Introduction
Anxiety is a common mental health problem, characterised by excess worry, fear, and hyperarousal that can be debilitating and interfere with normal daily functioning (Olthuis, Watt, Bailey, Hayden, & Stewart, Reference Olthuis, Watt, Bailey, Hayden and Stewart2016). Fear is a state of immediate threat and anxiety is the anticipation of future threats (Craske & Stein, Reference Craske and Stein2016). People with anxiety symptoms experience these responses to internal or external triggers out of proportion to the actual danger posed. Anxiety disorders have a widespread global prevalence of around 7.3% in adults (Baxter, Scott, Vos, & Whiteford, Reference Baxter, Scott, Vos and Whiteford2013) and 6.5% in children and adolescents (Polanczyk, Salum, Sugaya, Caye, & Rohde, Reference Polanczyk, Salum, Sugaya, Caye and Rohde2015). While anxiety disorders frequently co-occur with depression (Kessler et al., Reference Kessler, Gruber, Hettema, Hwang, Sampson and Yonkers2008), the diagnoses have different symptomologies (Simpson, Neria, Lewis-Fernández, & Schneier, Reference Simpson, Neria, Lewis-Fernández, Schneier, Simpson, Neria, Lewis-Fernandez and Schneier2010). Collectively, anxiety disorders represent the sixth leading cause of disability worldwide (World Health Organisation, 2017) and are associated with long-term physical health complications, including cardiovascular disease (Batelaan, Seldenrijk, Bot, van Balkom, & Penninx, Reference Batelaan, Seldenrijk, Bot, van Balkom and Penninx2016) and premature mortality (Walker, McGee, & Druss, Reference Walker, McGee and Druss2015).
Most anxiety symptoms first occur during adolescence (Kessler, Chiu, Demler, Merikangas, & Walters, Reference Kessler, Chiu, Demler, Merikangas and Walters2005) and cause substantial disruptions to the social, education, and family lives of young people (Woodward & Fergusson, Reference Woodward and Fergusson2001) and their functioning in later life (Essau, Lewinsohn, Olaya, & Seeley, Reference Essau, Lewinsohn, Olaya and Seeley2014). Identifying risk factors for anxiety symptoms that are modifiable during adolescence are essential to strategies for reducing the prevalence and burden of anxiety disorders. Interventions that simultaneously address the long-term physical and mental health outcomes could be particularly important for reducing the mortality gap between people with anxiety disorders and the general population (Firth et al., Reference Firth, Siddiqi, Koyanagi, Siskind, Rosenbaum, Galletly and Stubbs2019; Kandola et al., Reference Kandola, Vancampfort, Herring, Rebar, Hallgren, Firth and Stubbs2018).
Sedentary behaviour refers to any waking activity in a sitting, lying, or reclining position with a low energy expenditure of ⩽1.5 metabolic equivalents, which are standardised units of energy expenditure (Tremblay et al., Reference Tremblay, Aubert, Barnes, Saunders, Carson, Latimer-Cheung and Chinapaw2017). Some population-based studies have found that higher levels of self-reported sedentary behaviour and lower levels of physical activity are associated with an increased risk of anxiety symptoms and disorders (Allen, Walter, & Swann, Reference Allen, Walter and Swann2019; Hoare, Milton, Foster, & Allender, Reference Hoare, Milton, Foster and Allender2016; McDowell, Dishman, Gordon, & Herring, Reference McDowell, Dishman, Gordon and Herring2019; Schuch et al., Reference Schuch, Stubbs, Meyer, Heissel, Zech, Vancampfort and Hiles2019; Teychenne, Costigan, & Parker, Reference Teychenne, Costigan and Parker2015). Sedentary behaviour is directly modifiable through increasing physical activity in the day. Several randomised controlled trials have demonstrated that structured physical activity interventions can reduce the symptoms of anxiety in adults with and without anxiety disorders (Gordon, McDowell, Lyons, & Herring, Reference Gordon, McDowell, Lyons and Herring2017; Herring, O'Connor, & Dishman, Reference Herring, O'Connor and Dishman2010; Stonerock, Hoffman, Smith, & Blumenthal, Reference Stonerock, Hoffman, Smith and Blumenthal2015; Stubbs et al., Reference Stubbs, Vancampfort, Rosenbaum, Firth, Cosco, Veronese and Schuch2017).
However, there has been little research on adolescents. Most studies in this area are cross-sectional and unable to account for reverse causality (Allen et al., Reference Allen, Walter and Swann2019; Hoare et al., Reference Hoare, Milton, Foster and Allender2016; Schuch et al., Reference Schuch, Stubbs, Meyer, Heissel, Zech, Vancampfort and Hiles2019; Teychenne et al., Reference Teychenne, Costigan and Parker2015). From four recent systematic reviews (Allen et al., Reference Allen, Walter and Swann2019; Hoare et al., Reference Hoare, Milton, Foster and Allender2016; Schuch et al., Reference Schuch, Stubbs, Meyer, Heissel, Zech, Vancampfort and Hiles2019; Teychenne et al., Reference Teychenne, Costigan and Parker2015), only three prospective studies focus on sedentary behaviour (Gunnell et al., Reference Gunnell, Flament, Buchholz, Henderson, Obeid, Schubert and Goldfield2016; Khouja et al., Reference Khouja, Munafò, Tilling, Wiles, Joinson, Etchells and Cornish2017) or physical activity (Ströhle et al., Reference Ströhle, Höfler, Pfister, Müller, Hoyer, Wittchen and Lieb2007) and anxiety symptoms in adolescents. These studies used self-reported measures of activity that are subject to mood, attention, and recall biases that reduce the validity of their estimates in adolescents (Adamo, Prince, Tricco, Connor-Gorber, & Tremblay, Reference Adamo, Prince, Tricco, Connor-Gorber and Tremblay2009). Self-reported measures are particularly poor at detecting sedentary behaviour or light-intensity activities, such as walking at a casual pace or light housework (Matthews, Moore, George, Sampson, & Bowles, Reference Matthews, Moore, George, Sampson and Bowles2012).
Accelerometers are electromechanical devices that can reliably and continuously estimate activity across a wide range of intensities throughout the day (Ainsworth, Cahalin, Buman, & Ross, Reference Ainsworth, Cahalin, Buman and Ross2015). Accelerometer-based studies show that light activity makes up most of waking daily activity, but is progressively displaced by sedentary behaviour throughout adolescence (Colley et al., Reference Colley, Garriguet, Janssen, Wong, Saunders, Carson and Tremblay2013; Kandola, Lewis, Osborn, Stubbs, & Hayes, Reference Kandola, Lewis, Osborn, Stubbs and Hayes2020; Ortega et al., Reference Ortega, Konstabel, Pasquali, Ruiz, Hurtig-Wennlöf, Mäestu and Sjöström2013; Spittaels et al., Reference Spittaels, Van Cauwenberghe, Verbestel, De Meester, Van Dyck, Verloigne and De Bourdeaudhuij2012). We recently found that this activity shift was associated with an increased risk of depressive symptoms by age 18 in the ALSPAC study (Kandola et al., Reference Kandola, Lewis, Osborn, Stubbs and Hayes2020), but we currently lack evidence regarding the impact on anxiety symptoms. Most prospective studies of sedentary behaviour or physical activity interventions focus on depressive symptoms (Bond, Stanton, Wintour, Rosenbaum, & Rebar, Reference Bond, Stanton, Wintour, Rosenbaum and Rebar2020; Schuch et al., Reference Schuch, Vancampfort, Firth, Rosenbaum, Ward, Silva and Stubbs2018, Reference Schuch, Stubbs, Meyer, Heissel, Zech, Vancampfort and Hiles2019), creating a knowledge gap regarding their relationship with anxiety symptoms. Anxiety symptoms are associated with a substantial global health burden and likely have different underlying mechanisms from depressive symptoms that warrant further investigation in their own right.
Physical activity could promote mental health through several mechanisms, such as stimulating neuroplasticity or promoting self-esteem (Kandola, Ashdown-Franks, Hendrikse, Sabiston, & Stubbs, Reference Kandola, Ashdown-Franks, Hendrikse, Sabiston and Stubbs2019). High sedentary behaviour may forgo some of these possible benefits and cause additional problems that increase mental health risks, such as social isolation or sleep problems (Li et al., Reference Li, Buxton, Lee, Chang, Berger and Hale2019; Werneck, Collings, Barboza, Stubbs, & Silva, Reference Werneck, Collings, Barboza, Stubbs and Silva2019). While increasing activity will be essential to reducing sedentary behaviour, associations between sedentary behaviour and anxiety symptoms may be independent of total physical activity volume. This independence would suggest that the risks of sedentary behaviour are more than simply a product of low energy expenditure. For example, unengaging sedentary behaviours may induce prolonged bouts of minimal cognitive stimulation that could pose mental health risks, such as watching television (Hallgren et al., Reference Hallgren, Owen, Stubbs, Zeebari, Vancampfort, Schuch and Trolle Lagerros2018; Hallgren, Dunstan, & Owen, Reference Hallgren, Dunstan and Owen2020).
Advances in traditional regression-based methods are also necessary to account for the reality that reducing sedentary behaviour requires increasing other forms of activity to displace it. Traditional methods only estimate the impact of increasing time in one activity. For example, we estimated in our previous study that a 1 hour increase in daily sedentary behaviour was associated with around a 10% increase in depressive symptoms (Kandola et al., Reference Kandola, Lewis, Osborn, Stubbs and Hayes2020). But this estimation does not account for whether the increase in daily sedentary behaviour comes at the expense of light activity or moderate to vigorous activity, both of which may impact anxiety symptoms differently. Iso-temporal substitution models (ISMs) are a novel method for estimating how substituting time in one activity (e.g. sedentary behaviour) for the time in another (e.g. light activity) affects an outcome (e.g. anxiety symptoms) (Mekary et al., Reference Mekary, Lucas, Pan, Okereke, Willett, Hu and Ding2013; Mekary, Willett, Hu, & Ding, Reference Mekary, Willett, Hu and Ding2009).
We prospectively investigated associations between sedentary behaviour, light activity, and moderate-to-vigorous physical activity (MVPA) measured using accelerometers during adolescence with anxiety symptoms at age 18 with ISMs. We specifically assessed whether associations between sedentary behaviour and anxiety symptoms were independent of total physical activity volume. To the best of our knowledge, this is the first prospective study to examine associations between device-measured activity and anxiety symptoms in adolescents.
Our directional hypotheses were:
1. Higher sedentary behaviour at ages 12, 14, and 16 is associated with increased anxiety symptoms at age 18
2. Associations between sedentary behaviour and anxiety symptoms are independent of total physical activity volume
3. Substituting periods of daily sedentary behaviour for the light activity or MVPA during adolescence is associated with reductions in anxiety symptoms at age 18
Methods
Participants and study design
This is a prospective study with repeated measures using data from the Avon Longitudinal Study of Parents and Children (ALSPAC) cohort. The sample is broadly representative of the general population and full details of the cohort are described elsewhere (Boyd et al., Reference Boyd, Golding, Macleod, Lawlor, Fraser, Henderson and Smith2013; Fraser et al., Reference Fraser, Macdonald-Wallis, Tilling, Boyd, Golding, Davey Smith and Lawlor2013). Information about the variables is available through an online data dictionary and variable search tool (http://www.bristol.ac.uk/alspac/researchers/our-data/). The ALSPAC cohort consists of 15 454 women and 14 901 children alive at 12 months of age. All pregnant women in the Avon area of South-West England with an expected delivery date between 1 April 1991 and 31 December 1991, were invited to join the ALSPAC study (Boyd et al., Reference Boyd, Golding, Macleod, Lawlor, Fraser, Henderson and Smith2013; Fraser et al., Reference Fraser, Macdonald-Wallis, Tilling, Boyd, Golding, Davey Smith and Lawlor2013). The ALSPAC Law and Ethics committee and the local research ethics committees gave ethical approval for this study. All participants gave informed consent for the use of data collected via questionnaires and clinics following the recommendations of the ALSPAC Ethics and Law Committee at the time. We defined our sample as any participant with complete Clinical Interview Schedule-Revised (CIS-R) anxiety symptoms score at age 17.8 (n = 4257), or age 18 for simplicity. Online Supplementary Fig. s1 contains a flowchart of ALSPAC participants for this study.
Measures
Outcome: anxiety symptoms
The primary outcome in our study was anxiety symptoms, measured using a computerised version of the CIS-R anxiety score at age 18. The CIS-R is a common tool for assessing depression and anxiety symptoms in community-based samples, using criteria from the International Statistical Classification of Diseases, 10th Revision (ICD-10) (Lewis, Pelosi, Araya, & Dunn, Reference Lewis, Pelosi, Araya and Dunn1992). It performs similarly to diagnosis by a trained psychiatrist and shows good reliability (r = 0.90) (Lewis et al., Reference Lewis, Pelosi, Araya and Dunn1992). The anxiety score ranges from 0 to 16 and indicates the number and severity of recent anxiety symptoms, including feelings of anxiety, nervousness, tenseness, or physical symptoms, such as changes in heartrate. We used a discrete-continuous score for our primary outcome as this better reflects how anxiety symptoms manifest than a dichotomised outcome, such as a clinical diagnosis.
To measure baseline anxiety, we used data from the Development and Well-being Assessment (DAWBA) (Goodman, Ford, Richards, Gatward, & Meltzer, Reference Goodman, Ford, Richards, Gatward and Meltzer2000), completed by the mother around age 11 (10.8 years) and 14 (14.2 years). The DAWBA consists of questions about mental health symptoms up to the last 6-months following diagnostic criteria from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (American Psychiatric Association, 1994) and ICD-10. A computer algorithm creates ordered categorical measures to indicate the probability of an anxiety disorder (see Goodman, Heiervang, Collishaw, & Goodman, Reference Goodman, Heiervang, Collishaw and Goodman2011 for details).
Exposure: sedentary behaviour and physical activity
Activity data were collected at ages 11.8, 13.9, and 15.5 (which we refer to as ages 12, 14, and 16) using MTI Actigraph 7164 or 71 256 accelerometers (Actigraph LLC, Fort Walton Beach, FL, USA) worn during waking hours on the right hip for 7 days. We derived total activity volume, time in sedentary behaviour, light activity and MVPA based on counts per minutes (CPM) using methods that we describe in detail elsewhere (Kandola et al., Reference Kandola, Lewis, Osborn, Stubbs and Hayes2020). We used thresholds established by a calibration study in ALSPAC participants (Mattocks et al., Reference Mattocks, Ness, Leary, Tilling, Blair, Shield and Riddoch2008) of ⩾3600 CPM for MVPA, 200–3599 CPM for light activity, and ⩽199 CPM for sedentary behaviour. We only included data from participants with at least 10 h of wear time for 3 or more days (Mattocks et al., Reference Mattocks, Ness, Leary, Tilling, Blair, Shield and Riddoch2008).
Confounding variables
We based our confounding variable adjustments on a Directed Acyclic Graph (DAG), which represents our understanding of the proposed causal associations between sedentary behaviour, physical activity, and anxiety symptoms (online Supplementary Fig. s2). According to this DAG, the necessary adjustments for estimating the total effect of sedentary behaviour on anxiety symptoms include: Sex, ethnicity, social class (maternal manual or non-manual occupation), IQ (measured at age 8), parental psychiatric history (prior diagnosis of depression or schizophrenia), parental education (secondary or degree/higher level education), total physical activity volume (mean daily CPM), baseline anxiety symptoms (DAWBA at age 11 and 14), and total accelerometer wear time. Models at age 16 used the DAWBA at age 14 as the closest baseline measure of anxiety. Total physical activity volume is measured in counts rather than minutes to reduce the risk of collinearity with sedentary behaviour.
Our sensitivity analyses (detailed below) included the covariates: alcohol use and smoking (age 16), baseline depressive symptoms (Short Moods and Feelings Questionnaire at ages 12, 14, and 16), body mass index (BMI) (ages 12, 13, and 16) and physical health status (presence of a severe physical illness before age 17).
Analysis
For normally distributed, continuous variables, we calculated means and standard deviations. For non-normally distributed variables, we used medians and interquartile ranges.
For the main analysis, we used negative binomial regression models as the distribution of anxiety scores had a high positive skew and were over-dispersed (online Supplementary Fig. s3). Our main analysis consists of two sets of models. The first set (models 1–9) use single-exposure models (described below) to assess associations between sedentary behaviour, light activity, MVPA, and anxiety symptoms (hypothesis 1) and the possible independence of sedentary behaviour from total physical activity volume (hypothesis 2). The second set (models 10–13) use ISMs to examine substitution effects (hypothesis 3). We present the results of our models as percentage changes in anxiety scores.
We carried out our analysis in Stata 16 (StataCorp LLC).
Single-exposure models
Single-exposure models estimate the ‘total’ association between each activity category (sedentary behaviour, light, and MVPA) at ages 12, 14, and 16 and anxiety symptoms at age 18. We used three separate models for each time point of sedentary behaviour measurement. The single-exposure models assess total associations because they do not mutually adjust for other activity categories but do adjust for all other covariates. In addition to crude and adjusted models, we ran additional analyses for sedentary behaviour that also adjusted for total physical activity volume (mean daily CPM). These models assessed whether any association between sedentary behaviour and anxiety symptoms were independent of total physical activity volume (aim 2).
Iso-temporal substitution models
The single models assume time is infinite and only estimate the impact of increasing time in one activity on anxiety. These models do not account for the reality that increasing time in one activity must displace time in another activity as time is finite throughout the day.
ISMs estimate the percentage change in anxiety symptoms from substituting a unit of sedentary behaviour time for an equivalent unit of light activity or MVPA time. ISMs use the same set of linear parameters and operate as any generalised linear model. They include all three exposure variables and a total time variable, which is the summation of the three exposure variables. A simple illustration of these parameters using a linear set of parameters would look like:
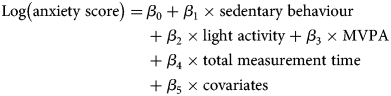
ISMs assume that time in one activity displaces an equal amount of time in another while holding total measurement time (β 4) and other covariates (β 5) constant. As all three exposure variables (β 1, β 2, and β 3) are from the same measure and in the same units of time (e.g. 60-minute blocks), we then drop sedentary behaviour (β 1) from the model. The resulting exposure coefficients (β 2 and β 3) now represent their association with anxiety, absent sedentary behaviour, while total measurement time is still held constant (β 4). We then interpret these coefficients as the consequence of substituting a unit of sedentary behaviour time for a unit of light activity or MVPA time per day on anxiety scores.
We entered activities into these ISMs in units of 60-minutes. As there are no specific guidelines for reducing daily sedentary behaviour, we based this on national recommendations for adolescents to achieve at least 60 min of MVPA per day (Department of Health and Social Care, 2019).
Sensitivity analyses and missing data
We conducted a series of sensitivity analyses to explore other possible explanations for our results and test the robustness of our main findings from the ISMs. Some analyses include possible covariates that were measured after the exposure and could represent mediators or colliders, such as physical illness, smoking, and alcohol use. Sensitivity models included: (1) baseline depressive symptoms as a covariate (ages 12, 14, and 16), (2) smoking and alcohol use (age 16 only) as covariates, (3) serious physical illness before age 17, (4) excluding anyone with a possible anxiety disorder at baseline, (5) linear, instead of negative binomial, regression models, (6) sex as an interaction term, (7) baseline BMI as a covariate (ages 12, 13, and 16).
To examine the extent to which any attrition bias from the missing data affected our results (see online Supplementary Fig. s1), we repeated the main analyses in a full cohort with imputed data. The missing data were estimated by multiple imputation models by chained equations (see Supplementary Methods s1 for details). We also calculated the e-value to examine the risk of unmeasured confounding (44). The e-value estimates the minimum strength that an unmeasured confounding variable would have to have to nullify the observed association between exposure and outcome while considering all other measured covariates (VanderWeele & Ding, Reference VanderWeele and Ding2017). The e-value helps to assess the plausibility of unmeasured confounding and contributes towards the evidence for causality (Haneuse, VanderWeele, & Arterburn, Reference Haneuse, VanderWeele and Arterburn2019).
Results
Participants
Our sample included 4257 participants with a complete CIS-R anxiety score at age 18. Over the 6-year follow-up period, the complete case analyses at ages 12, 14, and 16, included 2292, 18,66, and 1128 participants, respectively. Online Supplementary Table s1 contains a comparison of baseline characteristics between included and excluded participants from the full ALSPAC sample.
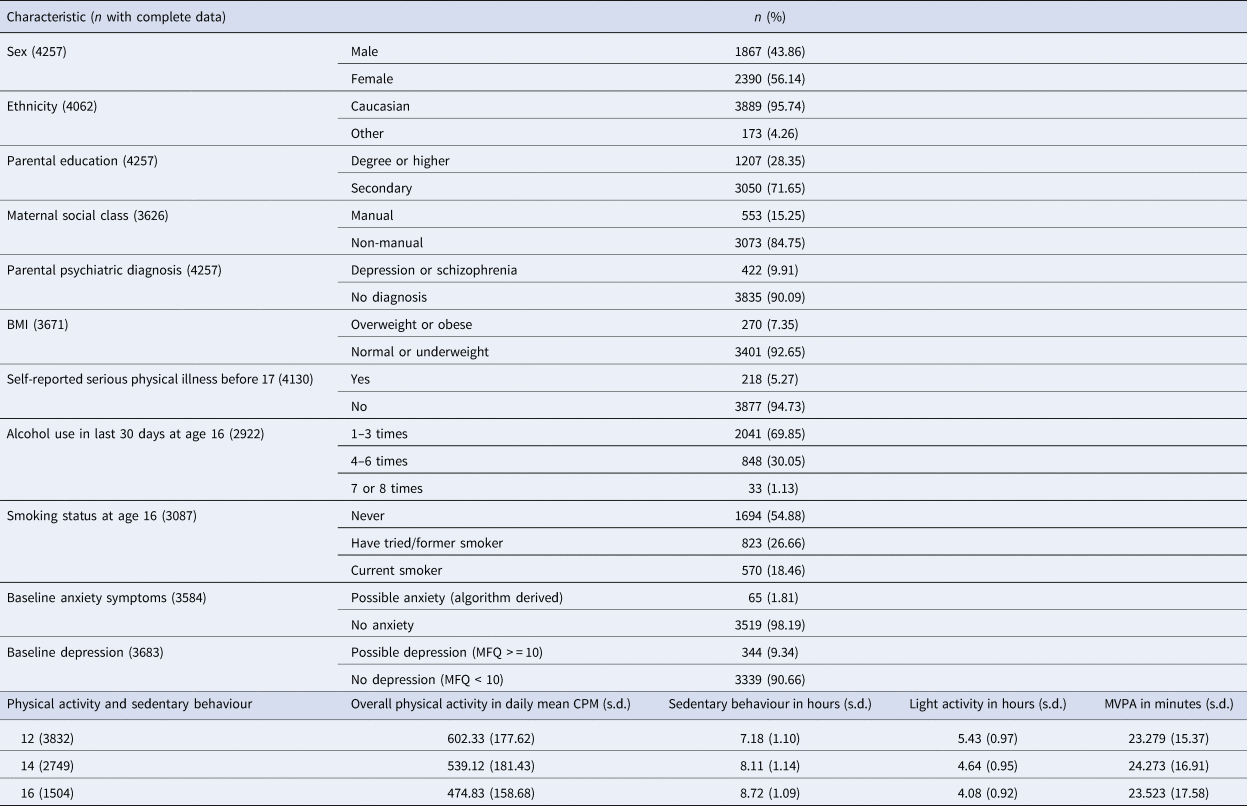
Table 1 contains participant characteristics and physical activity levels. Overall physical activity levels decline from age 602.33 CPM (s.d. 177.62) to 474.83 CPM (s.d. 158.68) between the ages of 12 and 16. Within the same waking period, sedentary behaviour increases from 7.18 (s.d. 1.10) to 8.72 (s.d. 1.09) hours per day, light activity decreases from 5.43 (s.d. 0.97) to 4.08 (s.d. 0.92) hours per day, and MVPA stays relatively stable.
Table 1. Participant characteristics and activity changes of included participants (n = 4257)
Single-exposure models
Table 2 shows results from the single-exposure models. The adjusted models without total activity volume (models 2, 5, and 8) suggest that an additional 60-minutes of sedentary behaviour at ages 12, 14, and 16, was associated with an 18.22% (95% CI 10.10–26.87), 10.19% (95% CI 1.37–19.79), and 15.75% (95% CI 3.71–29.12) higher anxiety score at age 18. An additional 60-minutes of light activity at 12, 14, and 16, was associated with a −16.82% (95% CI −22.99 to −10.16), −11.57% (95% CI −19.30 to −2.98), and −14.81% (95% CI −24.29 to −4.14) decrease in anxiety score. The model estimates suggest no association between MVPA and anxiety scores in this sample.
Table 2. Associations between sedentary behaviour, light activity, and MVPA and anxiety symptoms in single negative binomial models
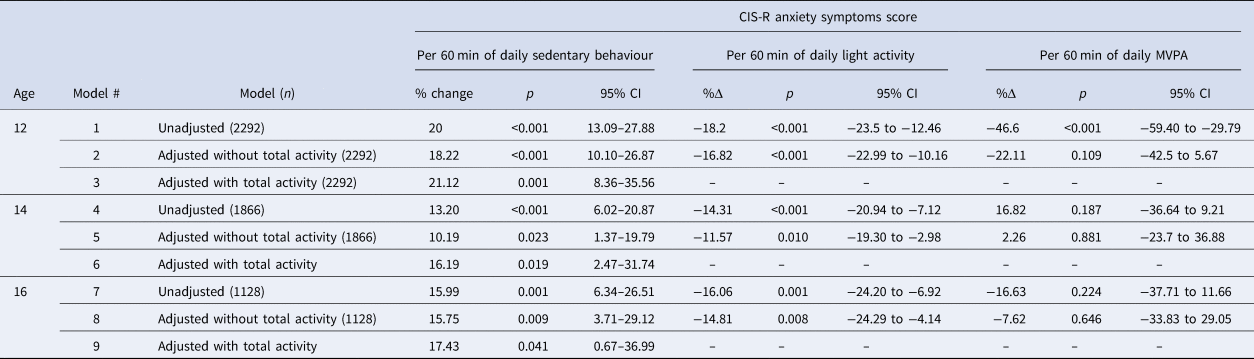
% change: Percentage change in anxiety score based on incident rate ratios; 95% CI: 95% confidence intervals; MVPA: moderate-to-vigorous physical activity.
Adjusted without total activity (models 2, 5, and 8): Conditioned on sex, ethnicity, maternal social class, baseline anxiety, parental psychiatric history, parental education, IQ, and total wear time.
Adjusted with total activity (models 3, 6, and 9): Conditioned on same as models 2, 5, and 8, with total activity volume (average daily CPM).
When adjusting for total activity (models 3, 6, and 9), sedentary behaviour at ages 12, 14, and 16 was associated with a 21.12% (95% CI 8.36–35.65), 16.19% (95% CI 2.47–31.74), and a 17.43% (95% CI 0.67–36.99) higher anxiety scores at age 18.
Iso-temporal substitution models
Table 3 displays results from the ISMs. These models suggest that substituting 60 min of sedentary behaviour for 60 min of light activity at ages 12, 14, and 16, was associated with a 16.4% (95% CI 9.4–22.7), 12.1% (95% CI 3.4–20.1), and 14.7% (95% CI 4–24.2) reduction in anxiety scores at 18. We found no associations between MVPA and anxiety.
Table 3. Iso-temporal substitution models for replacing sedentary behaviour with light activity and MVPA

% change: Percentage change in anxiety score based on incident rate ratios 95% CI: 95% confidence intervals; MVPA: moderate-to-vigorous physical activity.
Adjustment for all models: light activity, MVPA, total measurement time (SB + light activity + MVPA), sex, ethnicity, maternal social class, anxiety disorder (ages 10 and 14), parental psychiatric history, parental education, and IQ.
Sensitivity analyses
We reran our main analyses in a full cohort (n = 4257) with imputed missing data (Methods s1) and the results did not substantially differ from our main findings (online Supplementary Table s2). There were also no substantial differences in the results of all other sensitivity analyses and our main findings, and we found no evidence of an interaction with sex. Sensitivity analyses results are presented in online Supplementary Tables s2–s8.
The e-values (rate ratios) for the point estimate and lower confidence bound were 1.68 and 1.44 at age 12, 1.53 and 1.23 at age 14, and 1.62 and 1.25 at age 16, respectively. To nullify the observed association, an unmeasured confounding variable must be associated with both the exposure and outcome by a risk ratio of at least the e-value of each time point, having conditioned on the other confounding variables. For example, an unmeasured confounding variable would need to be associated with sedentary behaviour and anxiety score by a risk ratio of at least 1.68 at age 12, independent of the other confounding variables in the model. Most risk ratios for included covariates range between 1 and 1.3 at age 12, except for sex (IRR = 1.59, 95% CI 1.37–1.84).
Discussion
Main findings
This population-based study is the first to prospectively examine associations between device-measured sedentary behaviour and anxiety symptoms in adolescents. We consistently found that higher sedentary behaviour at ages 12, 14, and 16 was associated with higher anxiety symptoms at age 18. Higher light activity during the same period was associated with a decrease in anxiety symptoms. After adjusting for physical activity, an hour of daily sedentary behaviour during adolescence was independently associated with 16–21% higher in anxiety symptoms at age 18. Our use of ISM techniques demonstrates that theoretically substituting an hour of daily sedentary behaviour for light activity during adolescence was associated with a 12–16% reduction in anxiety symptoms at age 18. We found no associations between MVPA and anxiety. There were no substantial changes to these results following a series of sensitivity analyses or in the cohort following multiple imputations for missing data.
These results support previous cross-sectional and prospective findings that self-reported high sedentary behaviour is associated with a greater risk of anxiety symptoms in adolescents (Allen et al., Reference Allen, Walter and Swann2019; Hoare et al., Reference Hoare, Milton, Foster and Allender2016; Schuch et al., Reference Schuch, Stubbs, Meyer, Heissel, Zech, Vancampfort and Hiles2019; Teychenne et al., Reference Teychenne, Costigan and Parker2015). Sedentary behaviour is an established risk factor for physical health (Biswas et al., Reference Biswas, Oh, Faulkner, Bajaj, Silver, Mitchell and Alter2015) and our findings suggest it may also be a risk factor for anxiety symptoms independently of physical activity. Activity will be essential for reducing sedentary behaviour, but these findings suggest that other factors than energy expenditure are relevant to its association with anxiety symptoms. We previously found that sedentary behaviour is a possible risk factor for depressive symptoms in adolescents, where we did not assess sedentary behaviour as independent from physical activity (Kandola et al., Reference Kandola, Lewis, Osborn, Stubbs and Hayes2020). Here we showed that the associations between sedentary behaviour and anxiety symptoms are independent of baseline depressive symptoms.
For example, substituting mentally-passive (e.g. watching television) for mentally-active (e.g. reading) sedentary behaviours is associated with a reduced risk of depression in adults (Hallgren et al., Reference Hallgren, Nguyen, Owen, Stubbs, Vancampfort, Lundin and Lagerros2020). Engaging activities could help to distract young people from pathological thought patterns leading to states of anxiety. Stimulating activities could also approximate a form of cognitive training that elicits some resilience to attentional biases associated with developing anxiety symptoms in young people, such as threat detection (Telzer et al., Reference Telzer, Mogg, Bradley, Mai, Ernst, Pine and Monk2008; Waters, Henry, Mogg, Bradley, & Pine, Reference Waters, Henry, Mogg, Bradley and Pine2010).
The timing and bouts of activity may also be relevant. For example, long bouts of sitting could increase the duration within which pathological thought patterns might occur and develop into anxiety. Recent studies have found that breaking up prolonged bouts of sitting using light activity in adults have benefits for the brain and mental health, such as reducing fatigue and promoting brain plasticity and cognitive performance (Bojsen-Møller, Ekblom, Tarassova, Dunstan, & Ekblom, Reference Bojsen-Møller, Ekblom, Tarassova, Dunstan and Ekblom2020; Wennberg et al., Reference Wennberg, Boraxbekk, Wheeler, Howard, Dempsey, Lambert and Dunstan2016; Wheeler et al., Reference Wheeler, Green, Ellis, Cerin, Heinonen, Naylor and Dunstan2020). These benefits may accumulate to reduce the risk of anxiety symptoms developing. Young people who frequently break up bouts of sitting with activity could reduce this risk while still having a similar overall activity level. It may also interrupt other behaviours that occur with long bouts of sitting, which might also increase the risk of anxiety symptoms. For example, watching television is associated with a less healthy diet, including higher consumption of energy-dense snacks and sugar-sweetened drinks (Hobbs, Pearson, Foster, & Biddle, Reference Hobbs, Pearson, Foster and Biddle2015), which may increase the risk of anxiety symptoms in adolescents (Oddy et al., Reference Oddy, Allen, Trapp, Ambrosini, Black, Huang and Mori2018).
While the biological mechanisms underlying associations between sedentary behaviour and anxiety symptoms will overlap with physical activity, there may also be some unique pathways. For example, avoiding long bouts of sedentary behaviour could maintain constant mitochondrial activity throughout the day, reducing the risk of mitochondrial dysfunction in the brain leading to anxiety symptoms (Filiou & Sandi, Reference Filiou and Sandi2019). It is worth noting that research to identify possible biological mechanisms that differentiate the influence of sedentary behaviour from physical activity on health is an emerging area (Thyfault, Du, Kraus, Levine, & Booth, Reference Thyfault, Du, Kraus, Levine and Booth2015).
Strengths and limitations
There are several strengths to this study, including the use of accelerometers, a prospective, repeated measures study design with a 6-year follow up, and large sample size. Strengths in our analysis include using ISMs to account for the reciprocal relationship between changes in time-use variables. These methods produce a more realistic estimation of how interventions to reduce sedentary behaviour using different intensities of activity might affect anxiety symptoms. We also included adjustments for baseline anxiety symptoms to lower the risk of reverse causation and adequate adjustment of total physical activity volume. We used several sensitivity analyses, including calculating e-values to assess the strength of unmeasured confounding necessary to nullify our results, baseline depressive symptoms to examine anxiety symptoms independently, and multiple imputation models to account for potential selection bias due to attrition. The use of DAGs determined a priori also improve our ability to assess causal associations in the data.
A limitation of our study is the lack of 24-hour activity, which means that our ISMs only account for the waking portion of the day. Participants did not wear accelerometers while sleeping, so we were unable to examine how substituting sedentary behaviour for sleep might affect the risk of anxiety symptoms. There could also be gaps in the data if participants did not wear their devices for all waking behaviours. There is a further risk that our results are confounded by other unmeasured factors, such as social support, self-esteem, or physical health at baseline. Baseline adjustment was for the possible incidence of an anxiety disorder, which overlooks participants with sub-threshold anxiety symptoms. Baseline assessments of anxiety were also completed by the mother, rather than the participant. There was no assessment of anxiety symptoms at age 16, so in the age 16 models, we used anxiety symptom scores from age 14, which is a further limitation.
To assess anxiety symptoms independently of depressive symptoms, we adjusted for baseline depressive symptoms in our sensitivity analyses. However, there may still have been an overlap in the outcome of depressive and anxiety symptoms. The magnitude of the e-value relative to included confounding variables here suggests that an unmeasured confounding variable is unlikely to nullify the observed association between sedentary behaviour and anxiety symptoms. However, multiple unmeasured confounding variables may accumulate to impact our findings.
There was also substantial attrition within our sample during the study period that could have caused selection bias. Results did not differ in our multiple imputation models, which does not indicate a high risk of attrition bias in our sample. However, we only imputed missing data from the subsample with completed CIS-R anxiety scores at age 18 (n = 4257), not the entire ALSPAC sample (n = 14 901). Differences between our sample and the larger ALSPAC sample could have influenced our results. Participants also had low MVPA levels, with the average being around 40 min lower than the nationally recommended guidelines of 60 min of daily MVPA for adolescents in the UK. This may have contributed to the lack of an association between replacing sedentary behaviour with MVPA and changes in anxiety symptoms.
Accelerometers provide reliable estimates of activity, but they cannot record posture and differentiate between sitting and standing. Standing behaviours may be misclassified as sedentary behaviour in our study. Thigh-worn devices are preferable for recording sedentary behaviour, such as ActivPAL (PAL Technologies Ltd., Glasgow, UK). However, misclassifying light activity (standing) would increase sedentary behaviour, and its true association with anxiety symptoms could be even larger.
A broader issue with most devices is their inability to record the type and context of activities. This limitation prevented us from investigating how different types of sedentary behaviour are associated with anxiety, such as mentally-active v. mentally-passive behaviours (Hallgren et al., Reference Hallgren, Dunstan and Owen2020). However, the use of accelerometers still represents a methodological improvement in self-report measures for quantifying aspects of activity, such as time in sedentary behaviour (Atkin et al., Reference Atkin, Gorely, Clemes, Yates, Edwardson, Brage and Biddle2012).
Future directions and conclusion
Our findings suggest that sedentary behaviour could be a risk factor for anxiety symptoms in adolescents that is modifiable through light activity. These findings produce new insights to relate specifically to the development of anxiety disorders. Most research focuses only on depression, despite anxiety being a major cause of global disability (World Health Organisation, 2017). Sedentary behaviour may be a risk factor for both depressive and anxiety symptoms. Future research focusing on both paradigms together as common mental disorders may highlight common pathways. However, our results suggest that there may be independent pathways linking sedentary behaviour to anxiety symptoms, and the paucity of direct research into anxiety symptoms warrants further investigation.
There is a need to evaluate public health strategies and interventions to reduce anxiety symptoms in adolescents using light activity to replace sedentary behaviour. While just 29% of adolescents achieve national MVPA guidelines in developed nations (Steene-Johannessen et al., Reference Steene-Johannessen, Hansen, Dalene, Kolle, Northstone, Møller and Ekelund2020), efforts to increase light activity could be more successful. Compared with MVPA, light activity is less effortful and more pleasurable for most people, which is likely to stimulate higher motivation (Ekkekakis, Parfitt, & Petruzzello, Reference Ekkekakis, Parfitt and Petruzzello2011). Light activity is also sustainable over extended periods and does not require designated time during the day. Simple changes to incorporate more light activity at school could include standing desks or active breaks during classes. Approaches at home could include standing up during commercial breaks or doing house chores while watching television, more frequent trips to pick up groceries, or walking during phone calls.
Strong public health messaging on the importance of light activity should be made in tandem with the MVPA guidelines, which have essential physical health and developmental implications. Simply increasing movement will likely benefit more young people. It should be possible to increase light activity beyond the current target of 1 hour for MVPA, which may have a substantial impact on anxiety. For example, our models suggest that substituting 2 hours of sedentary behaviour for light activity per day during adolescence could lead to a 24–32% reduction in anxiety symptoms by age 18.
We also found that associations between sedentary behaviour and anxiety symptoms were independent of activity. Some sedentary behaviours may be particularly detrimental and represent specific targets for intervention, such as watching television. More research is necessary to assess the differential impact of various sedentary behaviours on anxiety symptoms.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291720004948.
Data
Details for accessing the data used in this study can be found on the ALSPAC website: http://www.bristol.ac.uk/alspac/researchers/our-data/.
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Funding/sponsors
AK is supported by the ESRC (ES/P000592/1), BS by a Clinical Lectureship (ICA-CL-2017-03-001) funded by Health Education England and the National Institute for Health Research (NIHR), and JH by the Wellcome Trust (211085/Z/18/Z). BS is part-funded by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust. DO, GL, and JH are supported by the UCLH NIHR Biomedical Research Centre. DO and JH are also part supported by the NIHR Collaboration for Leadership in Applied Health Research and Care North Thames at Bart's Health NHS Trust.
The UK Medical Research Council and Wellcome (Grant ref: 217065/Z/19/Z) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and AK, DO, GL, BS, and JH will serve as guarantors for the contents of this paper. A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf). Collection of the variables for this research was specifically funded by NIH (5R01HL071248-07) Wellcome Trust (08426812/Z/07/Z).
Conflict of interest
The authors declare no conflicts of interest.
Role of funders
No funder played any role in the conception, analyses, or writing of this research.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.





