shown in Figure 5. The overall odds ratio deduced was 1.99 (95% CI: 1.60–2.48). Sensitivity analyses revealed that two studies were responsible for major deviation in the effect size; hence, they were excluded. Absence of publication bias was confirmed by symmetrical DOI plot (Supplementary Appendix Figure 9).
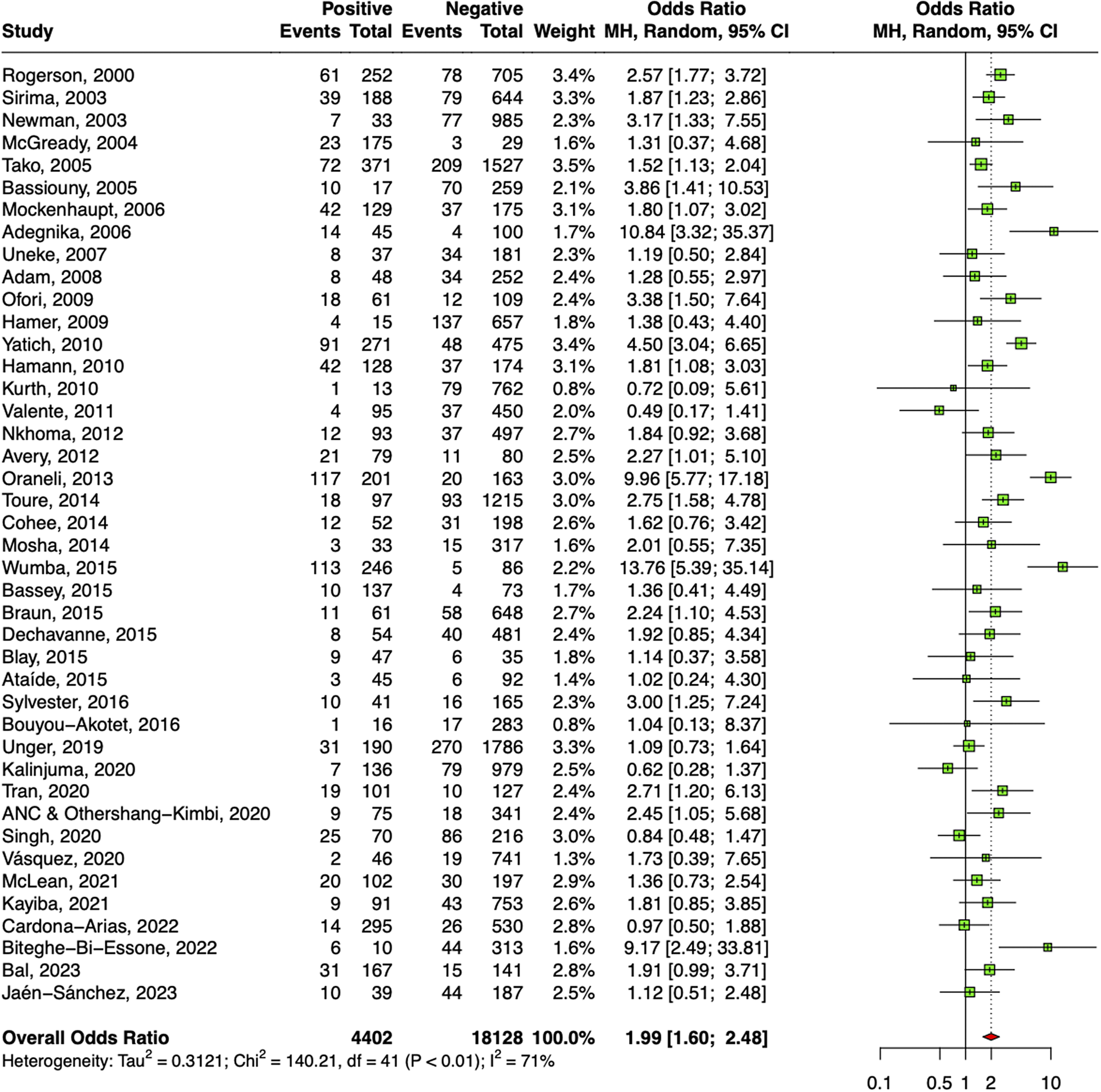
Figure 5. Forest plot confirming association of malaria in pregnancy and LBW.
Pre-term birth
A positive relation between malaria in pregnancy and preterm births was observed in 24 studies with an overall odds ratio of 1.65 (95% CI: 1.29–2.10) as shown in Figure 6. The random-effects model took into consideration the heterogeneity of 49% as calculated by I-squared value. Sensitivity analysis revealed that the effect size of meta-analysis was deviating significantly due to one study; hence, it was excluded. The DOI plot showed major asymmetry, thus indicating positive publication bias (Supplementary Appendix Figure 11).
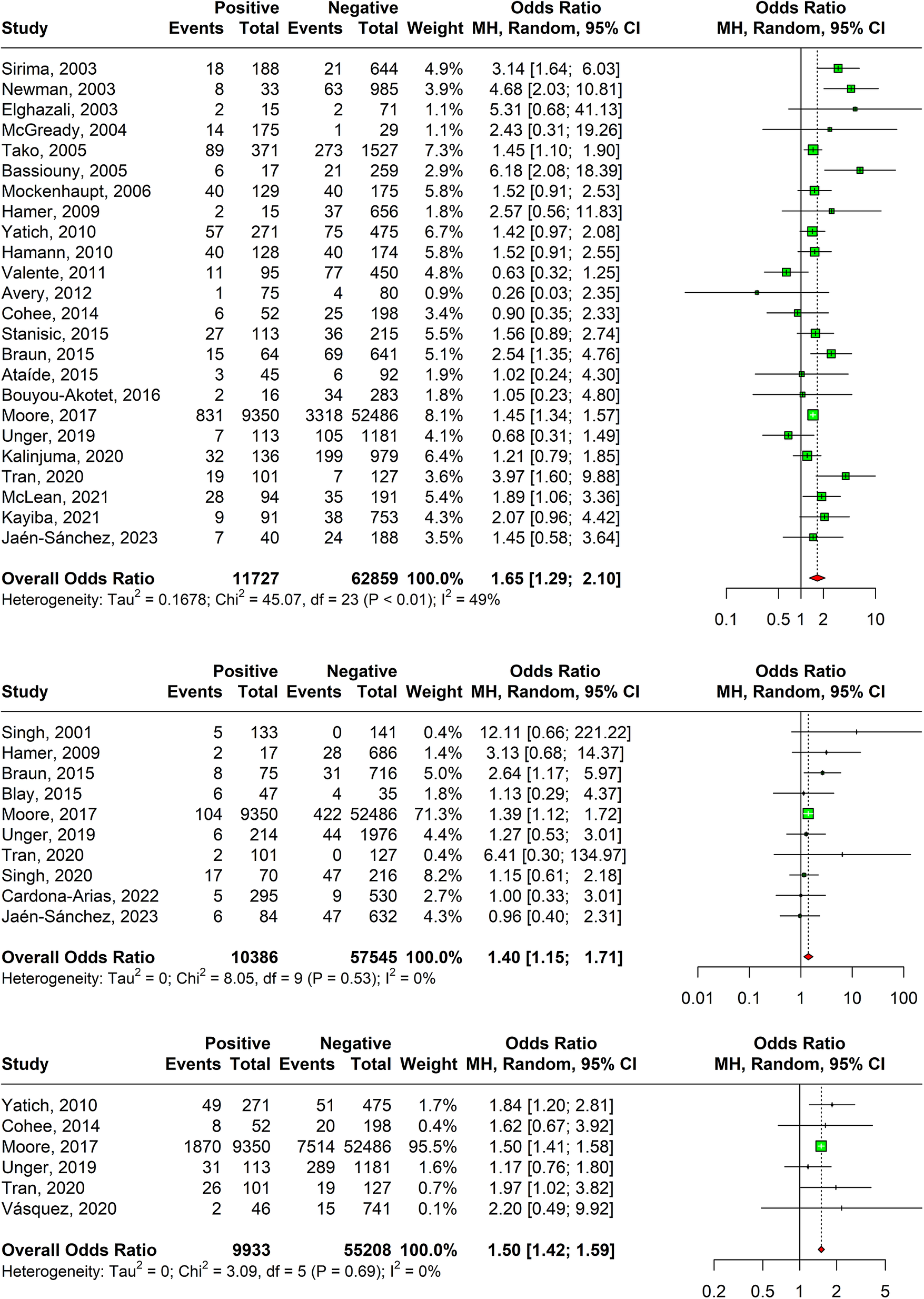
Figure 6. (a) Forest plot confirming association of malaria in pregnancy and preterm births. (b) Forest plot confirming association of malaria in pregnancy and stillbirths. (c) Forest plot confirming association of malaria in pregnancy and SGA.
Stillbirth
A statistically significant association was observed between stillbirths amongst malaria test-positive pregnant women with and odds ratio of 1.40 (95% CI: 1.15–1.71) based on ten studies as shown in Figure 6b. Sensitivity analyses revealed that one study was responsible for major deviation in the effect size; hence, it was excluded. The DOI plot showed major asymmetry, thus indicating positive publication bias (Supplementary Appendix Figure 13).
Small for gestational age (SGA)
A significant association has been observed between SGA and pregnancy malaria with an overall odds ratio of 1.50 (95% CI: 1.42–1.59) 1.39 (95% CI: 0.99–1.96) using estimates of six studies as shown in Figure 6c. Sensitivity analysis revealed that the effect size of meta-analysis was deviating significantly due to one study; hence, it was excluded. The DOI plot shows minor asymmetry, thus depicting minimal publication bias (Supplementary Appendix Figure 15).
Abortion
An insignificant statistical association was observed in abortion and malaria in pregnancy with an odds ratio of 0.85 (95% CI: 0.21–3.48) using estimates from five studies (Supplementary Appendix Figure 16). Sensitivity analyses revealed that two studies were responsible for major deviation in the effect size; hence, they were excluded. The DOI plot showed major asymmetry, thus confirming negative publication bias (Supplementary Appendix Figure 17).
Preeclampsia
A statistically insignificant association was seen with pre-eclampsia using the estimates from three studies with an odds ratio of 0.82 (95% CI: 0.16–4.34). Sensitivity analyses revealed that one study was responsible for major deviation in the effect size; hence, it was excluded (Supplementary Appendix Figure 18). The DOI showed no asymmetry, thus confirming absence of publication bias (Supplementary Appendix Figure 19).
Growth restriction
A statistically insignificant association was seen with growth restriction using the estimates from two studies with an odds ratio of 1.21 (95% CI: 0.04–35.52, n= 508). There was no change in effect observed during sensitivity analysis (Supplementary Appendix Figure 20). The DOI showed major asymmetry, thus confirming negative publication bias (Supplementary Appendix Figure 21).
Meta regression
Results of meta regression analyses for region, diagnostic test, and specie variables are displayed in Table 2 Test of moderators were found significant in both region (p < 0.001) and specie (p-value < 0.01), indicating a significant influence on the effect sizes. The R-squared for region showed that 10.45% of the difference in the true effect sizes can be explained by the region, and 3.67% by the specie, and 1.22% by the diagnostic variable.
Table 2. Meta regression analysis of effect size with respect to region, diagnostic tests, and specie
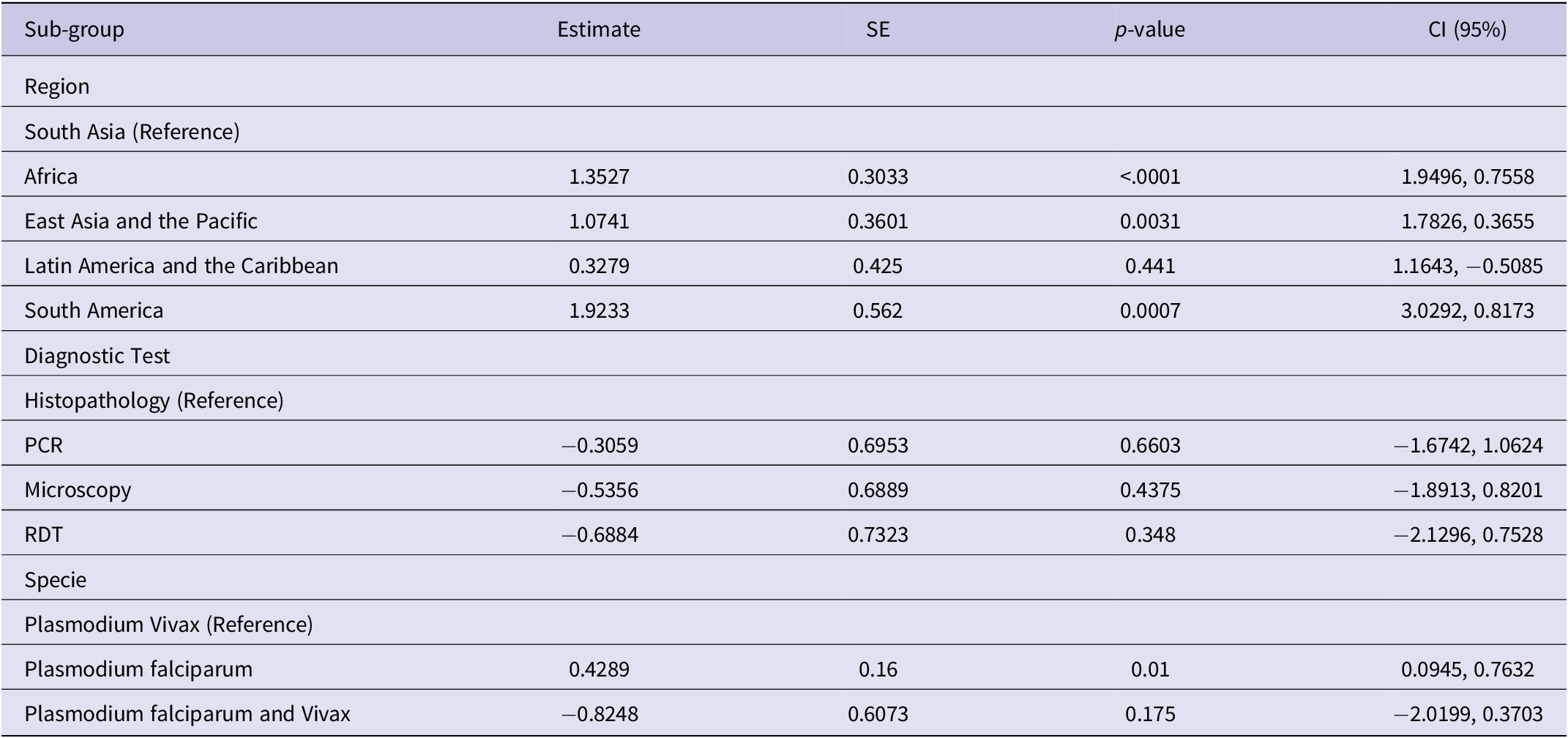
For meta-regression analysis by region, South America had the highest effect sizes when compared with South Asia (b=1.92, p < 0.001) which was followed by Africa (b=1.35, p < 0.001). Conversely, the effect sizes for the East Asia and Pacific were relatively lower (b=1.07, p < 0.01).
None of the diagnostic tests showed a significant difference in effect sizes when compared with histopathology, as evident. With respect to specie, Plasmodium falciparum was the only specie with significantly higher effect size when compared to Plasmodium vivax in the meta regression analysis by specie.
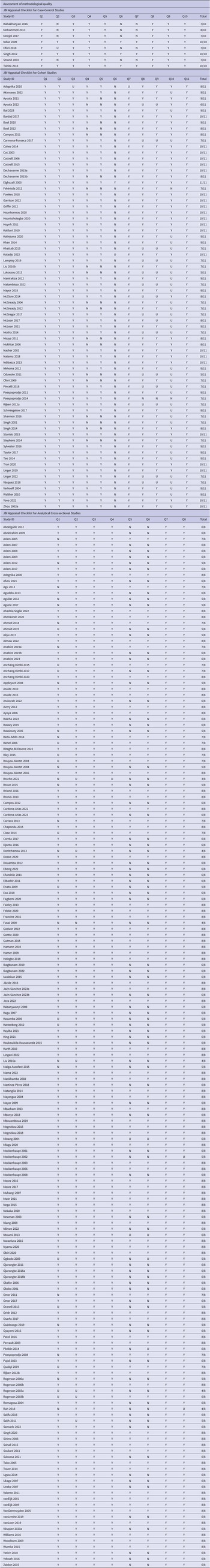
Quality assessment
All studies were included in the review after quality assessment. The JBI checklists for case–control, cohort, and cross-sectional studies were used according to the study designs (Table 3). Each study was scored out of the number of questions included in the checklist. The highest score was 10 for case–control studies, 11 for cohort studies, and 8 for cross-sectional studies.
Table 3. JBI appraisal checklist for included studies
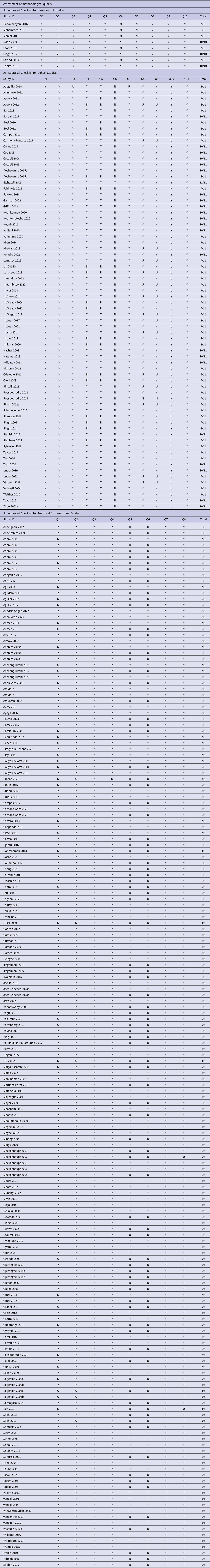
Abbreviations: Y, Yes; N, No; U, Unclear; N/A, Not Applicable.
Out of the 8 case–control studies, three studies scored 10/10, one study scored 8/10, and four studies scored 7/10. Of the 71 cohort studies, one study scored 11/11, twenty-two studies scored 10/11, seventeen studies scored 8/11, nineteen studies scored 7/11, one study scored 6/11, and two studies scored 5/11. Of the 174 cross-sectional studies, seventy-one studies scored 8/8, fifteen studies scored 7/8, sixty-three studies scored 6/8, nineteen studies scored 5/8, five studies scored 4/8, and one study scored 3/8.
The most common problems that came across overall were the identification of confounding factors and strategies to deal with confounding factors were not mentioned clearly. In the cohort studies, the most common problem was that the subjects were not free of the outcome at the start of the study and strategies to deal with incomplete follow-up were not clearly mentioned.
Discussion
Malaria in pregnancy is a cause of extensive morbidity and mortality globally, both among infectious diseases and overall. While numerous studies have estimated the rate of infection in different regions, this meta-analysis synthesizes an immense volume of data to describe the overall prevalence and distribution of the disease. The findings of our study highlight that prevalence of malaria varies geographically, temporally, and species specifically. Amongst the many virulent species, Plasmodium falciparum has been the cause of highest incidence of infection. Similarly, African region has shown highest regional prevalence amongst the other regions. In addition, prevalence was higher during the antenatal visits as opposed to at delivery.
In addition, we have secondarily analysed and demonstrated that several morbid disease states and outcomes, such as anaemia, low birthweight, preterm birth, and stillbirth, may be significantly associated with malaria during pregnancy. These detrimental factors to the well-being and survival of mothers and their infants may influence maldevelopment and poor health in individuals throughout the life-course if left unaddressed.
As estimated by our study, Africa presents with the highest burden of malaria in pregnancy. This is in line with studies conducted earlier in the region and the report presented by the World Health Organization [Reference Aleem and Bhutta2, Reference Matteelli18–Reference Moya-Alvarez, Abellana and Cot20]. This may be due to malarial endemicity of the region as it is considered as the most tropical continent, coupled with higher transmissibility of the infection. This endemicity is the product of a complex interplay of environmental, biological, and socio-economic factors. Tropical climates with appropriate temperature, humidity, and rainfall conditions encourage endemicity of the disease as they are conducive to the reproduction of the parasite within the anopheles’ mosquito, which is itself native to these environments [Reference Mabrouk17].
However, this natural localization of malaria is compounded by a lack of robust and resilient health systems in many of the affected countries, where poverty, conflict, and natural disasters often further limit the impact of concerted public health efforts to tackle the disease [Reference Kaforau14, Reference Kattenberg15]. To counter, preventive measures and immunogenicity of the population play a very significant role in combatting the pathogenesis of disease in any geographical region. Thus, the prevalence has reduced within Africa but is still the highest amongst other regions [Reference Munn21]. Even though the studies of Africa have shown a significant reduction in the prevalence of malaria, it is worth noting that these measures have not accounted for all the countries in the region, hence limiting its generalizability [Reference Furuya-Kanamori, Barendregt and Doi11].
In this study, we also observed that Plasmodium falciparum was responsible for the pathogenicity of the majority of infections. Several systematic reviews have confirmed that P. falciparum is the highest inhabited organism in pregnancy to cause the infection [Reference Dellicour7, Reference Ndifreke Edem, Okon Mbong and Hussain22]. Our study’s findings of a disproportionately high prevalence of this organism of malaria underscore the importance of taking strong measures to prevent and manage the disease, especially among pregnant women. While the WHO malaria 2016 report found that over 99% of malaria cases were attributable to P. falciparum, our analysis found a smaller proportion of P. falciparum-causing illnesses [Reference Otten23]). Extreme seasonal, interannual, and geographical fluctuation may be responsible for these shifts. Possible causes include dissimilarities in development and housing patterns, population migration, as well as climatic (temperature, precipitation, and relative humidity) factors.
The study assessment also revealed that malaria-positive women were more prone to encounter anaemia. Several meta-analyses support our findings as the overall odds of malaria of anaemia are higher amongst pregnant women with malaria [Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald, McGuinness, Stewart, Thomas, Tricco, Welch, Whiting and Moher24]. According to a review, malaria is responsible for an estimated 26% of the severe anaemia experienced by pregnant women of all gravities (population attributable fraction) [Reference Dellicour7]. Anaemia is strongly linked to malaria, although the underlying pathophysiology is poorly understood. Nonetheless, illness-related inadequate food intake, haemolysis, and a lack of micronutrients are all viable justifications for anaemia and malaria.
Association of low birthweight with the presence of maternal malaria was amongst the deductions from our study. This is validated by other reviews conducted that suggest the same statistically significant association between malaria in pregnancy and low birthweight of the baby [Reference Rayco-Solon, Fulford and Prentice25]. Around 19% of LBWs and 6% of LBW-related infant fatalities are attributed to malaria in regions where the disease is endemic. According to these estimates, over 100,000 infants die each year in parts of Africa where malaria is common because to LBW [Reference Rogerson and Unger26].
Augmenting with the findings of our study related to preterm babies and malaria exposure, several reviews have reported malaria to be the primary infection in pregnancy that can be associated with the PTB [Reference Willis and Riley27]. Moreover, PTB seasonality patterns were also observed in some studies to be paralleling those of malaria infection, with its peak occurring with periods of high malaria infection [28].
Our study also revealed that proportions of stillbirths were higher with women with malaria in pregnancy. This has been validated by other reviews conducted earlier that have reported a widespread effect of malaria and risk of stillbirths [Reference Falade10, 29]. Amongst the major modifiable risk factors of stillbirths, risk attributed to malaria is approximately 8% which can be prevented if exposure minimized [Reference Yimam, Nateghpour, Mohebali and Afshar30].
Amongst the major strengths of the review, the inclusion of 253 studies determining the burden of malaria in pregnancy creates a substantial mark. It gives us a holistic global standpoint of prevalence of the disease and its association with adverse pregnancy outcomes on both the maternal and neonatal health. To further strengthen the robustness of the review, sensitivity analyses were performed which refined the effect sizes of the meta-analyses eliminating the influential studies. In addition, assessment of publication bias was also undertaken to identify the presence of biases via relevant plots.
The limitations of the review include the non-uniformity of diagnostic test used. Multiple approaches, varying in sensitivity and specificity, were used to detect malaria during pregnancy. Not all studies utilize PCR for logistical reasons, and microscopy and rapid diagnostic tests are vulnerable to errors depending on reagents, personnel, mutant strains, and other factors. It is also pertinent to note that we lacked access to individual patient data from the studies that yielded adjusted estimates; thus, we were unable to account for this variation. Since the factors adjusted were not uniform in all studies, dichotomous data were preferred as a measure of reported and studies that failed to report dichotomous data were excluded. Further, confounding was also not taken into consideration when deducing associations with adverse outcomes and we also could not conduct the association analysis by strain due to paucity and diversity of data, which did not allow us to do a sub-group analysis.
Conclusion
Despite significant work being done to control the spread of the disease, the burden of malaria persists. A substantial impact of unfavourable pregnancy outcome also adds up to the seriousness of the issue and requires urgent attention and concern. Large-scale interventional studies are the need of the time to address this public health issue along with global level policy formulations to target the vulnerable populations living with such elevated burden of disease.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0950268824000177.
Data availability statement
Data are available upon reasonable request. All data relevant to the study is included in the article.
Author contribution
Conceptualization: S.L., J.K.D., S.K., Z.A.P., M.A.B.; Data curation: S.L., F.S., J.K.D., S.K.N., Z.R.; Formal analysis: S.L.; Investigation: S.L., F.S., J.K.D., Z.A.P., Z.R., M.A.B.; Methodology: S.L., F.S., J.K.D., A.R.R., Z.A.P.; Project administration: S.L., J.K.D., Z.A.P., M.A.B.; Writing – original draft: S.L., H.J., O.M.; Writing – review & editing: S.L., H.A.N., J.K.D., S.K., Z.A.P., M.A.B.; Supervision: J.K.D., Z.A.P., M.A.B.; Validation: J.K.D., S.K., M.A.B.; Resources: A.R.R.; Software: A.R.R.
Funding statement
There was no funding available for the review.
Competing interest
There is no competing interest declared.
Ethical standard
Ethical approvals were acquired from the Ethics Review Committee of the Aga Khan University Hospital and the Institution Review Board of the Jinnah Postgraduate Medical Center. Patient privacy and confidentiality were maintained at every stage of the study.