Introduction
Important ear problems can affect the outer ear, the middle ear and the inner ear. Globally, the most significant ear diseases are associated with otorrhoea and hearing loss. Pain and discomfort and balance problems (or a combination of symptoms) can also occur. The commonest health problems affect the middle and outer ear, including otitis media, otitis externa, wax impaction and foreign bodies. Of these, otitis media has the greatest impact on children and is the most likely to cause long-term problems.
Epidemiology of common ear conditions
Recent epidemiological studies highlighted that around 300 million individuals have permanent hearing loss; around 50 per cent of these cases were preventable.Reference Tucci, Merson and Wilson1, Reference Chadha and Cieza2 A review of studies from Africa found that approximately 7 per cent of children were affected by hearing loss.Reference Mulwafu, Kuper and Ensink3 Cryptogenic causes (presumably genetic) and infections were the most important causes of severe to profound hearing loss. Otitis media and wax impaction were the most important causes of hearing impairment in the broader school age population.Reference Mulwafu, Kuper and Ensink3
In their global review of otitis media, Monasta et al. estimated that there are 709 million cases of otitis media per year, 31 million cases of chronic suppurative otitis media (CSOM) and 21 000 dying from complications of otitis media.Reference Monasta, Ronfani, Marchetti, Montico, Brumatti and Bavcar4 Children under five years of age in the poorest countries were the worst affected. In populations of children at standard risk for acute otitis media, the annual incidence rate of acute otitis media episodes is approximately 45 per cent in the first year of life, and 61 per cent per annum from age one to four years, with approximately 53 per cent per annum over the first two years of life.Reference Monasta, Ronfani, Marchetti, Montico, Brumatti and Bavcar4
Other global systematic reviews have highlighted the heterogeneity of findings, problems of ascertainment of disease and the high rates in Indigenous populations.Reference Gunasekera5 Recent large epidemiological surveys emphasise the importance of CSOM even when rates of otitis media with effusion or acute otitis media are not high.Reference Anggraeni, Hartanto, Djelantik, Ghanie, Utama and Setiawan6, Reference Simoes, Kiio, Carosone-Link, Ndegwa, Ayugi and Macharia7
Features of a high-risk population
Communities that experience high rates of tympanic membrane perforation in pre-school children have a major otitis media problem. Aboriginal communities in central and northern Australia have participated in many research studies and increased our understanding of severe otitis media.Reference Leach8 It is likely that the findings can be applied to other populations where CSOM is prevalent.
Prospective studies in Australian Aboriginal infants have described early nasopharyngeal bacterial colonisation with Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis in the first few months of life.Reference Leach, Boswell, Asche, Nienhuys and Mathews9, Reference Leach, Wigger, Andrews, Chatfield, Smith-Vaughan and Morris10 This is associated with high rates of otitis media with effusion (OME) and frequent episodes of acute otitis media infections (acute otitis media without perforation).Reference Leach, Wigger, Andrews, Chatfield, Smith-Vaughan and Morris10 This can lead to perforation of the tympanic membrane (acute otitis media with perforation).
In some children, the middle-ear discharge persists and the child develops CSOM. Rates of CSOM greater than 4 per cent indicate a massive public health problem that requires urgent attention.11, 12 A similar association between early bacterial colonisation and high rates of bacterial pneumonia has also been described in Papua New Guinea.Reference Montgomery, Lehmann, Smith, Michael, Joseph and Lupiwa13 Interestingly, cross-sectional studies suggest that it may be possible to have high rates of CSOM without high rates of OME in some settings.Reference Anggraeni, Hartanto, Djelantik, Ghanie, Utama and Setiawan6, Reference Simoes, Kiio, Carosone-Link, Ndegwa, Ayugi and Macharia7 This needs to be confirmed through prospective birth cohort studies, with standardised assessment of middle-ear disease and nasopharyngeal colonisation.
Scenario
Imagine you are a primary care doctor who has just moved to a poor rural area 300 km away from the nearest city. One of the local teachers tells you that some school children are having difficulty hearing when the classroom is noisy. She has noticed that about 5 per cent of the children have pus coming from their ears every day. These are the ones that seem to have most difficulty hearing. She asks you to help. There are a small number of ENT surgeons in the country (approximately 1 per 1 million population), but none of them live in your region.
Primary care approach
Everyone should have access to primary care (or community care) services that can manage common ear health conditions. Serious conditions (with risk of death or permanent disability) will still need to be managed at larger centres where ENT surgeons are available (or through an ENT surgical outreach service). Because funding is limited, it is important to be aware of the benefits and harms of interventions.
The World Health Organization (WHO) has endorsed the grading of recommendations assessment, development and evaluation (‘GRADE’) approach for assessing the certainty of any estimates of effect resulting from interventions (Table 1).14 From a practical point of view, high or moderate certainty of effect is needed to have confidence in the impact of an intervention. However, there are many potentially important interventions that are yet to be evaluated in rigorous studies (or where the effect varies substantially in different settings). If there are several outcomes with ‘good evidence’, it is appropriate to describe the impact on the most important outcomes first. We use a hierarchy that prioritises the long-term impact (12 months or more), and ranks speech and language development, hearing, persistent middle-ear disease, and frequent middle-ear disease in that order.
Table 1. Four categories of evidence quality used in GRADE approach for interventions

GRADE = grading of recommendations assessment, development and evaluation
A list of interventions for otitis media with high or moderate certainty of effect is shown in Table 2.Reference Fortanier, Venekamp, Boonacker, Hak, Schilder and Sanders15–Reference Macfadyen, Acuin and Gamble24 Importantly, many of the interventions only provide modest benefit, and (in most cases) the long-term impact on development and hearing remains uncertain.
Table 2. Summary of Cochrane Reviews of RCTs describing interventions with moderate to high evidence relevant to otitis media management

RCT = randomised controlled trial; AOM = acute otitis media; CI = confidence interval; RR = relative risk; NNT = number needed to treat; OME = otitis media with effusion; mth = months; TTO = tympanostomy tube otorrhoea; CSOM = chronic suppurative otitis media
Prevention of common ear conditions
Prevention of common conditions is often a better investment than cure. This is especially true for preventative interventions that are low in cost and which provide other health benefits. To date, most of the relevant evidence on prevention comes from high-income settings.
Hygiene practices
Infections in children are usually spread by direct contact (or indirect contact via fomites). The important otitis media pathogens (S pneumoniae, H influenzae and M catarrhalis) are carried in the nasopharynx. Children who touch their nose and then touch other children increase the risk of transmission.Reference Rovers, Schilder, Zielhuis and Rosenfeld25 The respiratory viruses that often precede bacterial infection are also transmitted by contact with an infected person or fomites. Hence, handwashing has been shown to be an effective strategy in interrupting transmission.Reference Jefferson, Del Mar, Dooley, Ferroni, Al-Ansary and Bawazeer26–Reference Mbakaya, Lee and Lee28 Keeping a safe distance (beyond the spread of droplets created by coughing or sneezing) from anyone with a respiratory infection is sensible. Children with nasal discharge (usually due to upper respiratory infection) are likely to be good transmitters.
While there is a lack of evidence demonstrating that hygiene interventions will reduce rates of otitis media, the overall benefit of good hygiene practice is now accepted. The WHO recommends the use of standard precautions in the care of all patients (e.g. handwashing, use of personal protective equipment when indicated, and application of cough and respiratory illness etiquette).Reference Pittet, Allegranzi, Storr, Bagheri Nejad, Dziekan and Leotsakios29 A risk assessment should also consider whether additional droplet or airborne precautions are needed.
Breastfeeding
Two recent meta-analyses have investigated the data around otitis media and breastfeeding. Bowatte et al. focused on acute otitis media and used data from 22 observational studies (approximately 39 000 subjects).Reference Bowatte, Tham, Allen, Tan, Lau and Dai30 Zhang et al. addressed chronic and recurrent otitis media (particularly otitis media with effusion (OME) and CSOM) and drew on 3 observational studies of approximately 6500 patients.Reference Zhang, Xu, Zhang, Zeng, Wang and Zheng31
Evidence for the protective effect of breastfeeding on otitis media has low certainty. This is largely attributable to the observational nature of studies, the risk of recall bias in reporting breastfeeding exposure, parental reporting of ear disease, and the limitations of point-prevalence data in relation to actual otitis disease burden. However, if we assume that the annual incidence rate of acute otitis media episodes is approximately 53 per cent per annum over the first 2 years of life,Reference Monasta, Ronfani, Marchetti, Montico, Brumatti and Bavcar4 breastfeeding exclusively for 6 months might be associated with a reduction of approximately 14 episodes of acute otitis media per year per 100 children.
Breastfeeding has also been shown to protect against OME, though the data are less robust. A meta-analysis of 3 cohort studies of approximately 6500 children found a significant reduction in point prevalence of OME during primary school screening in children who had been breast fed at some stage compared to those who had never been breast fed (odds ratio = 0.70, 95 per cent confidence interval (CI) = 0.51–0.95).Reference Zhang, Xu, Zhang, Zeng, Wang and Zheng31
Data from large cohort studies in Western Australia suggest that the protective effect of breastfeeding wanes over time.Reference Brennan-Jones, Eikelboom, Jacques, Swanpoel, Atlas and Whitehouse32, Reference Brennan-Jones, Whitehouse, Park, Hegarty, Jacques and Eikelboom33 Exclusive breastfeeding for six months did not make a significant difference to the prevalence of OME (measured by tympanometry) when the same patient group was followed up at age six years. This is consistent with meta-analysis findings of other observational studies, which showed no difference in annual acute otitis media incidence beyond two years of age across different exposure groups.Reference Bowatte, Tham, Allen, Tan, Lau and Dai30
Tobacco and indoor smoke exposure
Observational evidence shows an association between ear disease and tobacco smoke exposure in utero or in childhood.Reference Jones, Hassanien, Cook, Britton and Leonardi-Bree34 Some authors have drawn parallels between tobacco smoke exposure and exposure to the smoke from cooking fires (which may also be related to the risk of otitis media).Reference Gordon, Bruce, Grigg, Hibberd, Kurmi and Lam35
A recent meta-analysis of 61 observational studies (with approximately 30 000 participants included) addressed second-hand tobacco smoke exposure.Reference Jones, Hassanien, Cook, Britton and Leonardi-Bree34 Overall, the evidence was low in certainty because of: the observational nature of the studies, the risk of bias inherent in parent-reported second-hand tobacco smoke exposure measurement, the lack of representativeness of study samples and/or the lack of adjusted analyses. In their pooled analysis, the risk of all-cause middle-ear disease (acute otitis media, OME, CSOM) was significantly increased in children whose mothers smoked (odds ratio = 1.53, 95 per cent CI = 1.22–1.92, 124 studies) and in those who lived with smokers in the household (odds ratio = 1.32, 95 per cent CI = 1.20–1.45, 37 studies). The association between household smoking and rates of surgery for middle-ear disease was also important (odds ratio = 1.62, 95 per cent CI = 1.31–1.98).Reference Jones, Hassanien, Cook, Britton and Leonardi-Bree34
One large study of approximately 20 000 Norwegian mother–infant pairs specifically examined maternal pre-natal smoking, and found that this was associated with an increased risk of acute otitis media in the first 6 months of life (odds ratio = 1.34, 95 per cent CI = 1.06–1.68).Reference Haberg, Bentdal, London, Kvaener, Nystad and Nafstad36 The association weakened as children became older (and when adjusted for post-natal maternal smoking). However, acute otitis media in early life has been shown to be an important predictor of increased middle-ear disease throughout childhood.
Jacoby et al. studied second-hand smoke exposure and otitis media in a high-risk rural population in Western Australia.Reference Jacoby, Coates, Arumugaswamy, Elsbury, Stokes and Monck37 Middle-ear disease (as diagnosed by ENT surgeons) was markedly more common in children exposed to tobacco smoke at home (odds ratio = 3.54, 95 per cent CI = 1.68–7.47). Smoke exposure was very high in these populations (64 per cent of Aboriginal children exposed vs 40 per cent of non-Aboriginal children).
Indoor air pollution from cooking stoves in low- and middle-income countries is associated with an increased risk of acute respiratory infection in young children. The assumption is that this exposure will also increase the risk of otitis media.Reference Gordon, Bruce, Grigg, Hibberd, Kurmi and Lam35 While avoiding excessive indoor pollution is sensible, recent randomised controlled trials (RCTs) assessing the impact of reduced cooking biomass smoke exposure has not found that rates of pneumonia are substantially reduced.Reference Haberg, Bentdal, London, Kvaener, Nystad and Nafstad36, Reference Schilmann, Riojas-Rodriguez, Ramirez-Sedeno, Berrueta, Perez-Padilla and Romieu38, Reference Smith, McCracken, Weber, Hubbard, Jenny and Thompson39 The impact on otitis media has not been assessed.
Vaccinations
Vaccines are a well-established part of the effective care of children. Severe to profound hearing loss in children has been prevented by the measles and rubella vaccines, and the protein conjugate bacterial meningitis vaccines (targeting H influenzae type b, pneumococcal and meningococcal disease). Some of the more recent vaccines (conjugate pneumococcal vaccine and influenza vaccine) also have the potential to reduce otitis media.
Pneumococcal conjugate vaccines
There is good evidence from high-quality RCTs regarding the impact of pneumococcal conjugate vaccines on otitis media.Reference Fortanier, Venekamp, Boonacker, Hak, Schilder and Sanders15, Reference Ewald, Briel, Vuichard, Kreutle, Zhydkov and Glliy40 We reviewed data for 7-, 10- and 11-valent pneumococcal conjugate vaccines, as well as the 10-valent pneumococcal polysaccharide non-typeable H influenzae protein D conjugate vaccine (‘PHiD-CV’), which has been shown to confer protection against H influenzae (also a prominent pathogen in otitis media). Evidence was of high certainty overall (large double-blind, placebo-controlled randomised trials, without significant risk of bias). However, the overall effect on otitis media was modest because of the replacement of vaccine serotypes with non-vaccine serotypes and other pathogens.
Analysis of the efficacy of 7-valent pneumococcal conjugate vaccine versus placebo revealed vaccine efficacy of 7 per cent in preventing acute otitis media episodes over the first two years of life. Decreased rates of tympanostomy tube insertion are also associated with pneumococcal conjugate vaccine, with vaccine efficacy of 20 per cent (95 per cent CI = 11–29 per cent). Assuming a baseline tympanostomy tube insertion rate of 3 per cent, vaccination will prevent 6 operations per 1000 children vaccinated.
The pneumococcal polysaccharide non-typeable H influenzae protein D conjugate vaccine has the potential to reduce disease due to non-typeable H influenzae. A meta-analysis of 3 RCTs (including approximately 15 000 children) revealed a vaccine efficacy of 14 per cent (95 per cent CI = 10–18 per cent), when comparing the proportions of children who had at least 1 acute otitis media episode during 18 months to 2 years’ follow up.Reference Prymula, Peeters, Chrobok, Kriz, Novakova and Kaliskova41–Reference Vesikari, Forsten, Seppa, Kaijalainen, Puumalainen and Soininen43 Ascertainment of acute otitis media episodes was low and this is likely to be related to methodological issues (passive case finding,Reference Tregnaghi, Saez-Llorens, Lopez, Abate, Smith and Poselman42 parental text-message requestsReference Vesikari, Forsten, Seppa, Kaijalainen, Puumalainen and Soininen43 and the requirement of ENT specialist confirmation of diagnosisReference Prymula, Peeters, Chrobok, Kriz, Novakova and Kaliskova41, Reference Tregnaghi, Saez-Llorens, Lopez, Abate, Smith and Poselman42). However, this is not likely to have altered the estimate of the relative effect of vaccination.
Seasonal influenza vaccines
Influenza virus is not often identified as the sole causative organism in acute otitis media. However, it may establish conditions amenable to the emergence of a bacterial acute otitis media. A recent meta-analysis described 10 RCTs.Reference Norhayati, Ho and Azman16
The trials of live attenuated influenza vaccine (‘LAIV’) and trivalent inactivated influenza vaccine (‘TIV’) were of moderate to high quality. The evidence was limited by variations in the type of vaccine used, otitis media not being included as a primary end-point, and variation in acute otitis media case finding and definition. The analysis of five studies with appropriate outcome measures found that influenza vaccination (at the beginning of the influenza season) can significantly reduce the proportion of children with at least one acute otitis media episode over the subsequent season (some studies followed patients to 36 months, the majority for approximately 6 months), with a vaccine efficacy of 20 per cent (95 per cent CI = 4–33 per cent). A significant difference was also found in the number of antibiotic courses prescribed to vaccinated versus unvaccinated children, with vaccine efficacy of 30 per cent (95 per cent CI = 17–41 per cent).Reference Norhayati, Ho and Azman16
On the negative side, there is a small but significant increase in minor adverse events attributable to vaccination seen in vaccine groups for fever following vaccination, with a relative risk of 1.15 (95 per cent CI = 1.06–1.24).Reference Norhayati, Ho and Azman16
In summary, vaccination remains an important strategy for reducing morbidity and mortality worldwide. However, the specific impact of available vaccines on otitis media is modest. Although never studied directly, measles vaccine has probably had a substantial impact on severe otitis media rates (as tympanic membrane perforation was a recognised complication). In the future, the largest gains in ear and hearing health are likely to come from: (1) the more widespread use of currently available vaccines; (2) more effective meningitis vaccines (protecting a greater range of pneumococcal and meningococcal serotypes); (3) a more effective tuberculosis vaccine; (4) a reduction in exposure to ototoxic medications; and (5) the development and implementation of cytomegalovirus and human immunodeficiency virus vaccines.
Noise exposure
In the USA, noise-induced hearing loss (determined by the presence of a high-frequency notch on the audiogram) is thought to affect approximately 12 per cent of children (aged 6–18 years)Reference Niskar, Kieszak, Holmes, Estaban, Rubin and Brody44 and approximately 24 per cent of adults (aged 18–65 years).Reference Carroll, Eichwald, Scinicariello, Hoffman, Deitchman and Radke45 Among adults who report exposure to loud noise at work, one-third have noise-induced hearing loss.Reference Carroll, Eichwald, Scinicariello, Hoffman, Deitchman and Radke45 This problem can go unrecognised until hearing loss associated with ageing (presbycusis) progresses more rapidly than anticipated. Prevention of noise-induced hearing loss can be achieved by limited exposure to noise greater than 85 dB for long periods. Hearing protection by use of ear plugs (or ear muffs) is recommended to anyone exposed to very loud noises (over 120 dB) even for very brief periods.
Resources for common ear conditions
Accurate examination of the ear requires special equipment. An otoscope is essential. It is traditionally a handheld device. Alternatives include the binocular headlamp plus magnification systems, video-otoscopes, or smart phone attachments that allow photography and video of the tympanic membrane. Lower cost options are being developed, such as the Fred Hollows Foundation ophthalmoscope and otoscope combination called the ‘Arclight’, which is portable, hardy and a fraction of the cost of traditional models.46 Whatever system you choose, pneumatic otoscopy or tympanometry are needed to reliably identify otitis media with effusion.
The ear canal needs to allow a clear view of the tympanic membrane. There is a lack of research on cleaning methods. For clearing debris, simple (washable and reusable) instruments should include a couple of serrated dental probes (wool carriers) and wax rings for removing foreign bodies. Alligator forceps are best for ‘graspable objects’. Probes with angled rings can be used to remove ‘non-graspable objects’. The micro-suction systems (favoured by ENT surgeons) require training and a reliable power supply.
Simple irrigation with a syringe and a soft, flexible tip is an excellent method for cleaning the ear of pus and debris, in order to facilitate tympanic membrane assessment. For children with CSOM, we find that syringing the ear canal with dilute betadine solution (1 in 20) is well tolerated. A trial assessing its impact as an adjunctive treatment (prior to topical antibiotic insertion) is currently underway (see Figure 1a–c for instructions on the use of dilute betadine wash). The cheapest safe technique for removing pus involves the use of ‘tissue spears’. These are made of regular tissues or toilet paper and can be done by families.
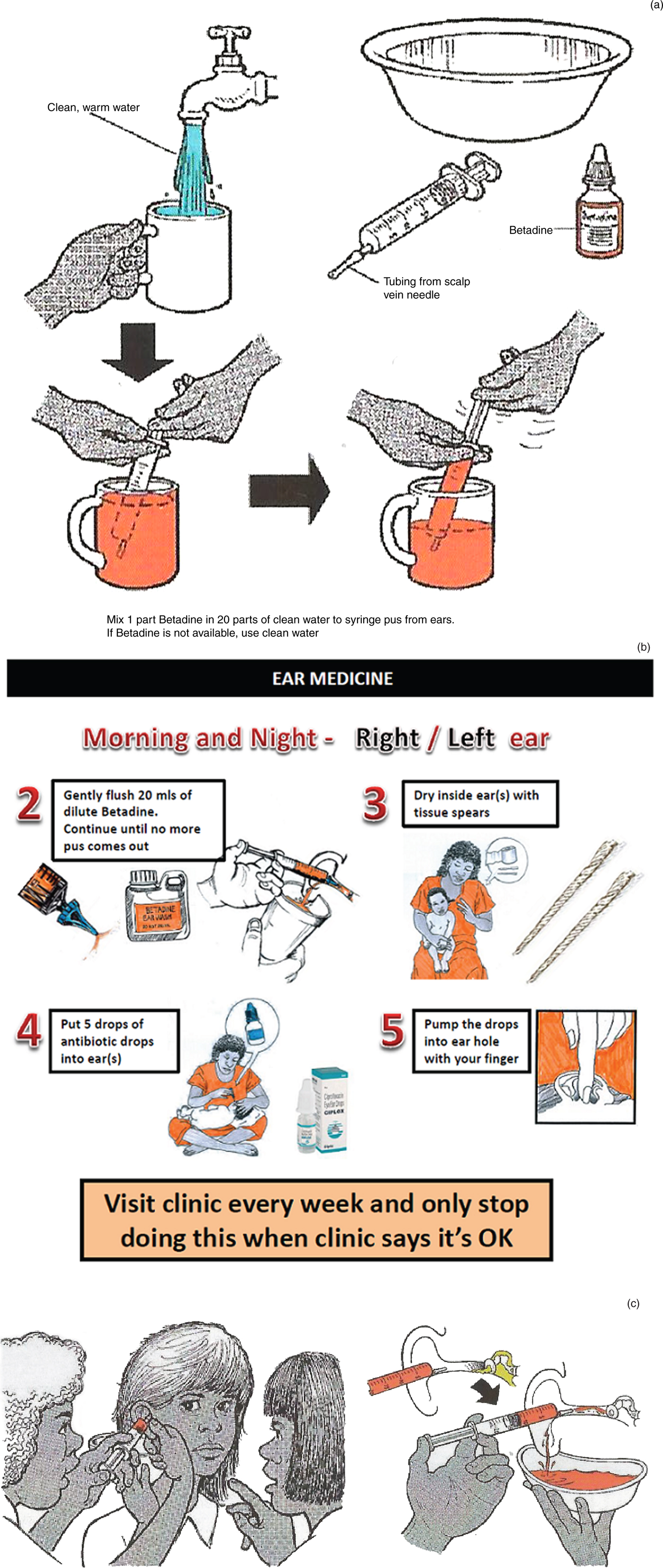
Fig. 1. (a) Drawing showing parents how to make up the dilute betadine solution, used to treat their child's chronic suppurative otitis media (CSOM). (b) Information provided to parents explaining how to use dilute betadine ear washes as part of their treatment for CSOM. (c) Drawing showing parents how to syringe dilute betadine ear wash into the ear.
The most important complication of ear disease is the impact on hearing loss. As well as documenting parents’ concerns, observation of communication during play is helpful. The ‘whisper test’ (whispering 2–3 syllable words or numbers from a distance of 2 feet (0.6 m)) will identify children most likely to have a significant hearing loss (positive likelihood ratio of over 9 for thresholds of 25–35 dB).Reference Pirozzo, Papinczak and Glasziou47 However, this test can be difficult to administer in young children. For an accurate threshold across multiple frequencies, play audiometry will determine the pure tone average hearing loss in co-operative children (from around three years of age).Reference Sininger48 For younger children, visual reinforced audiometry and brainstem evoked response testing can be used.
Access to appropriate medication is not always straightforward. The WHO suggests that oral antibiotics (amoxicillin and co-trimoxazole) and topical antibiotics (quinolone containing drops is preferred) are essential medicines.49
Viewing the tympanic membrane
Most clinicians examine the middle ear with a handheld otoscope and use the largest speculum that fits easily into the middle-ear canal. Because we care for large numbers of children with discharging ears, we prefer the spectacle-mounted Voroscope (which allows cleaning under direct binocular vision). Otomicroscopy or video-otoscopy with a high-quality camera probably provides the clearest image of the tympanic membrane. It is also possible to use a device that converts a mobile phone into an otoscope for photographing or videoing the tympanic membrane.
Making the diagnosis
Accurate diagnosis of the different forms of otitis media in primary care is difficult (especially in younger children). In a large study of 9 high-income countries, general practitioners were uncertain about the diagnosis of acute otitis media in: 42 per cent of children aged under 12 months, 34 per cent aged 13–30 months, and 26 per cent aged over 31 months.Reference Froom, Culpepper, Grob, Bartelds, Bowers and Bridges-Webb50
The diagnosis of otitis media with effusion is even more difficult (and especially challenging in resource-limited settings). The online training packages available through the Department of Pediatrics at the Children's Hospital of Pittsburgh and the EarInfoNet section of the Australian Indigenous HealthInfoNet are good resources.
Our approach to diagnosis is shown in Figure 2. The algorithm identifies four key questions that need to be answered: (1) ‘is discharge present in the ear canal?’; (2) ‘is there a perforation?’; (3) ‘is the tympanic membrane bulging?’; and (4) ‘does the tympanic membrane have normal mobility?’. Photographs that illustrate the important features of each condition are a helpful reminder and can also be used in discussion with the parents (Figures 3–6).
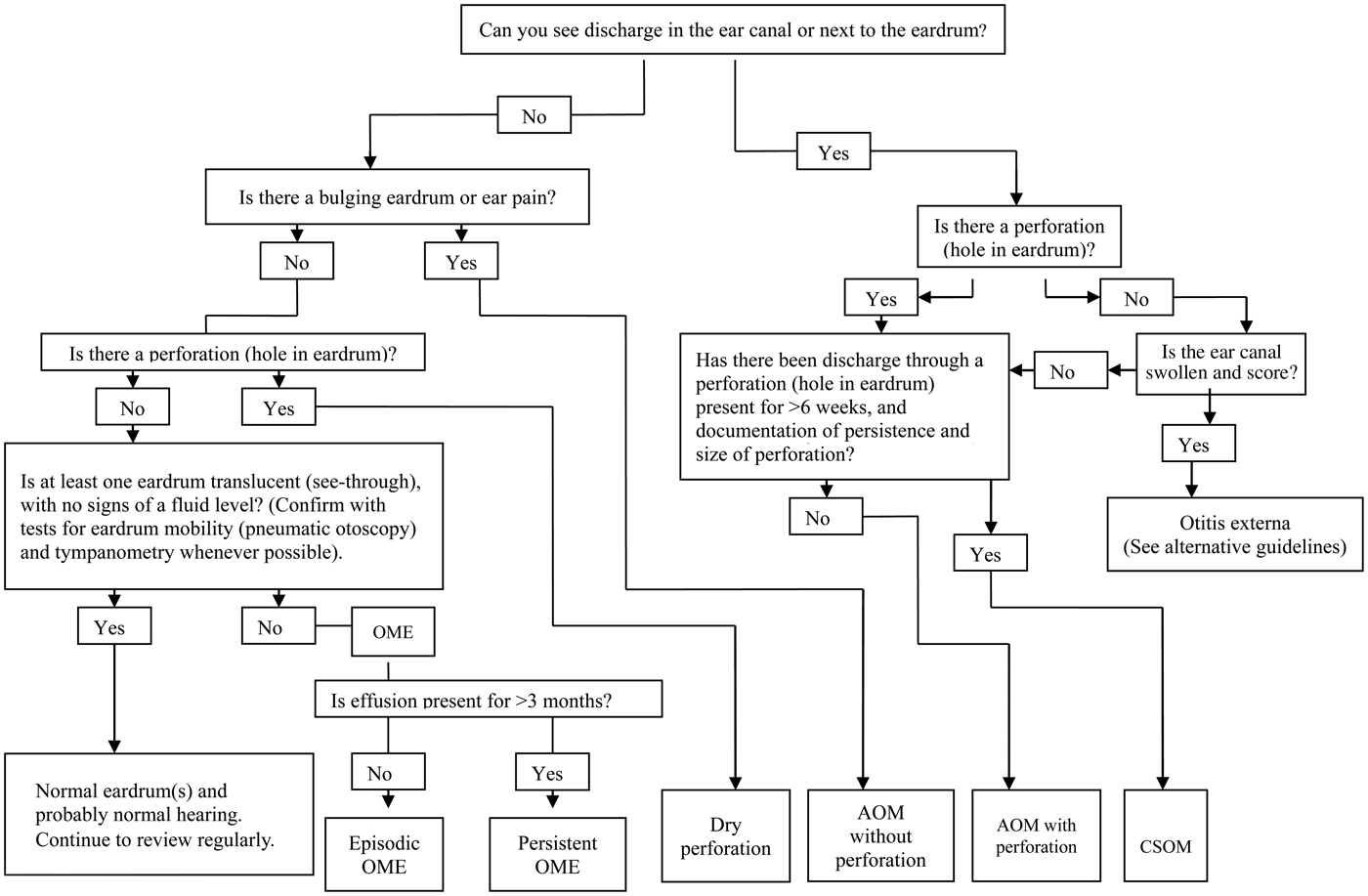
Fig. 2. Diagnostic algorithm for the different types of otitis media. OME = otitis media with effusion; AOM = acute otitis media; CSOM = chronic suppurative otitis media
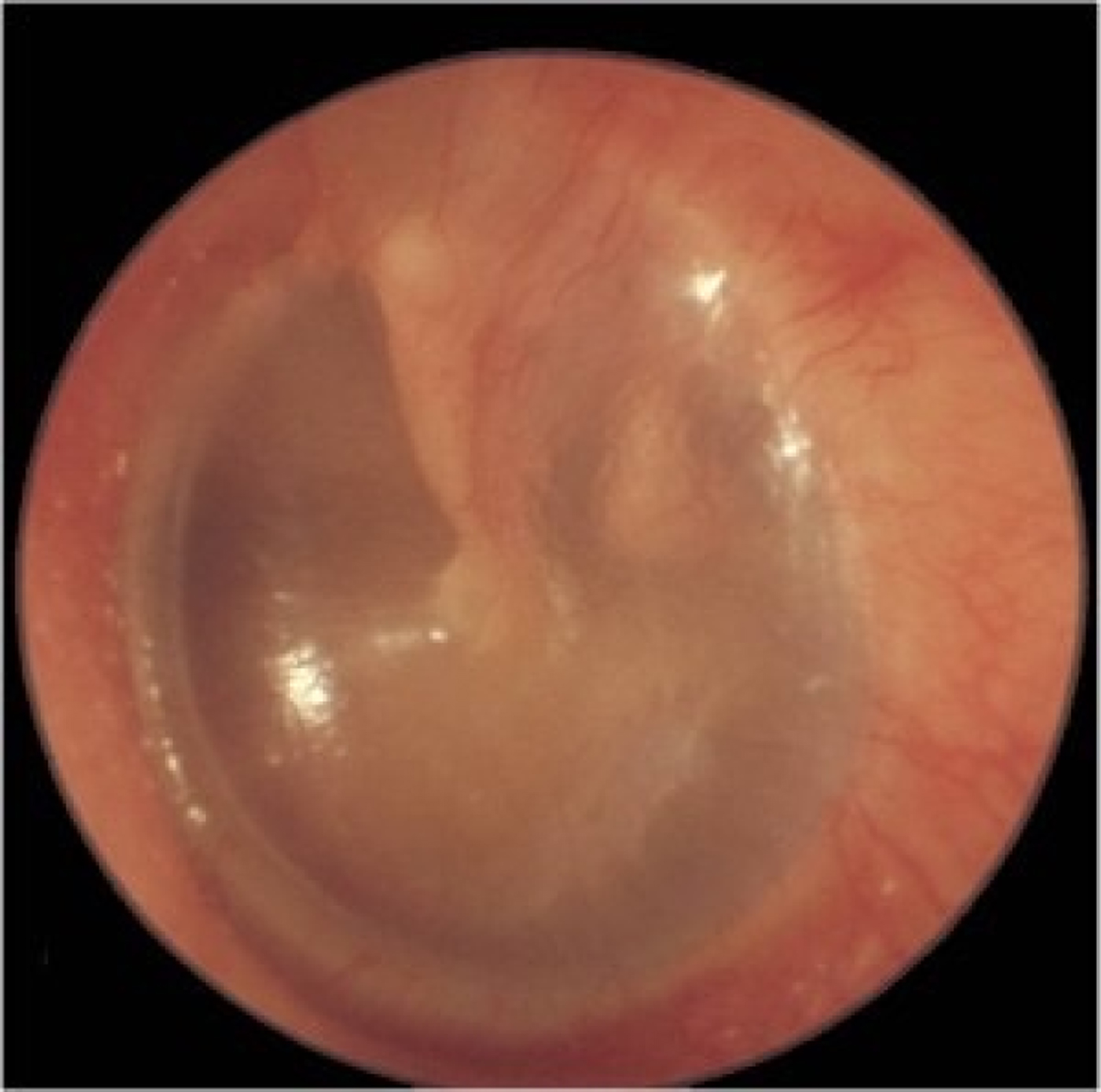
Fig. 3. Left tympanic membrane with features of a normal middle ear. The handle of the malleus is easily seen and situated in the neutral position and the tympanic membrane is translucent (so the incus is easily seen).
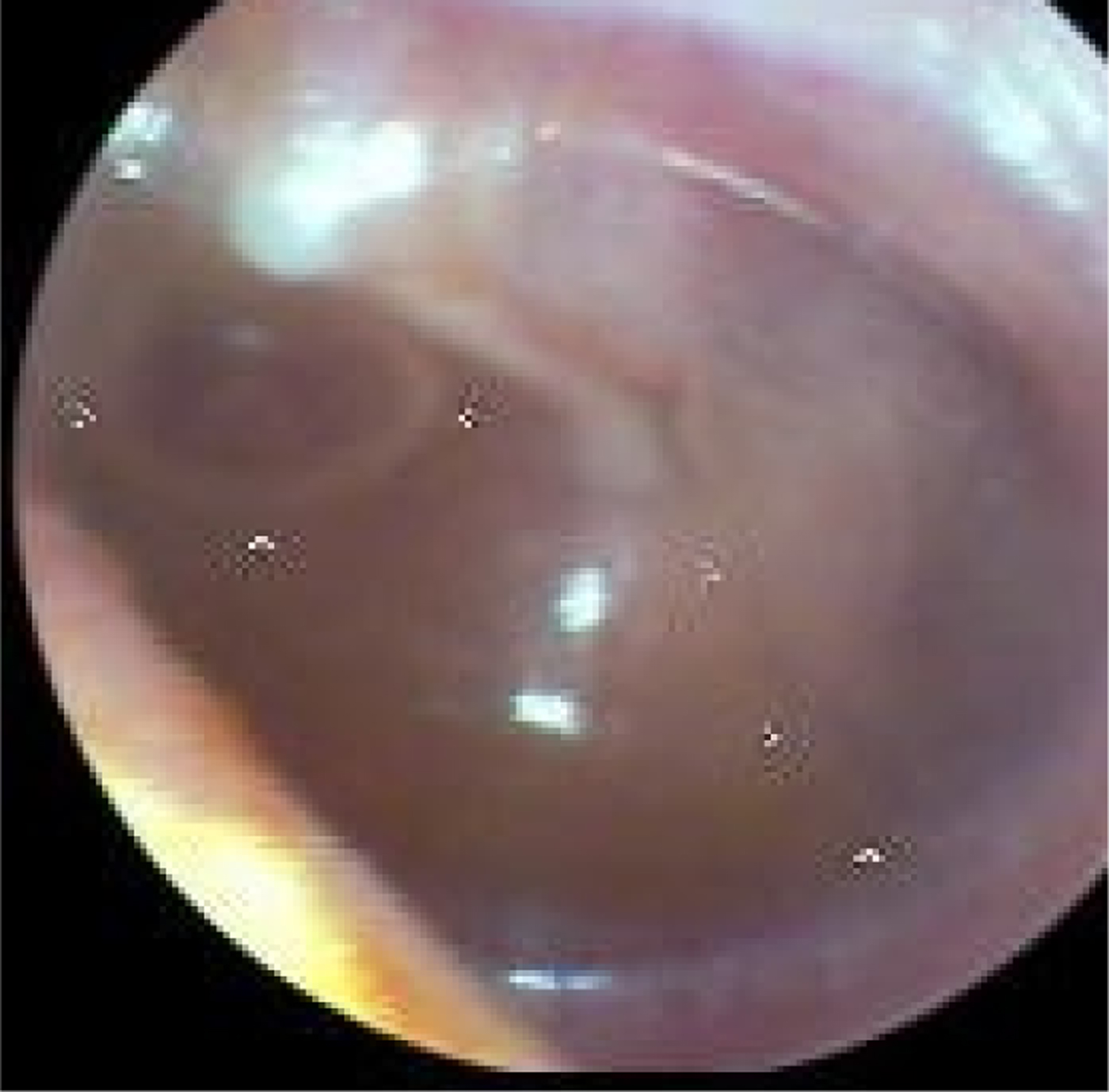
Fig. 4. Left tympanic membrane with features of otitis media with effusion. The handle of the malleus is easily seen and situated in a slightly retracted position. The tympanic membrane is not translucent and there is a fluid level in the anterior-superior quadrant.

Fig. 5. Right tympanic membrane with features of acute otitis media. The handle of the malleus is not easily seen (loss of landmarks). The tympanic membrane is white and the vessels are prominent. The tympanic membrane is very bulging with a central depression (where the handle of the malleus is attached to the tympanic membrane – the ‘donut sign’).
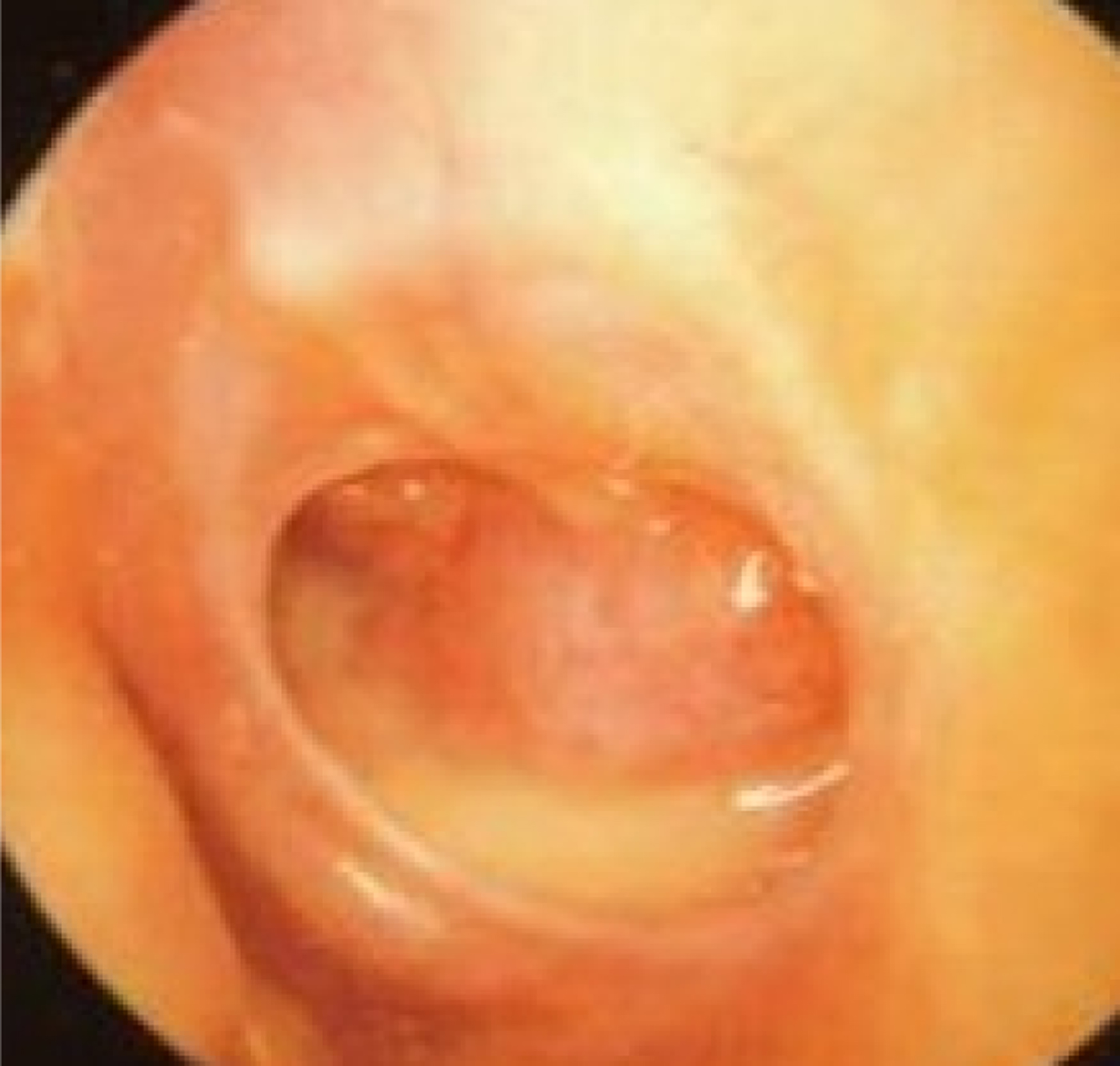
Fig. 6. Left tympanic membrane with features of chronic suppurative otitis media. The handle of the malleus may be difficult to see because of tympanic membrane thickening. There is a large perforation covering around 50 per cent of the pars tensa of the tympanic membrane. There is yellow pus pooling in the middle-ear space.
Perforation size is also important in determining management (Figure 7). Pinhole perforations (covering less than 2 per cent of the tympanic membrane) need oral antibiotics (unless topical antibiotics can be injected through the perforation). Medium-sized perforations (from over 2 per cent to 25 per cent of the tympanic membrane) usually (but not always) heal if they are kept dry. Around 2 per cent of children who have tympanostomy tube insertions are left with small chronic dry perforations.51 Large perforations (over 25 per cent of the tympanic membrane) are more likely to persist and may need tympanoplasty in the future.Reference Morris, Leach, Shah, Nelson, Anand and Daby52 Children with chronic otorrhoea despite treatment, or with an attic perforation (above the handle of the malleus), should be referred to an ENT surgeon to exclude cholesteatoma.Reference Bhutta, Williamson and Sudhoff53 For children without a perforation, normal tympanic membrane mobility or a peaked tympanogram excludes otitis media.Reference Margolis, Hunter and Giebink54
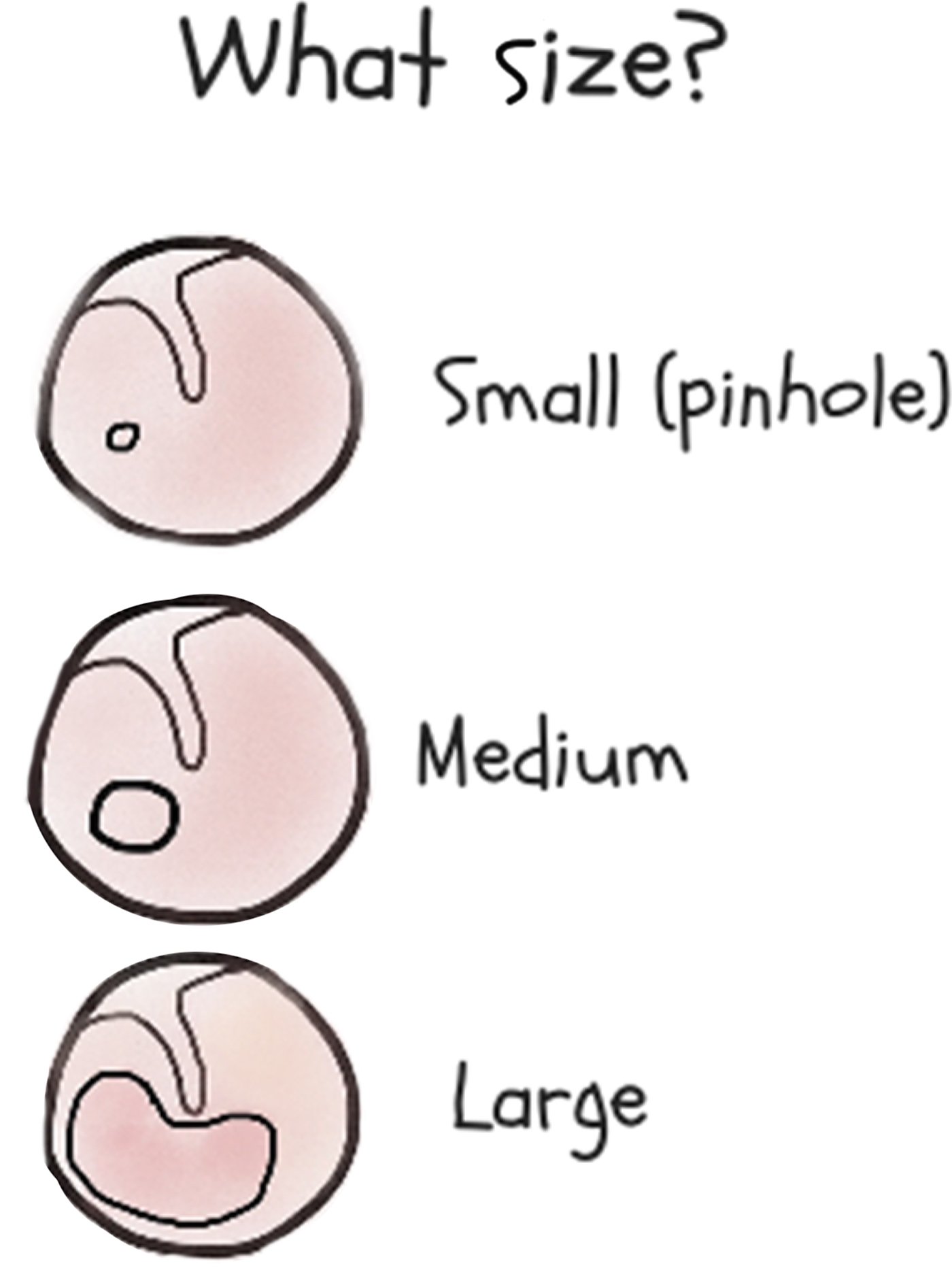
Fig. 7. Guide to establishing the size of a tympanic membrane perforation.
Treatment of common ear conditions
There have been a relatively large number of randomised controlled trials (RCTs) assessing various treatments for the different forms of otitis media. It is now clear that the benefits of most treatments are modest (and so small studies are not able to detect differences). The interventions for which there is moderate or high certainty of effect are listed in Table 2.Reference Fortanier, Venekamp, Boonacker, Hak, Schilder and Sanders15–Reference Macfadyen, Acuin and Gamble24
Acute otitis media with perforation
Around 7 per cent of children with acute otitis media will progress to acute otitis media with perforation, but rates vary from 2 per cent to 17 per cent in different studies.Reference Venekamp, Sanders, Glasziou, Del Mar and Rovers20 Children under the age of two years are also more prone to acute otitis media with perforation. Incidence decreases with increasing age. Children with acute otitis media with perforation have worse prognosis, and are more likely to experience prolonged pain and fever. Several studies have reported that children with acute otitis media with perforation, regardless of aetiology, are more prone to recurrences and repeated tympanic membrane perforation.
Treatment of acute otitis media with perforation should involve similar principles as treatment of acute otitis media without perforation. However, the benefit of antibiotic treatment is much greater (number needed to treat = 3).Reference Venekamp, Sanders, Glasziou, Del Mar and Rovers20, Reference Rovers, Glasziou, Appelman, Burke, McCormick and Damoiseaux55 Antibiotics will reduce the duration of pain and fever.Reference Venekamp, Sanders, Glasziou, Del Mar and Rovers20, Reference Rovers, Glasziou, Appelman, Burke, McCormick and Damoiseaux55 We recommend a longer duration of antibiotics than required for standard treatment of acute otitis media without perforation (i.e. over 7 days). Antibiotics should be continued until there is no discharge for at least 48–72 hours. As the perforation is usually very small (less than 2 per cent of the tympanic membrane), topical antibiotics cannot easily penetrate the perforation and reach the site of infection.
Chronic suppurative otitis media
Chronic suppurative otitis media refers to persistent discharge through a visible tympanic membrane perforation lasting at least six weeks (although definitions vary from greater than two weeks to greater than three months). A conductive hearing loss is common. While the average air–bone gap is around 30 dB, hearing levels can vary from normal hearing to a severe hearing loss.
Common bacteria found in CSOM are pseudomonas species, proteus species and Staphylococcus aureus.Reference Mittal, Lisi, Gerring, Mittal, Mathee and Narasimhan56 Formation of biofilm may mean that prolonged adherence to therapy is required for success.Reference Jensen, Johansen, Bjarnsholt, Eickhardt-Sørensen and Homøe57 Infection with resistant bacteria can lead to treatment failure even when adherence is good.
For all discharging tympanic membrane perforations over 2 per cent of the surface area, topical antibiotic treatment is recommended. This provides higher antibiotic concentration at the site of infection without unwanted systemic side effects. Families should be educated about the importance of clearing the ear canal of pus before applying ear drops (so they do reach the site of infection). Dry mopping of the ear canal with tissue spears can be performed. Alternatively (or in addition), ear syringing with dilute betadine is effective (Figure 1a–c). Meta-analysis has shown topical antibiotics to be superior to aural toileting (number needed to treat = 2) and topical antiseptics (number needed to treat = 4).Reference Macfadyen, Acuin and Gamble23 Topical quinolone antibiotics are preferred to aminoglycosides given the risk of ototoxicity. The addition of topical steroids has not been shown to have any benefit.
There is some evidence (low in certainty) to suggest a role for oral antibiotics. One RCT demonstrated that children treated with trimethoprim-sulfamethoxazole as an adjunct to topical therapy had less persistent otorrhoea after six weeks (number needed to treat = 4).Reference van der Veen, Rovers, Albers, Sanders and Schilder58 It is unclear whether this benefit persists long-term and whether the results are generalisable to other populations.
Once treatment for CSOM is commenced, patients should be reviewed weekly for resolution, and again four weeks after resolution of discharge. Secondary fungal infections (requiring topical antifungal treatment) may occur after broad spectrum topical antibiotic treatment.
Dry perforation
A dry perforation of the tympanic membrane is unlikely to cause symptoms other than a hearing loss. Respiratory illnesses and exposure to contaminated water may lead to further discharge. If there are identifiable factors that result in active disease (e.g. swimming), then advice should be provided on prevention. There is no strong evidence for universal water precautions.Reference Moualed, Masterson, Kumar and Donnelly59 A small RCT showed no difference in otorrhoea in Australian Aboriginal children who swam daily in a chlorinated pool.Reference Stephen, Leach and Morris60
Referral to an ENT specialist is recommended in cases where there is significant hearing loss or frequent recurrent active disease. Tympanoplasty involves repairing the tympanic membrane and possibly the ossicular chain. The success rate for closure is approximately 80–90 per cent and (if the ossicular chain is intact) there will also be improvement in hearing post-operatively.Reference Tan, Santa Maria, Eikelboom, Anandacoomaraswamy and Atlas61
Acute otitis media without perforation
A bulging tympanic membrane is the most reliable indicator of acute otitis media without perforation. Treatment involves several strategies of pain relief, watchful waiting, antibiotics and medical review.
A Cochrane Review described low-certainty evidence that paracetamol (or non-steroidal anti-inflammatory medication) is better at relieving pain at 48 hours compared with placebo (number needed to treat = approximately 6).Reference Sjoukes, Venekamp, van de Pol, Hay, Little and Schilder62 Additional topical anaesthetic drops resulted in a 50 per cent reduction in pain scores at 10 minutes (low-certainty evidence).Reference Bolt, Barnett, Babl and Sharwood63
Prescribing antibiotics routinely for acute otitis media without perforation remains controversial.Reference Lieberthal, Carroll, Chonmaitree, Ganiats, Hoberman and Jackson64 The decision should be based on age, extent of disease, history of previous otorrhoea and risk of subsequent CSOM. For children at low risk of CSOM, a ‘watchful waiting’ approach can be taken. A meta-analysis of children with acute otitis media who received immediate antibiotics compared to watchful waiting revealed that antibiotics were associated with modest benefits in terms of pain relief and no long-term benefits.Reference Venekamp, Sanders, Glasziou, Del Mar and Rovers20 A watchful waiting approach also reduced side effects from antibiotics (number needed to harm = 9).
A watchful waiting approach may not be the best option in a child at high risk of CSOM. We recommend that amoxicillin 50 mg/kg/day be prescribed for 7 days in these children. Clinical treatment failure should prompt an increase to 90 mg/kg/day, with the option of broadening the spectrum of antibiotics to include amoxicillin clavulanate. Longer courses of antibiotics (7 or more days) provide a modest benefit compared with shorter courses.Reference Hoberman, Paradise, Rockette, Kearney, Bhatnagar and Shope65 Outcomes from twice daily doses are very similar to antibiotics given three times a day. In cases where there is concern regarding adherence, or if the family has limited access to refrigeration, a single dose of azithromycin (30 mg/kg/dose) has a sufficiently long half-life to be effective.
The available evidence does not support the use of decongestants and/or antihistamines, and most guidelines recommend against their use. The limited data available means that there are no recommendations for complementary or alternative procedures in the treatment of acute otitis media.Reference Marom, Marchisio, Tamir, Torretta, Gavriel and Esposito66
Prophylactic antibiotics are not routinely recommended for recurrent acute otitis media, as the benefit is modest. A Cochrane Review found that long-term prophylactic antibiotics prevent around 50 per cent of infections. This translates to 1.5 episodes of acute otitis media for every 12 months of treatment per otitis-prone child (95 per cent CI = 1.2–2.1) who would otherwise experience an average of 3 episodes annually.Reference Leach and Morris19 Children with recurrent disease may also benefit from tympanostomy tube placement. Similar benefits have been described in RCTs of tympanostomy tubes with less certainty of effect.Reference McDonald, Langton Hewer and Nunez67
Otitis media with effusion
Otitis media with effusion (OME) often occurs after upper respiratory tract infections. It usually resolves within a few days to weeks. Only bilateral OME that persists for longer than three months plus a hearing loss of over 20 dB is a cause for concern. Even then, there does not seem to be a problem in waiting another three to six months before taking action. However, the impact on speech and language development or hearing is still uncertain.
In RCTs, antibiotics assist in the resolution of OME.Reference Venekamp, Burton, van Dongen, van der Heijden, van Zon and Schilder17 In developed countries, tympanostomy tube insertion is a common operation. Tympanostomy tubes normalise hearing for the life of the tube. This is typically 6–12 months.Reference Browning, Rovers, Williamson, Lous and Burton21 For children who receive tympanostomy tube insertions, we recommend ciprofloxacin-based ear drops for 3–5 days after the operation, to reduce the risk of discharge after the procedure.Reference Syed, Suller, Browning and Akeroyd68 Another option is combining tympanostomy tubes with adenoidectomy. In RCTs, adenoidectomy (compared to no surgery) reduces the risk of subsequent otitis media (especially for children aged four years and older).Reference van den Aardweg, Schilder, Herkert, Boonacker and Rovers69, Reference Boonacker, Rovers, Browning, Hoes, Schilder and Burton70 However, the benefits of adenoidectomy as an adjunctive therapy in addition to tympanostomy tubes is still unclear.
Discharge through a tube (tympanostomy tube otorrhoea) is the most common complication and may affect up to 50 per cent of children.Reference Froom, Culpepper, Grob, Bartelds, Bowers and Bridges-Webb50 It is usually not a major problem and can be treated with ototopical antibiotic drops.Reference Venekamp, Javed, van Dongen, Waddell and Schildern22 Sometimes the tubes come out prematurely (approximately 5 per cent are out before three months) or become blocked (5–10 per cent).Reference Froom, Culpepper, Grob, Bartelds, Bowers and Bridges-Webb50, Reference Steele, Adam, Di, Halladay, Balk and Trikalinos71 Occasionally the eardrum does not heal after the tube has extruded (2–3 per cent). Sometimes a tube will not extrude by itself, and an operation becomes necessary to remove it (5–10 per cent).Reference Froom, Culpepper, Grob, Bartelds, Bowers and Bridges-Webb50 More serious (rare) complications include: cholesteatoma due to invagination of skin through the tympanic membrane (approximately 0.8 per cent) and a new sensorineural hearing loss (less than 1 per cent).Reference Froom, Culpepper, Grob, Bartelds, Bowers and Bridges-Webb50
Around 20–30 per cent of children will meet the criteria for tympanostomy tube reinsertion. Many ENT surgeons would recommend an adenoidectomy at this point.Reference van den Aardweg, Schilder, Herkert, Boonacker and Rovers69, Reference Boonacker, Rovers, Browning, Hoes, Schilder and Burton70 The additional risk associated with adenoidectomy is justified if the child requires a second set of tubes.Reference Kadhim, Spilsbury, Semmens, Coates and Lannigan72
Treatment of otitis media complications
The commonest complication of acute otitis media is a progression along the disease pathway to CSOM.Reference Mittal, Lisi, Gerring, Mittal, Mathee and Narasimhan56 More serious (but less common) complications are classified as either intracranial or extracranial.Reference Schilder, Marom, Bhutta, Casselbrandt, Coates and Gisselsson-Solen73 The important intracranial complications are meningitis, brain abscess, venous sinus thrombosis, extradural abscess and subdural abscess.Reference Jain, Arora, Meher, Passey and Bansal74–Reference Yorgancilar, Yildirim, Gun, Bakir, Tekin and Gocmez76 Regarding intracranial complications, older children with CSOM are at greatest risk. The most important extracranial complication is mastoiditis (incidence of approximately 4–12 per 100 000 in young children, and approximately 1–6 per 100 000 across all ages in high-income countries).Reference Halgrimson, Chan, Abzug, Perkin, Carasone-Link and Simoes77–Reference Loh, Phua and Shaw79 Regarding mastoiditis, children under two years of age are at greatest risk.
All intracranial complications are potentially life-threatening and need prompt treatment with intravenous antibiotics, with or without surgery. Anticoagulation is usually appropriate for sinus venous thrombosis. Early surgical opinion is also recommended (ENT with or without neurosurgery if available).
There are many possible extracranial complications. Facial palsy and labyrinthitis complicating acute otitis media are both well recognised. Cholesteatomas are slow-growing masses of squamous epithelial cells trapped behind the tympanic membrane. They can be associated with infection and should be considered when treatment for CSOM is unsuccessful.Reference Bhutta, Williamson and Sudhoff53 Acute mastoiditis occurs when the bacterial infection (most commonly S pneumoniae, H influenza, Streptococcus pyogenes or S aureus) spreads across the mucosal surface and through bone to cause subperiosteal infection. In a systematic review, this was the most common complication and occurred in approximately 60 per cent of mastoiditis cases.Reference van den Aardweg, Rovers, de Ru, Albers and Schilder80 Rarely, a Bezold's abscess (due to infection tracking inferomedially in muscles of the neck) may result.Reference Sheikh, Murday, Abbas, Main, King and Rao81 Alternatively (and very rarely), local spread of infection to the temporal bone apex may cause Gradenigo's syndrome.Reference Vitale, Amrit, Arora and Lata82 This syndrome is associated with ipsilateral retro-orbital pain, abducens nerve palsy (unable to abduct the eyeball) and otorrhoea. Local infection can also spread into the inner ear, resulting in acute vertigo and, in some cases, sensorineural hearing loss.
The initial treatment of mastoiditis is intravenous antibiotics. The lack of randomised controlled trials testing alternative treatments means that the non-surgical approach needs to be closely monitored. Clinical deterioration, persistent fever, or no improvement in symptoms and signs by 48–72 hours should be regarded as treatment failure.Reference Chesney, Black and Choo78 In these cases, needle aspiration or surgical incision and drainage (with or without myringotomy, and with or without tympanostomy tube insertion) is indicated. Cortical mastoidectomy (extensive surgical drainage) is not required in most cases of mastoiditis, but it can be life-saving. Early involvement of ENT surgeons will help establish criteria for surgical intervention in each patient.
Primary care of ear canal
There are three common problems affecting the outer ear canal: wax impaction, otitis externa and foreign bodies. These conditions are not usually associated with systemic illness, but they still create a substantial demand on the available services.
Wax impaction
Ear wax (cerumen) is a naturally occurring substance. It is a mix of secretions produced by the sebaceous glands and sweat glands of the outer two-thirds of the ear canal, combined with exfoliated skin and debris. Ear wax gradually moves along the canal to protect, clean and lubricate the ear canal.Reference Schwartz, Magit, Rosenfeld, Ballachanda, Hackell and Krouse83
In their recent evidence-based guidelines, the American Academy of Otolaryngology defined ‘impacted wax’ as any build-up of wax that either caused symptoms or prevented examination of the ear canal, tympanic membrane or audiovestibular system (i.e. total blockage is not required to meet their definition).Reference Schwartz, Magit, Rosenfeld, Ballachanda, Hackell and Krouse83 The guidelines made a strong recommendation that wax impaction should be treated.Reference Schwartz, Magit, Rosenfeld, Ballachanda, Hackell and Krouse83 They also highlighted six clinical strategies: (1) give advice on appropriate ear canal hygiene in those at risk of wax impaction; (2) only treat wax impaction when it causes symptoms or prevents a necessary assessment; (3) check for wax impaction in patients with hearing aids; (4) check for factors that might modify treatment in anyone with wax impaction (e.g. tympanic membrane perforation, previous ear surgery or radiotherapy, diabetes, exostoses, canal stenosis); (5) use appropriate interventions for the treatment of wax impaction (ceruminolytic agents, syringing or manual removal); and (6) follow up anyone who undergoes treatment.
A Cochrane Review of ear drops for the removal of ear wax described 9 randomised controlled trials (RCTs) involving 679 participants and 11 different treatments.Reference Burton and Doree84 The authors concluded that the studies were small and heterogeneous. While it appears that ear drops are probably better than no treatment, it is not clear if any of the drops tested are superior. Furthermore, most of the participants in the trials still needed syringing after treatment. This suggests that ear drops are unlikely to be more than an adjunctive treatment for many patients.
There are no RCTs of syringing, but it is generally agreed that it is an effective option. There is a small risk (less than 1 per cent) of serious complications such as tympanic perforation or vertigo.Reference Bird85 Manual removal under direct vision is also thought to be an effective treatment. To date, this approach has not been compared against other options in RCTs.
Otitis externa
Acute otitis externa is defined as a diffuse inflammation of the external ear canal, which may also involve the tympanic membrane and the pinna.Reference Rosenfeld, Schwartz, Cannon, Roland, Simon and Kumar86 It is usually very painful, and the tender, swollen canal wall makes visualisation of the tympanic membrane difficult. Pain on movement of the tragus and/or pinna can help to distinguish otitis externa from acute otitis media. The common principal pathogens associated with otitis externa are Pseudomonas aeruginosa and staphylococcus species (including S aureus).Reference Roland and Stroman87 They are usually part of a polymicrobial infection. Secondary fungal infection (with candida or aspergillus) can also occur.Reference Martin, Kerschner and Flanary88
In its severe form (‘necrotising otitis externa’ or ‘malignant otitis externa’), destruction of the adjacent temporal bone and its mastoid process occurs. Necrotising otitis externa should be suspected in patients with severe pain (keeping them awake at night), and those with diabetes or who are immunocompromised. Necrotising otitis externa can be a life-threatening condition in the immunocompromised patient, and requires treatment with intravenous antibiotics, with or without surgery.
Otitis externa (‘swimmer's ear’) is more common in hot, humid climates.Reference Hoadley and Knight89 People who have narrow external auditory canals, hearing aid users, anyone with eczema near the ear canal skin, and those who have experienced recent ear canal trauma or syringing are at increased risk. In the USA, around 1 in 123 people are affected each year, and half of these are children aged 5–14 years old.90 Overall, it is estimated that around 10 per cent of the population are affected by otitis externa at some time in their life.Reference Hajioff and MacKeith91
Recent evidence-based guidelines for the management of otitis externa make strong recommendations to manage pain effectively with appropriate analgesics and to avoid initial treatment with systemic antibiotics (unless there is extension of infection beyond the ear canal or a high risk of complications).Reference Rosenfeld, Schwartz, Cannon, Roland, Simon and Kumar86 They highlight six clinical strategies: (1) ensure that there is not another explanation for the ear pain; (2) consider other factors that might modify treatment (e.g. presence of tympanic membrane perforation, immunodeficiency etc.); (3) use topical therapy initially; (4) ensure that delivery of topical treatment is maximised (by cleaning, using wicks etc.); (5) use a non-ototoxic topical treatment if a tympanic membrane perforation is present; and (6) review the patient if there is no improvement in 48–72 hours. In populations with high rates of CSOM, a follow-up examination (after ear canal swelling subsides) is appropriate to ensure that middle-ear discharge has not caused otitis externa.
A Cochrane Review of interventions for acute otitis externa described 19 RCTs with 3382 participants. They concluded that most of the trials were at a high risk of bias and few were conducted in the primary care setting.Reference Kaushik, Malik and Saeed92 A single trial demonstrated that topical antibiotic treatment was superior to placebo. Most of the studies comparing one topical treatment with another did not find significant differences. The Clinical Evidence Otitis Externa Review reached similar conclusions.Reference Hajioff and MacKeith91 The authors advised that the combination of topical antibiotics plus steroids is effective. Topical treatments should generally be used for at least one week and continued for a second week if the patient is still symptomatic.Reference Kaushik, Malik and Saeed92 The role of cleaning and ear irrigation by an experienced clinician has not been formally studied, but is standard practice in referral centres.
Foreign bodies
Foreign bodies in the external auditory canal are a common problem in children (and also occur in adults). They can present early to the doctor or emergency department for removal, or be found incidentally on a routine ear examination in children. In an Australian study of children and adults, the common foreign bodies in children were beads, cotton tips, insects and paper.Reference Ryan, Ghosh, Wilson-Boyd, Smit and O'Leary93 In adults, the common foreign bodies were cotton tips, insects, silicon plugs, cotton wool and paper.Reference Ryan, Ghosh, Wilson-Boyd, Smit and O'Leary93 In US studies, foreign bodies that were smooth and firm (non-graspable), or were associated with previous removal attempts, were more likely to result in further failed attempts at removal.Reference Thompson, Wein and Dutcher94, Reference Marin and Trainor95 If there is no infection present and the foreign body is not causing pain, removal can be done electively. It may be appropriate to wait until someone more experienced (or with appropriate equipment) is available.
Foreign body removal requires a similar approach to that described for cleaning the ear. If the foreign body is an insect and it is still alive, fill the canal with oil (e.g. olive oil) for 5 minutes to kill it before attempting removal. Exposure to water needs to be avoided if the foreign body has the capacity to expand when wet. Sometimes a general anaesthetic is needed for removal in children, but this is rarely necessary in adults.
Conclusion
The effective management of ear conditions is an important part of primary healthcare. Otitis media is common in all populations. Treatment effects are more substantial in children with acute otitis media with perforation, and with CSOM. This means that initial watchful waiting of otitis media with effusion and acute otitis media without perforation is sensible (especially where resources are limited). For children at risk of more severe infections, antibiotic treatment is appropriate. Antibiotic treatment needs to continue until the infection has resolved. Globally, there needs to be greater recognition that CSOM is a preventable condition that can have a profound impact on quality of life over many years.
Healthy ears are needed for good hearing. Sometimes it is not possible to restore the hearing back to normal levels. An explanation to parents about how communication can be improved is always appropriate. Unfortunately, access to appropriate hearing aids for children and adults with significant hearing loss is beyond the reach of many families. This means that the prevention of severe disease will remain a priority for the foreseeable future. Over time, improved living conditions and washing facilities, better access to vaccines, early identification of severe disease, and appropriate use of antibiotics will reduce the burden of ear disease around the world.
Acknowledgements
We thank Mr Mahmood Bhutta for his encouragement, helpful suggestions and editorial advice. We thank Professor Harvey Coates for providing the drawings that demonstrate the use of dilute betadine ear washes. Preparation of the manuscript was supported by the National Health and Medical Research Council of Australia through funding of the Centre for Research Excellence in Ear and Hearing Health of Aboriginal and Torres Strait Islander Children.
Competing interests
None declared











