The MS phenotype in the African-American (AA) patient is an ideal model to study drug efficacy since the disease follows a rapidly disabling course.Reference Kister, Chamot, Bacon, Niewczyk, De Guzman and Apatoff1 AA MS patients admitted to US nursing homes are 6 years younger but more disabled compared to Caucasian American (CA) patients with MS.Reference Buchanan, Wang, Huang and Graber2 Since phenotypes between CA and AA can be clinically distinct, it is remarkable that not a single study has compared how drugs perform in such diverse groups.
In 2014, I reported that enrollment numbers in clinical trials for AAs with MS had dropped from 7.7% (2002, interferon beta-1a, Rebif, EMD Serono) to 2% (2013, dimethyl fumarate, Tecfidera, Biogen) over a course of 11 years.Reference Avasarala3 More recently, investigation of raw numbers and datasets for the US Food and Drug Administration (FDA)-approved drugs Zinbryta (daclizumab, approved May 2016, now withdrawn) and Ocrevus (ocrelizumab, approved March 2017) supports my observations. Significantly, MS drugs approved by the FDA do not contain efficacy data for minorities and therefore clinicians are unable to discuss the efficacy data of any MS drug with their non-Caucasian patients. The lack of any drug data in non-Caucasian patients with MS in published clinical trials is troublesome.
In 2014, partly to address such concerns, the FDA via its Center for Drug Evaluation and Research (CDER) launched an initiative called drug trials snapshots. One of the goals of this initiative was to encourage the inclusion of women and people from different racial, ethnic, and other minority groups in clinical studies. For the first time, these data provided consumers with information about the demographic distribution in clinical trials that supported the FDA approval of new drugs. However, package inserts for drugs continue to contain no demographic information on patient ethnicity participation. Absent such critical information, physicians cannot extrapolate if drugs work in such poorly represented populations. Additionally, published literature of phase 3 clinical trials are vexingly vague about patient demographic data.
Data evaluation of 2 recently approved drugs, Zinbryta and Ocrevus, will be presented in this analysis since the FDA has publicly provided information on them on their website, under the ‘drug trials snapshots’ categorization. Specifically, in the DECIDE study for Zinbryta,Reference Kappos, Wiendl and Selmaj4 89.5% or the 823/919 patients in the Zinbryta arm were Caucasian. What the 10.5% was made up of is unclear but the study had principal investigators from 28 countries and yet the demographic makeup is unsatisfactory.
Without the publication of information on the ethnic composition of patients included in this study on the FDA website, this specific piece of information would be unavailable in the public domain. If companies cannot or will not recruit patients with probably the worst burden of disease (AA MS), there at least ought to be a disclaimer in the package insert or a statement in the publication that the study does not apply to minority communities. It is time to consider changing the package labeling by law, and no publication ought to be accepted unless a minimum percentage of the recruited patients are represented by the AA cohort.
A more recent example in a phase 3 clinical trial publication, viz OPERA I and OPERA II concerning Ocrevus (ocrelizumab), does not report any data on ethnicity.Reference Hauser, Bar-Or and Comi5 The OPERA I and II studies are identical and compared Ocrevus to Rebif (interferon beta-1a). In the OPERA I trial, patients from 141 trial sites across 32 countries were randomized, while in OPERA II, patients from 24 countries across 166 trial sites were randomized into the study. Despite the impressive clinical trial design, the published literature does not show data on the raw numbers or percentages of the minorities or non-Caucasian groups enrolled in these studies. Only the fda.gov website shows such data: for OPERA I and II studies, Caucasians comprised 90.5% (1500/1656), AA were 4.3% (72/1656), and others were 5% (84/1656), as shown in Figure 1. Another phase 3 clinical trial, the ORATORIO study,Reference Montalban, Hauser and Kappos6 enrolled 732 patients with primary progressive MS in the USA, Canada, and European countries; the trial compared efficacy of Ocrevus to placebo. In the ORATORIO study, the ethnic distribution as per the fda.gov website was 94% Caucasian (689/732), 1.9% AA (14/732), 0.6% American Indians (5/732), and 3.2% others (24/732), as depicted in Figure 2. However, no ethnicity listing was included in the publication itself.Reference Montalban, Hauser and Kappos6 Reporting baseline patient demographic data characteristics in the published literature must be made mandatory.
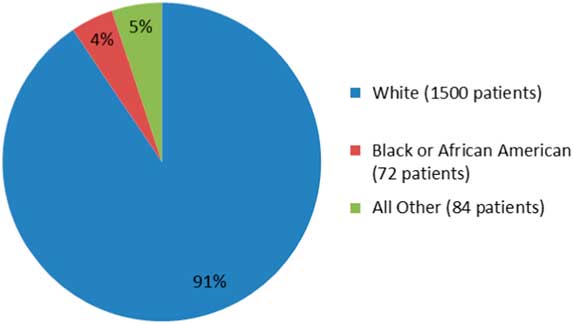
Figure 1 The percentage of patients by race in OPERA I and OPERA II studies (adapted from fda.gov).
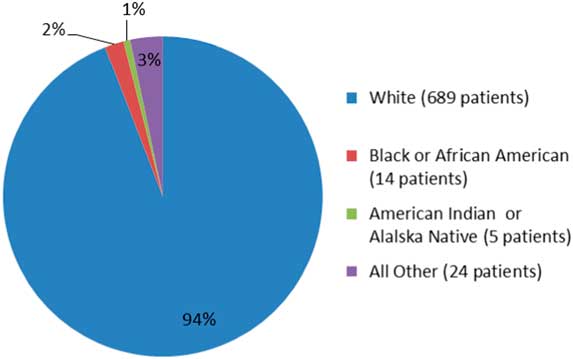
Figure 2 The percentage of patients by ethnicity in the ORATORIO trial (adapted from fda.gov).
If AAs or other minorities cannot be recruited into clinical trials, my recommendations are as follows:
1. An efficacy statement regarding lack of conclusions in non-Caucasian groups in drug package inserts should be included.
2. No publication should be accepted without a statement or disclaimer on the lack of data to make reasonable conclusions in non-Caucasian minorities.
3. Pharmaceutical companies ought to collect post-marketing data in all minority groups to assess their drug’s efficacy statistics and report them to the FDA.
If the REMS (Risk Evaluation and Mitigation Strategy) program was developed in 2007 via a new law that gave FDA new authorities and responsibilities to monitor a drug safety during its use in patients, something similar can be created to address inherent deficiencies in a drug’s efficacy and disability data.
Disclosures
Jagannadha Avasarala has nothing to disclose.