Introduction
Welfare can be assessed using different approaches, such as management-based, resource-based and animal-based measures (Welfare Quality®, 2009). Another approach to assess welfare is measuring the stress response using glucocorticoids, particularly cortisol (Cook, Reference Cook2012). Welfare scores using Welfare Quality® Assessment Protocol and cortisol concentration in pooled hair samples have been compared to evaluate if these two methods are in agreement in dairy cows (Vesel and Pavić, Reference Vesel and Pavić2019).
Cortisol, also sometimes called the stress hormone, is a glucocorticoid hormone. Its blood level is controlled by the hypothalamic–pituitary–adrenal (HPA) axis. In an organism cortisol exhibits catabolic activity with accelerated glucose, fat, and protein metabolism. As a result of high HPA axis activity during stress, blood cortisol level increases as a metabolic coping adaptation. Since cortisol is released in response to stress (Sjaastad et al., Reference Sjaastad, Hove and Sand2010), it has been increasingly used as a biomarker of stress in animals (Romero, Reference Romero2004). The relation between HPA axis activity and hair cortisol concentration has been confirmed in dairy cows and calves (Gonzalez-de-la-Vara Mdel et al., Reference González-de-la-Vara Mdel, Valdez, Lemus-Ramirez, Vázquez-Chagoyán, Villa-Godoy and Romano2011; Talló-Parra et al., Reference Talló-Parra, Manteca, Sabes-Alsina, Carbajal and Lopez-Bejar2015). In contrast to cortisol concentration in blood, saliva, faeces, urine and milk, hair cortisol concentration is a marker of chronic stress that reflects a period of weeks or months (Comin et al., Reference Comin, Prandi, Perić, Corazzin, Dovier and Bovolenta2011; Meyer and Novak, Reference Meyer and Novak2012). The exact mechanism by which cortisol is incorporated into hair is not yet fully understood. The main source of incorporation is thought to be the vascular supply during hair growth, additionally cortisol is likely to incorporate into the hair from the surrounding tissues and possibly fluids, such as sweat and sebum. Some studies report that the hair follicle itself is a potential source of cortisol synthesis, however most authors assume hair cortisol reflects systemic levels (Meyer and Novak, Reference Meyer and Novak2012). Hair cortisol concentration is not likely to be affected by manipulation during sampling and daily physiological or acute changes (Mastromonaco et al., Reference Mastromonaco, Gunn, McCurdy-Adams, Edwards and Schulte-Hostedde2014). It is important to emphasize that hair sampling is non-invasive, simple (Mesarcova et al., Reference Mesarcova, Kottferova, Skurkova, Leskova and Kmecova2017) and painless (Ouschan et al., Reference Ouschan, Kuchar and Moestl2013). Furthermore, hair can be stored at room temperature (Gow et al., Reference Gow, Thomson, Rieder, Van Uum and Koren2010), and only a small amount of hair is needed for analysis (Stalder and Kirschbaum, Reference Stalder and Kirschbaum2012). Because of these advantages, hair cortisol seems to be a very promising non-invasive biomarker of animal welfare and has already been associated with housing, management and handling of animals (Heimbürge et al., Reference Heimbürge, Kanitz and Otten2019).
Fixed factors affecting hair cortisol concentration
Studies that detected effects of different factors on hair cortisol concentration in cattle are shown in Table 1. Some of these suggest an effect of age on hair cortisol concentration, for instance, mature Busha cows had a higher concentration than Busha heifers (Nedić et al., Reference Nedić, Pantelić, Vranješ-Đurić, Jovanović, Čebulj-Kadunc, Kobal, Snoj and Kirovski2017). Busha cows live under challenging environmental factors, which could inhibit shedding of hair in cows (Dowling, Reference Dowling1958; Nedić et al., Reference Nedić, Pantelić, Vranješ-Đurić, Jovanović, Čebulj-Kadunc, Kobal, Snoj and Kirovski2017). As a result, cortisol concentration in cows, which had been subject to environmental challenge for a longer period, could be higher compared to heifers due to a difference in hair age. On the other hand, higher hair cortisol concentrations were found in 15-day-old female calves compared to 2-year-old cows, which could be a result of foetal pituitary-adrenal axis stimulation in late pregnancy (Gonzales-de-la-Vara Mdel et al., Reference González-de-la-Vara Mdel, Valdez, Lemus-Ramirez, Vázquez-Chagoyán, Villa-Godoy and Romano2011).
Table 1. Fixed factors affecting hair cortisol levels in cattle
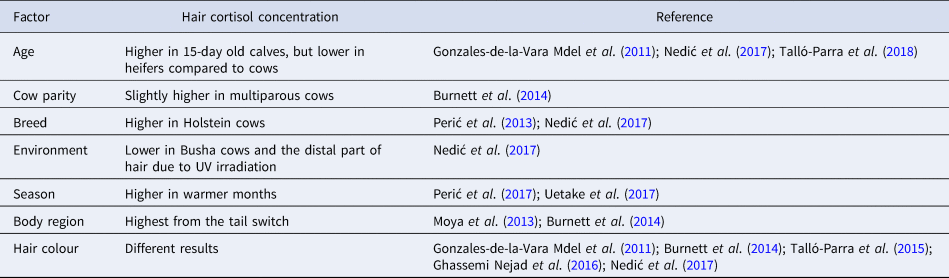
In a study by Talló-Parra et al. (Reference Talló-Parra, Carbajal, Monclús, Manteca and Lopez-Bejar2018), no effect of age and parity was detected, although all cows were adult at the time of sampling. Slightly higher hair cortisol concentration was detected in multiparous cows compared to primiparous cows in a study by Burnett et al. (Reference Burnett, Madureira, Silper and Nadalin2014). These results are contrary to the belief that primiparous cows suffer more stress because of the changes during the transition period (Burnett et al., Reference Burnett, Madureira, Silper and Nadalin2014) and suggest older cows are not necessarily more adapted to handling and herd routine (Talló-Parra et al., Reference Talló-Parra, Carbajal, Monclús, Manteca and Lopez-Bejar2018). Overall, age-related variation may be due to physiological state rather than age per se.
It has been reported that hair cortisol concentration is influenced by breed. In dairy cattle, higher concentrations were found in the hair of Holstein-Friesian cows in comparison to crossbreed F1 heifers with Swedish Red and Montbéliarde breeds (Perić et al., Reference Perić, Comin, Corazzin, Montillo, Cappa, Campanile and Prandi2013). Higher concentrations were reported in Holstein cows and heifers in comparison to Busha cows and heifers as well (Nedić et al., Reference Nedić, Pantelić, Vranješ-Đurić, Jovanović, Čebulj-Kadunc, Kobal, Snoj and Kirovski2017), possibly as a result of higher milk production and intensive production systems, and hence greater metabolic demand and stress related to this. Lower hair cortisol concentrations of Busha cows in comparison to Holstein cows could also be explained by the effect of UV irradiation, which has been reported by Wester et al. (Reference Wester, van der Wulp, Koper, de Rijke and van Rossum2016). This could be a reason for higher cortisol concentrations in the proximal part of the hair (closer to the skin) compared to the distal part in Busha cows on mountain pasture (Nedić et al., Reference Nedić, Pantelić, Vranješ-Đurić, Jovanović, Čebulj-Kadunc, Kobal, Snoj and Kirovski2017).
Regarding the effect of season, the highest concentrations were found in hair sampled in June, followed by December, March, and September. In cows from colder climate regions of Japan, the difference of hair cortisol concentration between March and June was higher compared to hair of cows from warmer climate regions in the same period probably due to the absence of acclimatization to heat (Uetake et al., Reference Uetake, Morita, Sakagami, Yamamoto, Hashimura and Tanaka2017). In a study by Perić et al. (Reference Perić, Corazzin, Romanzin, Boloventa, Prandi, Montillo and Comin2017), cows kept indoors in tie-stall barns showed significantly higher hair cortisol concentrations from August to October. This study suggests that heat stress can cause an increase in hair cortisol concentration. Heat stress is indeed a well-known welfare issue (Polsky and von Keyserlingk, Reference Polsky and von Keyserlingk2017).
Cortisol concentration was higher in hair sampled from the tail switch compared to hair sampled from the backline and hip and significantly higher compared to hair sampled from the shoulder in dairy cows (Burnett et al., Reference Burnett, Madureira, Silper and Nadalin2014). In a study on beef cattle, hair from the tail switch had higher concentrations compared to the neck and hip hair, as well as hair from the head and shoulder (Moya et al., Reference Moya, Schwartzkopf-Genswein and Veira2013). Tail switch hair grows 10 times faster than hair from the hips and shoulders (Burnett et al., Reference Burnett, Madureira, Silper and Nadalin2014). We can speculate tail switch hair needs more blood supply to grow, meaning it is more exposed to cortisol in blood that could be incorporated into hair.
Some studies found higher concentrations in white hair compared to black hair (Gonzales-de-la-Vara Mdel et al., Reference González-de-la-Vara Mdel, Valdez, Lemus-Ramirez, Vázquez-Chagoyán, Villa-Godoy and Romano2011; Burnett et al., Reference Burnett, Madureira, Silper and Nadalin2014), while another found the opposite (Talló-Parra et al., Reference Talló-Parra, Manteca, Sabes-Alsina, Carbajal and Lopez-Bejar2015) and Ghassemi Nejad et al. (Reference Ghassemi Nejad, Kim, Lee and Sung2016) and Nedić et al. (Reference Nedić, Pantelić, Vranješ-Đurić, Jovanović, Čebulj-Kadunc, Kobal, Snoj and Kirovski2017) did not find differences in hair concentrations of black and white hair. Ghassemi Nejad et al. (Reference Ghassemi Nejad, Kim, Lee and Sung2016) detected lower hair cortisol concentrations in cows with over 85% of white coat colour compared to cows with over 80% of black coat colour in heat stress conditions. They concluded that Holstein cows with white coats are more resistant to heat stress. Further studies are needed to elaborate the effect of hair colour on hair cortisol concentration.
Studies of hair cortisol in cattle under different stressful conditions
Table 2 summarizes the relationship between different stressful conditions and hair cortisol concentration in cattle. Higher hair cortisol levels were detected in hair from cows at parturition (which reflected the third trimester of pregnancy) compared to non-pregnant cows (Braun et al., Reference Braun, Michel, Baumgartner, Hässig and Binz2017), while in other studies differences between pregnant and non-pregnant cows were not observed (Talló-Parra et al., Reference Talló-Parra, Carbajal, Monclús, Manteca and Lopez-Bejar2018). Higher concentrations were detected on the day of calving and 21st day after calving in comparison to later measurements in multiparous cows (Burnett et al., Reference Burnett, Madureira, Silper and Nadalin2014). Comin et al. (Reference Comin, Perić, Corazzin, Veronesi, Meloni, Zufferli, Cornacchia and Prandia2013) detected lower hair cortisol concentrations of healthy cows compared to cows that calved one month before sampling. These findings confirm that the transition period, late pregnancy and the beginning of lactation is a stressful period for cows.
Table 2. Relationship between different factors and hair cortisol concentration in cattle

In a study by Burnett et al. (Reference Burnett, Madureira, Silper, Tahmasbi, Nadalin, Veira and Cerri2015) lower concentrations were observed on 42nd and 84th day in milk (DIM) in cows that were diagnosed pregnant at 100 DIM compared to cows that did not get pregnant by 100 DIM, but only in multiparous cows. Since animals that were not pregnant at 100 DIM had a higher prevalence of clinical diseases during the transition period, higher hair cortisol concentrations in these animals may be a reflection of diseases during the transition period, which consequently caused an increase in days open.
In a study by Burnett et al. (Reference Burnett, Madureira, Silper, Tahmasbi, Nadalin, Veira and Cerri2015) higher hair cortisol concentrations were detected in cows recently suffering a disease, such as clinical mastitis, clinical metritis, displaced abomasum, retained placenta, milk fever, clinical ketosis, and chronic lameness. However, animals diagnosed with subclinical endometritis did not differ from healthy cows. In another study, differences were also detected between healthy cows and cows that had recently suffered from a disease (mastitis, metritis or laminitis) (Comin et al., Reference Comin, Perić, Corazzin, Veronesi, Meloni, Zufferli, Cornacchia and Prandia2013). Differences between healthy and diseased cows were not found in the study by Talló-Parra et al. (Reference Talló-Parra, Carbajal, Monclús, Manteca and Lopez-Bejar2018), possibly due to rapid veterinary interventions, which shortened disease course and dilution of the period of the disease in 2-month-old hair samples. More sensitive classification concerning the type of disease and severity was missing. In a study by Fischer-Tenhagen et al. (Reference Fischer-Tenhagen, Ladwig, Heuwieser and Thöne-Reineke2018) no correlation was observed between chronic lameness and hair cortisol. A significant correlation was found for subclinical mastitis (Comin et al., Reference Comin, Perić, Corazzin, Veronesi, Meloni, Zufferli, Cornacchia and Prandia2013), a weak relationship was detected in the study between somatic cell count and hair cortisol concentration (Talló-Parra et al., Reference Talló-Parra, Carbajal, Monclús, Manteca and Lopez-Bejar2018), while in other studies connection between somatic cell count and hair cortisol was not detected (Comin et al., Reference Comin, Prandi, Perić, Corazzin, Dovier and Bovolenta2011; Burnett et al., Reference Burnett, Madureira, Silper, Tahmasbi, Nadalin, Veira and Cerri2015). Overall, most studies show a connection between higher hair cortisol concentration and clinically compromised cows. Higher hair cortisol concentrations in diseased cows suggest these animals were also under long-term stress, leading to an increased susceptibility to different diseases (Comin et al., Reference Comin, Perić, Corazzin, Veronesi, Meloni, Zufferli, Cornacchia and Prandia2013). Higher hair cortisol concentration could, therefore, be an indicator of future diseases and on the other hand an indicator of subclinical disease states, which are usually not detected promptly, meaning cows suffer from stress for a longer period. The methodology of hair sampling is crucial for proper evaluation of welfare and disease in such circumstances.
In a study by Nedić et al. (Reference Nedić, Kirovski, Vujanac, Prodanović, Jovanović, Kobal and Snoj2018) lower hair cortisol concentrations were found in cows treated with ectoparasiticide compared to non-treated cows on day 21 (July) and 42 (August) after the first treatment. Results indicate and confirm that ectoparasitic infestation is stressful (welfare issue) for cows and can be decreased using ectoparasiticide.
Thin (<2.75 on 1–5 scale) multiparous cows had lower hair cortisol levels compared to cows with moderate (>2.75) and average (2.75) body condition score (BCS), while differences between BCS were not detected in primiparous cows (Burnett et al., Reference Burnett, Madureira, Silper, Tahmasbi, Nadalin, Veira and Cerri2015). On the contrary, a moderate negative correlation was found between the average BCS and hair cortisol concentration on a farm-scale (Vesel and Pavić, Reference Vesel and Pavić2019). This suggests Hunger is a stressful condition for cows and influences hair cortisol concentration, but we can also speculate that cows with lower BCS have a subclinical condition that caused higher hair cortisol concentration.
Studies indicate hair cortisol concentration is affected by environmental stress, such as change of housing, feed, herd routine, change in social structure and others. Hair cortisol concentration increased from day 7 to day 40 after the beginning of pasture and remained constant until day 70 after pasture, which indicates stress related to change from winter indoor to high mountain conditions in the summer (change of social groups, diet, housing, transport) (Comin et al., Reference Comin, Prandi, Perić, Corazzin, Dovier and Bovolenta2011). Similarly, in a study by Perić et al. (Reference Perić, Corazzin, Romanzin, Boloventa, Prandi, Montillo and Comin2017) hair cortisol concentration increased during the first month of grazing. During the second month concentrations were similar to hair concentrations of cows kept indoors, suggesting animals adapted to a new environment, different feed and increased physical activity. A significant increase in cortisol concentration was found in hair samples from the last month of grazing, which reflected the period when cows were grazing on pasture at a higher altitude after the relocation. The increase in hair cortisol concentration could be due to nutrient-poor pasture and negative energy balance. Talló-Parra et al. (Reference Talló-Parra, Carbajal, Monclús, Manteca and Lopez-Bejar2018) failed to demonstrate a change in cortisol concentration after pen change and resulting social stress because of a new environment, perhaps due to the short duration of the stressful event.
Hair cortisol concentrations of veal calves reared under two different animal welfare production systems (concerning stocking density and access to an outdoor area and pasture) were not statistically significantly different between the two groups (Braun et al., Reference Braun, Wiest, Lutz, Riond, Stirn, Hilbe, Baumgartner and Binz2019). No effect of stocking density (20% difference between groups) on hair cortisol of Jersey cows in the prepartum period was detected at calving in a study by Silva et al. (Reference Silva, Lobeck-Luchterhand, Cerri, Haines, Ballou, Endres and Chebel2016), possibly because the high stocking density may not have been dramatic enough. In the study on beef heifers, higher cortisol concentrations were detected from the hair of animals kept in dry-lot pens with a stocking density 14 m2/heifer compared to animals kept on pastures with a stocking density 2.5 ha/heifer on day 98 after allocation (Schubach et al., Reference Schubach, Cooke, Brandão, Lippolis KD, Marques and Bohnert2017), showing stocking density can influence hair cortisol concentration.
Some studies detected a negative relationship between milk yield and hair cortisol levels (Burnett et al., Reference Burnett, Madureira, Silper, Tahmasbi, Nadalin, Veira and Cerri2015; Talló-Parra et al., Reference Talló-Parra, Carbajal, Monclús, Manteca and Lopez-Bejar2018). This suggests stress can result in lower milk production or that animals were inadequately feed and were in catabolic state. The latter study compared cortisol concentrations in 2 month-old-hair to the whole lactation period and revealed a strong negative connection as well.
Methods of sampling and analysis
To be able to detect higher cortisol concentration in hair as a result of a specific stressor, time of sampling is an important factor (Talló-Parra et al., Reference Talló-Parra, Carbajal, Monclús, Manteca and Lopez-Bejar2018). A shave–reshave approach (before the start of the studied period and after the end of the experiment) should be used to analyse cortisol that has incorporated into the hair in the study period (Meyer and Novak, Reference Meyer and Novak2012). A lag time of one to two weeks should be considered for cortisol deposition in the hair shaft, because of its initial deposition in the hair root, which is beneath the skin surface (Russel et al., Reference Russel, Koren, Rieder and Van Uum2012).
Length of hair has to be taken into account when evaluating a certain period of time (Talló-Parra et al., Reference Talló-Parra, Carbajal, Monclús, Manteca and Lopez-Bejar2018). Hair from the tail switch grows 0.51 ± 0.05 mm/day, which is over 10 times faster than hair growth from the hips and shoulders. To reflect the biologically important period of lactating cows, such as the transition period, a high growth rate of hair is a precondition (Burnett et al., Reference Burnett, Madureira, Silper and Nadalin2014).
White hair from the tail switch had higher concentrations than white hair from other regions in a study by Burnett et al. (Reference Burnett, Madureira, Silper and Nadalin2014). In a study on beef cattle, hair from the tail switch had higher cortisol concentrations and had a stronger connection to salivary and faecal cortisol compared to hair from other regions (Moya et al., Reference Moya, Schwartzkopf-Genswein and Veira2013). Because of this, the preferred sampling region seems to be the tail switch.
To avoid blood contamination, hair should not be pulled but cut or shaved near the skin (Meyer and Novak, Reference Meyer and Novak2012). Hair can be stored at room temperature (Gow et al., Reference Gow, Thomson, Rieder, Van Uum and Koren2010) in a dark place to avoid the possible effect of UV irradiation (Wester et al., Reference Wester, van der Wulp, Koper, de Rijke and van Rossum2016). In a study by Vesel and Pavić (Reference Vesel and Pavić2019) hair samples had to be cleaned manually because of dirt and faeces contamination to avoid possible faecal cortisol measurement. There is probably some deposition of cortisol on the hair shaft from saliva, sweat, and sebum (Meyer and Novak, Reference Meyer and Novak2012). It has been estimated that 21% of the entire hair cortisol in Holstein cows and 32% of the entire hair cortisol in Busha cows is located on hair surface (Nedić et al., Reference Nedić, Pantelić, Vranješ-Đurić, Jovanović, Čebulj-Kadunc, Kobal, Snoj and Kirovski2017). Because cortisol diffuses to these fluids from the bloodstream, concentration is influenced by acute stress. To evaluate chronic stress, saliva, sweat, and sebum should be removed from hair surface before analysis. Isopropanol has been recognized as an effective solvent of steroids and is therefore preferably used for the treatment of hair (Davenport et al., Reference Davenport, Tiefenbacher, Lutz, Novak and Meyer2006). Cortisol concentrations were higher in unwashed hair compared to washed hair using isopropanol (Nedić et al., Reference Nedić, Pantelić, Vranješ-Đurić, Jovanović, Čebulj-Kadunc, Kobal, Snoj and Kirovski2017; Vesel and Pavić, Reference Vesel and Pavić2019).
After washing, hair is minced with scissors to small fragments (1 mm) or ground to powder. Higher concentrations were found in powdered hair compared to minced hair in cattle (Burnett et al., Reference Burnett, Madureira, Silper and Nadalin2014). Hair can be placed in liquid nitrogen and ground in a mortar using a pestle (Vesel and Pavić, Reference Vesel and Pavić2019) or a ball mill (Nedić et al., Reference Nedić, Pantelić, Vranješ-Đurić, Jovanović, Čebulj-Kadunc, Kobal, Snoj and Kirovski2017).
Methanol is normally used as a solvent for cortisol extraction. It causes swelling of the hair, cortisol diffusion, and solubilization. After solvent evaporation hair extract is reconstituted into an appropriate medium (Meyer and Novak, Reference Meyer and Novak2012), such as PBS buffer (Vesel and Pavić, Reference Vesel and Pavić2019). Cortisol concentration can be analysed by EIA, RIA, chemiluminescent immunoassays, HPLC-mass spectrometry and HPLC with fluorescence detection (Meyer and Novak, Reference Meyer and Novak2012). RIA has been used to analyse cortisol in dairy cows using validation tests (Comin et al., Reference Comin, Prandi, Perić, Corazzin, Dovier and Bovolenta2011; Gonzales-de-la-Vara Mdel et al., Reference González-de-la-Vara Mdel, Valdez, Lemus-Ramirez, Vázquez-Chagoyán, Villa-Godoy and Romano2011). A complete validation of EIA has been made in beef cattle (Moya et al., Reference Moya, Schwartzkopf-Genswein and Veira2013), and acceptable repeatability and reliability have also been confirmed for EIA to detect hair cortisol concentration in dairy cattle (Talló-Parra et al., Reference Talló-Parra, Manteca, Sabes-Alsina, Carbajal and Lopez-Bejar2015). Detection of hair cortisol in dairy cows with ELISA was found effective in a study by Vesel and Pavić (Reference Vesel and Pavić2019).
Collection, preparation and analysis methods need full validation for dairy cattle to be used in everyday practice. High individual differences were found between cows in several studies (Comin et al., Reference Comin, Perić, Corazzin, Veronesi, Meloni, Zufferli, Cornacchia and Prandia2013; Talló-Parra et al., Reference Talló-Parra, Carbajal, Monclús, Manteca and Lopez-Bejar2018), but also analysed mean cortisol concentrations differ considerably between different reports. To our knowledge, there are no reference values for normal cortisol levels or thresholds in cattle that would differ between stressed and non-stressed animals. In a study by Vesel and Pavić (Reference Vesel and Pavić2019) cortisol concentrations were higher compared to some other research (Comin et al., Reference Comin, Prandi, Perić, Corazzin, Dovier and Bovolenta2011; Gonzalez-de-la-Vara Mdel et al., Reference González-de-la-Vara Mdel, Valdez, Lemus-Ramirez, Vázquez-Chagoyán, Villa-Godoy and Romano2011; Burnett et al., Reference Burnett, Madureira, Silper and Nadalin2014; Talló-Parra et al., Reference Talló-Parra, Manteca, Sabes-Alsina, Carbajal and Lopez-Bejar2015; Uetake et al., Reference Uetake, Morita, Sakagami, Yamamoto, Hashimura and Tanaka2017), however, they were similar to results of Nedić et al. (Reference Nedić, Pantelić, Vranješ-Đurić, Jovanović, Čebulj-Kadunc, Kobal, Snoj and Kirovski2017). This could be due to the usage of similar or dissimilar sampling and analysis protocols for the detection of hair cortisol and reinforces the need for standardization.
Assessment of welfare in dairy cattle using welfare assessment protocols
The first models for welfare assessment were focused on environmental and management factors. Nowadays welfare protocols are orientated towards animal-based measures, as animals represent an interpretation of environmental effects (Bertocchi and Fusi, Reference Bertocchi and Fusi2014). Animal-based measures probably show the most problematic welfare issues, whereas resource-based measures help identify the cause of problems and future welfare risks (EFSA, 2012). Protocols consist of animal assessment, assessment of the human-animal relationship, environmental parameters and documentation overview. Welfare Quality® Assessment Protocol uses mostly animal-based measures, except for the assessment of water provision (Welfare Quality®, 2009). Most welfare assessment protocols used today are variations and adaptations of Welfare Quality® Assessment Protocol for specificities of dairy cow production systems, for example year-round pastoral and loose housing systems (Stilwell et al., Reference Stilwell, Correia, Viveiros, Calouro, Arruda Rieff, Medeiros and Vasconcelos2018; Bertocchi and Fusi, Reference Bertocchi and Fusi2014).
Does hair cortisol concentration reflect the welfare assessment protocol score?
To the authors' best knowledge there are no published studies on this topic in international peer-review literature. In a study by Vesel and Pavić (Reference Vesel and Pavić2019) welfare scores of 8 farms (with 180–470 dairy cows) assessed by Welfare Quality® Assessment Protocol and hair cortisol concentrations in pooled samples (of 17–33 randomly selected dairy cows from the assessed herds) were compared using Pearson correlation coefficient. There was no real evidence of concordance: lower mean cortisol concentrations were not detected in pooled hair samples from farms that were given better scores using the Welfare Quality® Assessment Protocol. This could be interpreted in context with a study showing that chronic stress can lead to a decrease in the activity of the HPA axis under some circumstances, concerning stressor and individual response (Miller et al., Reference Miller, Chen and Zhou2007). This could be the reason why in the farms with the lowest welfare scores, where cows were supposedly subjected to long-term stress, cortisol concentrations were not higher compared to other farms. On the positive side, a moderate negative correlation with pooled hair cortisol concentration was detected for welfare criteria ‘Absence of prolonged hunger’ and ‘Absence of pain induced by management procedures’. The limitation of the study is that only 8 farms were studied and tested for the correlation of these two variables. Furthermore, the sample of cows from which hair was collected was small (on average 8% of the herd), so the results may not reflect the real welfare situation of the herd. Standardization of hair sampling and analysis is non-existent to our best knowledge and is highly needed. On the other hand, we also cannot be sure if scores obtained with the Welfare Quality® Assessment Protocol are best estimates of the welfare of assessed dairy cows (Vesel and Pavić, Reference Vesel and Pavić2019).
Conclusions and future directions
Studies show that stressful factors such as diseases, pregnancy and inappropriate environmental conditions affect hair cortisol. In comparison to cortisol concentration in blood, saliva, faeces, urine and milk, hair cortisol concentration is not likely to be affected by acute changes, but rather represents chronic stress (Comin et al., Reference Comin, Prandi, Perić, Corazzin, Dovier and Bovolenta2011; Meyer and Novak, Reference Meyer and Novak2012). Since we are interested in a long-term state of animals when referring to animal welfare, hair cortisol measurement could be a useful non-invasive indicator of welfare in dairy cattle (Mesarcova et al., Reference Mesarcova, Kottferova, Skurkova, Leskova and Kmecova2017).
To date, research by Vesel and Pavić (Reference Vesel and Pavić2019) seems to be the first to compare welfare assessment using welfare assessment protocol and cortisol concentration in a pooled sample of hair in dairy cows. The number of herds and cows from which hair was collected in each herd were too small to establish correlations between the welfare score obtained with the protocol and hair cortisol concentration. More research should be made with larger sample size.
Since it has been reported that chronic stress can lead to a decrease in the activity of the HPA axis from studies on plasma, saliva, and urine, further research should be made to see whether there is a possibility that chronic stress could lead to decrease in cortisol concentration in hair as well. Moreover, more research is needed to confirm the extent of impacts affecting hair cortisol concentration. There have been different results regarding some of the fixed factors on hair cortisol concentration. Still not enough is known about the mechanisms of cortisol deposition in hair, which is important to detect changes of cortisol concentration reflecting different stressors. The timing of hair sampling seems to be crucial to detect the change of concentration in response to a specific stressor and on the other hand to avoid the impact of a specific stressor when evaluating the long-term welfare state of animals. For example, cortisol concentration in hair could be higher in response to managemental procedures such as dehorning in cows at a certain period of hair sampling, but when sampled later on the increase in cortisol concentration could not be detected. When comparing different farms, the time of hair sampling should be determined taking into account such procedures.
To date, there is no standardized protocol for sampling and hair cortisol concentration measurement. It is probably because of this that there are huge differences in hair cortisol concentration in different studies, and effort should be put into finding the best sampling protocol and the best method for cortisol detection.
It is important to know how much hair from one cow is needed to represent her welfare and also what percentage of cows is needed for the pooled sample to represent the herd. For practical and financial reasons, the smallest number of cows in the pooled sample, that still represents the herd should be selected, including dry cows, fresh cows, cows in early, mid and late lactation.
In our opinion and according to studies that have been published, the most appropriate hair sampling region is the tail switch because of the highest hair cortisol concentration. To avoid blood contamination hair should be shaved or cut near the skin and not pulled out. The shave–reshave approach is preferred to get results that reflect cortisol incorporated in the hair at the same period for all animals. The hair sample should be stored at room temperature in a dark place and cleaned manually before analysis in case of faeces or dirt contamination. To evaluate chronic stress without detecting changes of cortisol concentration in response to short-term stressors, it is reasonable to wash hair samples with isopropanol to remove saliva, sweat, and sebum from the hair surface. Studies show that more cortisol is extracted from ground hair compared to hair minced with scissors, hence grinding hair to powder before extraction should be the method of choice. Methanol is an appropriate solvent for cortisol extraction and PBS buffer an appropriate medium for the reconstitution of hair extract according to our experience.
Less is known about the best method of hair cortisol concentration measurement. Research comparing EIA, RIA and other methods of analysis is needed to find the most suitable one.
After standardization of sampling and analysis protocol more research is needed to establish reference values for hair cortisol concentration in cows. The reference values should be based on healthy cows living under best-known conditions. In our opinion, it would be best to detect hair cortisol concentration in cows kept in more extensive farming system, compared to cows living in an intensive production system, since they seem to have elevated hair cortisol levels. However, it should be noted that hair cortisol concentration could be lower if cows are exposed to UV light, especially in the summer, when they are on pasture. Furthermore, the effect of the season should not be overlooked when interpreting hair cortisol concentration. Therefore, we suggest different reference values for indoor/outdoor cows and regarding the season of sampling. Additionally, because of the possible effect of age and parity, reference should be made for cows of similar age or parity.
Overall, measuring cortisol concentration in hair is a promising non-invasive tool for dairy cattle welfare assessment. In the future, hair sampling could be automated and incorporated into a milking robot. This would enable regular (monthly, for example) monitoring of welfare in a dairy cattle herd as a whole or a subgroup of cows, showing trends and identifying welfare deterioration at its beginning.
Acknowledgment
This article is based upon work from COST Action FA1308 DairyCare, supported by COST (European Cooperation in Science and Technology, www.cost.eu). COST is a funding agency for research and innovation networks. COST Actions help connect research initiatives across Europe and enable scientists to grow their ideas by sharing them with their peers. This boosts their research, career and innovation. The authors would like to thank Assoc. Prof. Dr John McNamara for critical reading of the manuscript and COST action DairyCare for inspiring this work.



