Signs and symptoms of infection due to the gram-positive anaerobic bacterium Clostridioides difficile (C. diff) that can colonize the human gut and release pathogenic toxins range from mild diarrhea to severe life-threatening inflammation of the colon. Epidemiological research has established advanced age and healthcare exposures common among those of older age, such as antibiotic use and hospitalization, as risk factors for C. diff infections (CDI).Reference McDonald, Gerding and Johnson1–Reference Baggs, Yousey-Hindes and Ashley4 However, studies on the burden of CDI for those <65 years of age, and in the Veterans Health Administration (VHA) population in particular, are limited.
In their 2015 publication, Lessa et alReference Lessa, Winston and McDonald5 reported that the adjusted incidence of healthcare facility (HCF)-associated and community-associated CDI in the United States were more than 4 and 13 times higher for patients aged ≥65 years compared to patients aged 45–64 and 18–44 years, respectively. Conversely, the proportion of community-associated CDI was nearly 3 times as high among those aged 18–44 and twice as high for those 45–64 years, compared with those 65 years and older. Furthermore, Gutierrez et alReference Gutiérrez, Riddle and Porter6 found a 10-fold increase in community-associated CDI from 1998 to 2010 among active-duty US military personnel, a population that is generally younger and healthier than the US general population.
Recent evidence offers additional support for these findings, as studies demonstrate patients with community-associated CDI are younger, have lower comorbidity, fewer instances of healthcare contact, and less exposure to antibiotics as compared to patients with HCF-onset or HCF-associated CDI.Reference McDonald, Gerding and Johnson1, Reference Dantes, Mu and Hicks7–Reference Kuntz, Chrischilles, Pendergast, Herwaldt and Polgreen11 These findings are expected as health deteriorates and contact with healthcare increases with advancing age; therefore, CDI onset among those of younger age is more likely to be community associated, while for those that are older, HCF onset or HCF-associated is more likely.
Ample evidence has documented a rise in overall CDI incidence across the US since the start of the twenty-first century, a trend partially explained by both the emergence of hypervirulent strains and the increased use of highly sensitive methods for detection of C. diff, such as nucleic acid amplification testing.Reference McDonald, Gerding and Johnson1, Reference Young-Xu, Kuntz and Gerding12–Reference Reveles, Pugh and Lawson15 Whether these increases are also seen among those of younger age, and for community-associated CDI, warrants further investigation in the context of prevention and identification of opportunities for which to intervene.
More than 40% of CDI in the VHA patient population occurs among those aged <65 years.Reference Lessa, Winston and McDonald5, Reference Gutiérrez, Riddle and Porter6, Reference Young-Xu, Kuntz and Gerding12, Reference Pinzon, Buie and Liou16, Reference Gutiérrez, Riddle and Porter17 The objective of this study was to describe the epidemiology of CDI for the VHA population aged 18–64 years to better understand its occurrence among this vulnerable population whose disease burden may be a continuation of, or impacted by, experiences during active duty.
Methods
A national, retrospective cohort study was conducted among patients aged 18–64 years as of January 1, 2011, with a VHA healthcare priority group rating of 1; at least 1 inpatient or 2 outpatient visits during calendar year 2011, and no evidence of CDI in the prior 90 days (October 1–December 31, 2010).
As the single largest integrated healthcare system in the United States, the VHA of the Department of Veterans Affairs (VA) provides comprehensive services to veterans of the armed forces that can be followed across the care continuum, from the nonurgent outpatient clinic to the emergency department and subsequent hospitalization to postdischarge extended care in rehabilitation and nursing facilities. A higher healthcare priority group rating (groups 1–4) assigned at the time of enrollment indicates that the VHA will pay for a greater amount of an individual’s care; thus, patients with these ratings are more likely to use its services for most, if not all, of their needs, especially before becoming eligible for Centers for Medicare and Medicaid Services coverage.
Data for this study were extracted from the integrated databases of national clinical and administrative datasets of the VHA Corporate Data Warehouse (CDW) comprised of raw data delivered directly from VHA’s Veterans Health Information and Technology Architecture (VistA) unified electronic medical record (EMR) system. Standardization of the laboratory data was performed in accordance with previously established methods.Reference Hauser, Quine and Ryder18 Each patient is assigned a unique identification number that allows longitudinal follow-up.
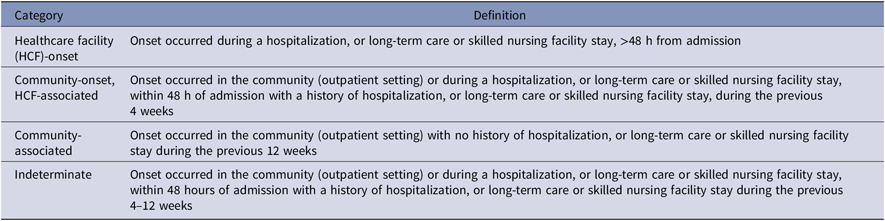
The CDI episodes were identified by one of the following criteria: (1) a diagnosis code for CDI (ICD-9-CM 008.45 or ICD-10 A04.7) during an inpatient hospital stay or from an outpatient encounter accompanied by metronidazole, oral vancomycin, or fidaxomicin therapy within 14 days of diagnosis; or (2) the presence of toxin or toxin gene in a stool sample detected by enzyme immunoassay (EIA) or polymerase chain reaction (PCR). Duplicate episodes, those occurrences of either criteria within 14 days of one another as defined for the Centers for Disease Control and Prevention (CDC) laboratory identification definition, were excluded.19 Episodes were classified as HCF-onset; community-onset, HCF-associated; community-associated; or indeterminate according to Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) definition guidelines (Table 1).Reference McDonald, Gerding and Johnson1
Table 1. Clostridioides difficile Infection Episode Definition per Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) ClassificationReference McDonald, Gerding and Johnson 1
The index date for the study cohort was defined as January 1, 2011. All patients were followed from the index date until the earliest of incident CDI, death, loss to follow-up, or December 31, 2016. Loss to follow-up was defined by a period of 2 consecutive years during which a patient was found to have no inpatient or outpatient visit and remained alive. Censoring occurred at the end of the calendar year during which she or he last had an encounter prior to the two-year period.
Demographic and clinical characteristics, including age, gender, race/ethnicity, region of care, healthcare utilization, Charlson comorbidity index score, and comorbidities (medical diagnoses), of patients with and without CDI were measured for the year prior to the index date (calendar year 2010) and compared using a standardized difference (SD) approach for which an absolute value > 10 may be indicative of a meaningful imbalance in a covariate between the 2 groups.Reference Austin and Stuart20 The incidence of CDI was calculated as the number of patients with an incident, or first new, CDI episode acquired during the study period divided by the person-years of observation and reported for three age group stratifications of 18–34, 35–49, and 50–64 years to allow for additional granularity by age. To determine whether linear trends in the incidence of CDI, as well as the proportion of episodes identified as and incidence of community-associated CDI, differed significantly by age group over time, their interactions were included in the model. A significance level was set at 0.01 after Bonferroni adjustment to account for the overall and three age group stratifications.Reference Perneger21
The study received institutional review board (IRB) approval from the Veteran’s IRB of Northern New England at the White River Junction VA Medical Center.
Results
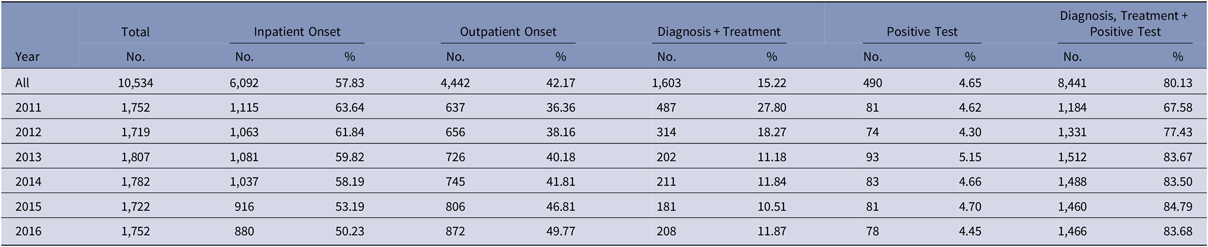
Between 2011 and 2016, 1,073,900 patients met the study inclusion criteria (Fig. 1). Of these, 10,534 patients (1%) were identified as having a CDI. The proportion of episodes identified as outpatient onset rose across the study period, from 36% in 2011 to nearly 50% in 2016 (Table 2). Although the proportion of episodes identified by the presence of a positive test alone was relatively stable across the study period, the proportion with a positive test rose from 68% in 2011 to 84% in 2016.
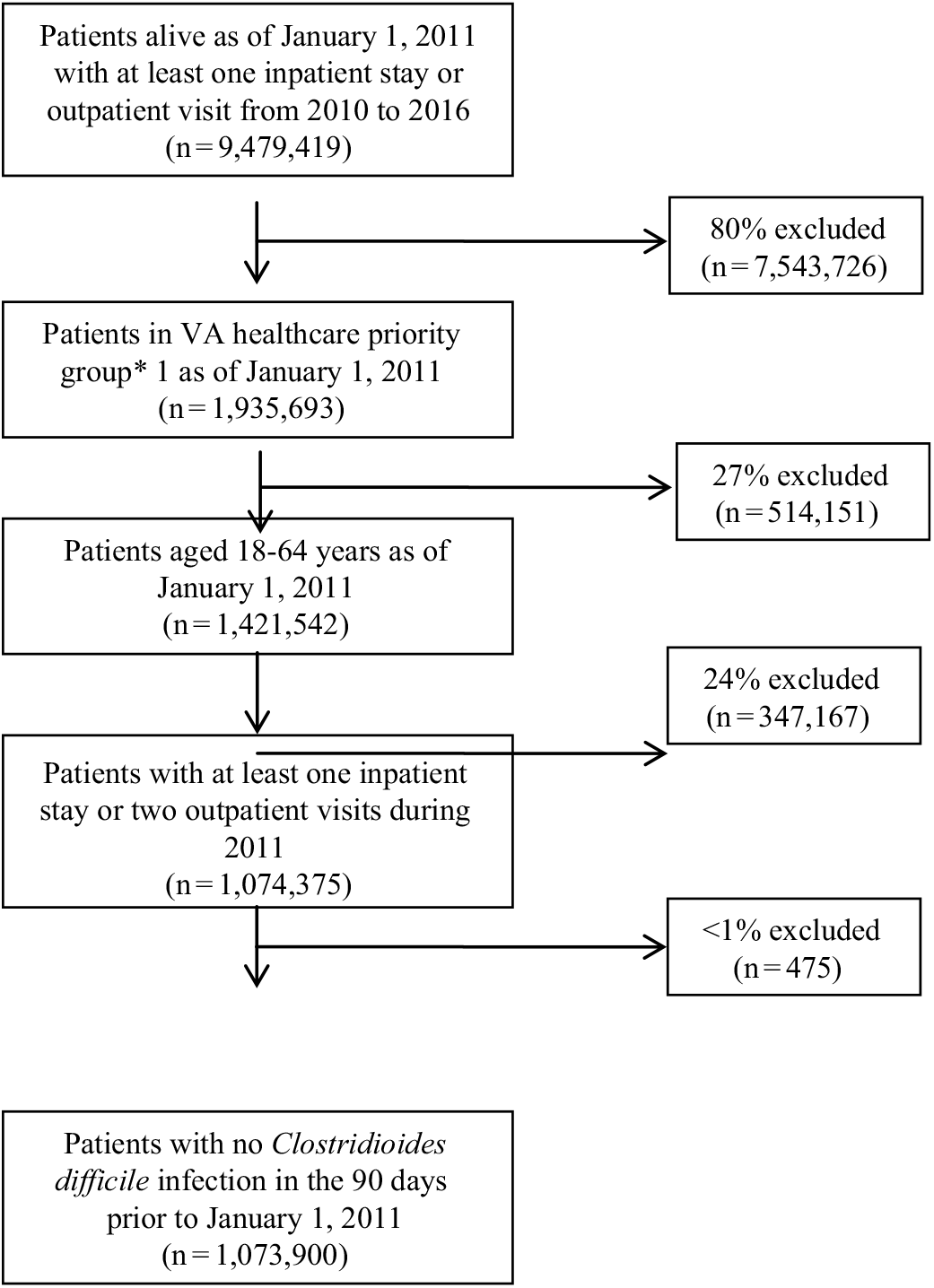
Fig. 1. Study population inclusion and exclusion criteria. *Priority is a classification assigned at the time of enrollment based on the severity of the Veteran’s claimed condition(s) deemed to be related to his/her military service (percent service connected), employability, disability, other insurance eligibility, as well as other factors. It is used to determine the level of monetary compensation and the extent of VHA benefits received by a Veteran. Veterans with a higher priority rating (groups 1–4) receive higher levels of compensation for the care they receive from VHA.
Table 2. Clostridioides difficile Infection Episode Characteristics
The overall incidence rate was 177 CDIs per 100,000 person years, steadily rising from 164 per 100,000 person years in 2011 to 189 per 100,000 person years in 2016 (Fig. 2). Overall incidence increased with increasing age: as compared to the youngest age group (18–34 years), CDI incidence was 1.3 times (or 29%) higher for those aged 35–49 years and 2.7 times higher (more than double) for those 50–64 years.
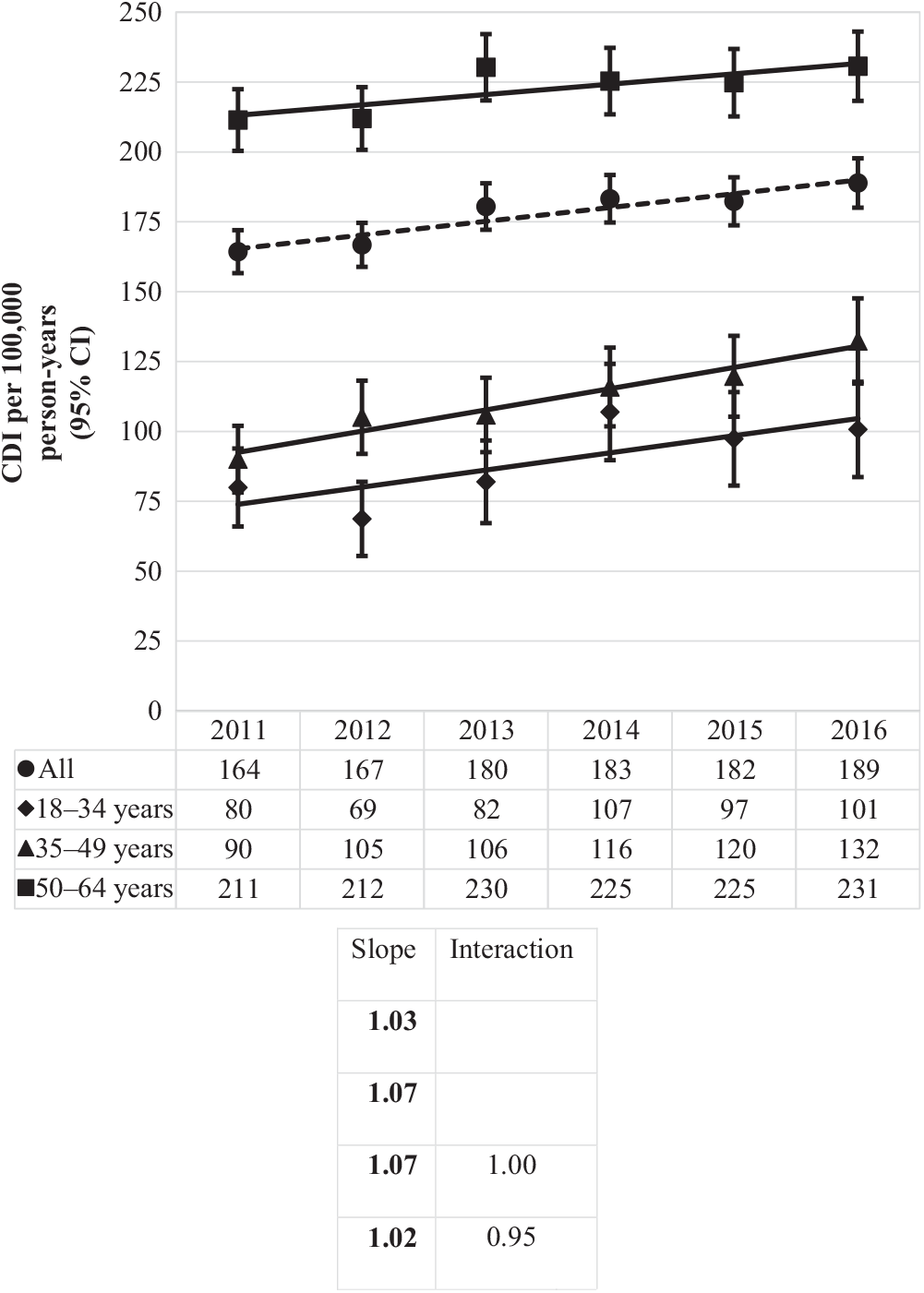
Fig. 2. Incidence of Clostridioides difficile infection (CDI) per 100,000 person years by year and age group. Bold values indicate P ≤ .01.
Of the CDIs identified during the study period, nearly half (48%) were community-associated. The proportion of CDIs that were community-associated rose from 41% in 2011 to 56% in 2016 (Fig. 3). Increases were observed for the 50–64 age group, from 35% to 52%, and 35–49 age group, from 56% to 64%, while a slight decrease was observed for the 18–34 age group, from 75% to 70%. The proportion of community-associated CDI decreased as age increased, from 72% of CDIs among patients aged 18–34 years to 44% of CDIs among those aged 50–64 years (Fig. 4). In contrast, the proportion of all CDIs that were HCF-onset increased with age, from 11% in the youngest age group to 34% in the oldest.
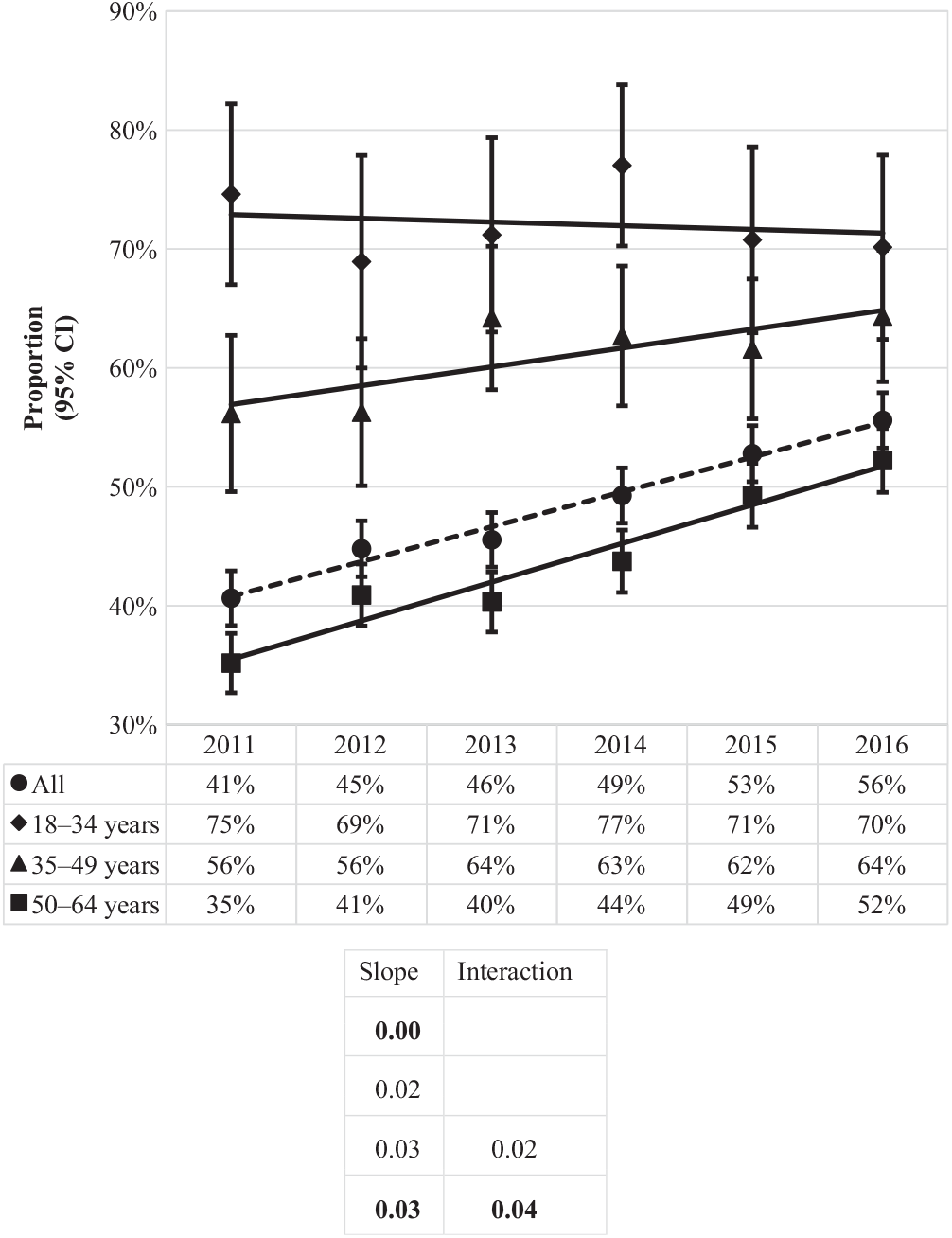
Fig. 3. Proportion of incident (first new) Clostridioides difficile infection episodes classified as community-associated by year and age group. Bold values indicate P ≤ .01.
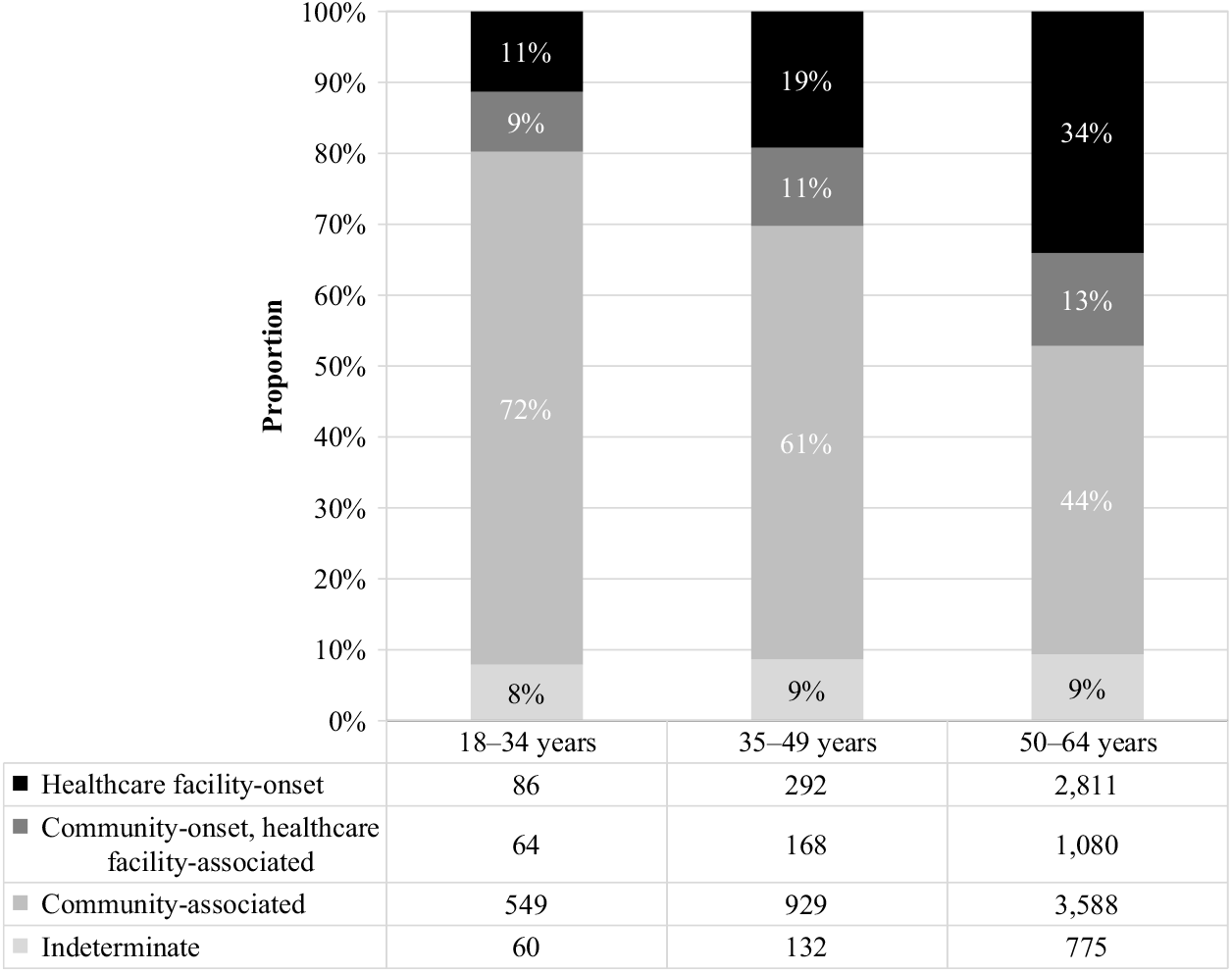
Fig. 4. Incident (first new) Clostridioides difficile infection episodes by IDSA-SHEA classification and age group.
Similarly, the incidence of community-associated CDI over time mirrored the rise of the proportion of this CDI type, up from 67 per 100,000 person years in 2011 to 105 per 100,000 person-years in 2016 (Fig. 5). Statistically significant increases were observed for all age groups, with the greatest increase occurring for the oldest age group (from 75 to 121 per 100,000 person years), as compared to the middle (from 51 to 85 per 100,000) and the youngest (from 60 to 71 per 100,000) age groups.
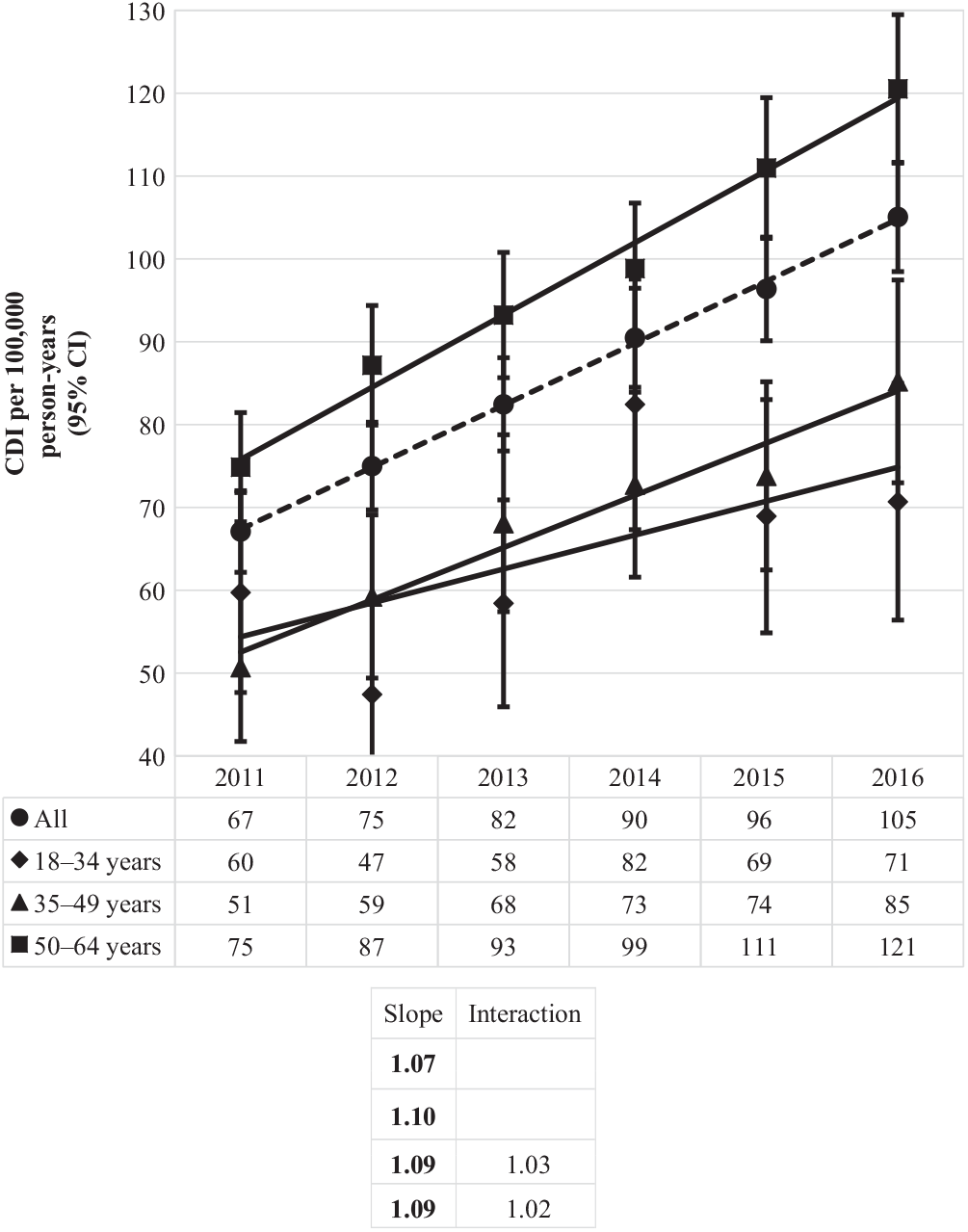
Fig. 5. Incidence of community-associated Clostridioides difficile infection (CDI) per 100,000 person-years by year and age group. Bold values indicate P ≤ .01.
Except for the 2 younger age groups of the proportion of community-associated CDI, all linear slopes were statistically significant at the 0.01 level (18–34 years, P = .72 and 35–49 years P = .06) (Figure 3). In contrast, the only interaction term found to be statistically significant at the 0.01 level was of the proportion of community-associated CDI for the age group of 50–64 years, indicating its linear slope is significantly different from the other 2 age groups (Fig. 3).
Most patients were male (89%) and white (61%) (Table 3). Patients with a CDI were significantly older (mean, 55 vs 51 years; SD > 10), utilized more healthcare during the 6-year study period (mean, 7 vs 3 inpatient stays; SD > 10 and 117 vs 72 outpatient visits; SD > 10), and had a higher baseline Charlson comorbidity index score (mean, 1.4 vs 0.5; SD > 10) than those without a CDI.
Table 3. Study Population Characteristics
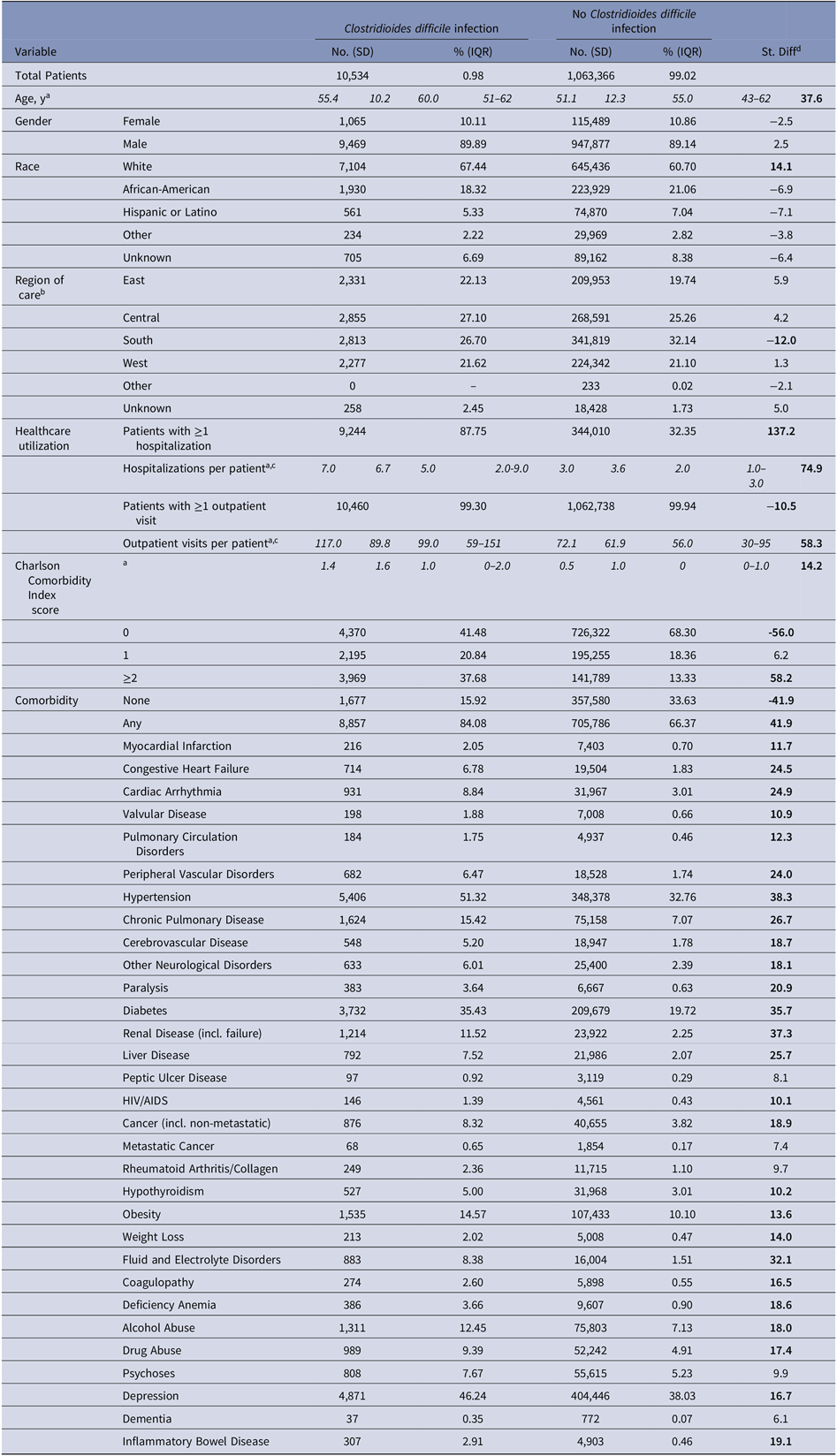
Variables are measured from the previous calendar year and are reported as of January 1, 2011.
a mean (standard deviation; SD) and median (interquartile range; IQR) are reported instead of count and percentage;
b East: CT, DE, IN, MA, MD, ME, MI, NH, NJ, NY, OH, PA, RI, VT; Central: AR, IA, IL, KS, LA, MN, MO, ND, NE, OK, SD, TX, WI; South: AL, Washington DC, FL, GA, KY, MS, NC, PR, SC, TN, VA, VI, WV; West: AK, AS, AZ, CA, CO, GU, HI, ID, MT, NM, NV, OR, PI, UT, WA, WY; Other: AA, AE, AP, BC, EU, FM, FG, JA, MH, MX, NB, NF, MP, ON, PW;
c Among those with at least one;
d Standardized difference (St. Diff), for which values indicative of a meaningful imbalance (>10 or <-10) between the two groups are bolded19.
Discussion
A substantial portion of CDI research efforts have focused on the incidence of and risk factors for infection among those that are 65 years and older as the disease burden disproportionately afflicts those of this age group. Undoubtedly an unintended consequence, this has resulted in a research gap among young and middle-aged adults that seems to have been acknowledged by the 2017 IDSA and SHEA clinical practice guideline update.Reference McDonald, Gerding and Johnson1 Our study addresses this gap by estimating CDI incidence, and examining more closely this vulnerable population, among the VHA population aged 18–64 years using 6 years of rich, longitudinal VHA EMR data.
We observed a sustained increase in overall CDI incidence for this younger patient population between 2011 and 2016, a finding consistent with Reveles et al’s study that reported increasing CDI incidence for VHA enrollees from 2003 until 2013 and a subsequent decline in 2014.Reference Reveles, Lawson and Mortensen14 Several recent studies, including that by Reveles et al, that document a decline in HCF CDI include patients of any age, usually adults aged 18 years and older.Reference Evans, Simbartl, Kralovic, Jain and Roselle13–Reference Reveles, Pugh and Lawson15, Reference Guh, Mu and Baggs22–Reference Evans, Kralovic, Simbartl, Jain and Roselle25 As such, HCF CDI accounts for most episodes identified, ranging from 65% to 89% for those that defined both HCF and community-associated CDI. Declines in overall incidence, therefore, are not unexpected in populations for which the majority of episodes are HCF CDI, nor are comparisons between this study and those published contradictory; rather, our findings suggest trends in disease burden among those of younger age might differ from those of older age.
Our results confirm that which is expected and perhaps inherent to the infection definitions employed: with advancing age comes deterioration of health and increasing healthcare contact, implying that the proportion of HCF CDI is likely to be higher for those of older age. We found that community-associated CDI was more common than HCF-onset and HCF-associated CDI among younger and middle-aged adults, and that the latter increased with increasing age group as well. Similarly, Lessa et alReference Lessa, Winston and McDonald5 demonstrated differences in these proportions, for which community-associated CDI was the predominant episode type among those aged 18–44 years, whereas HCF CDI accounted for 75% of CDIs among those ≥65 years.
In addition, we observed that the proportion of community-associated CDI increased during the study period, complementing the declines for HCF CDI noted by other recent studies. More striking, though, was the finding that while overall CDI incidence increased by 15% during our study period, the incidence of community-associated CDI rose by 20%. Again, the increase differs by age, however, and was more notable among the 2 older age groups in this younger population: we observed was a slight decline, marked by fluctuations over time, in the proportion for those aged 18–34 years. It is not clear whether this variation may be explained by limitations in the data source analyzed, as the low number of events, incompleteness of healthcare records, and undiagnosed infection might affect those in the youngest age group (<35 years) most. These findings are consistent with prior research by Reveles et al. that showed a gradual increase in the proportion of community-associated CDI for VHA patients aged 18 years and older from 2003 to 2014.Reference Reveles, Pugh and Lawson15
Our results suggest that younger populations are not only at risk for community-associated CDI but also an important and perhaps expanding reservoir of C. diff. Although evidence of carriage rates by age is currently limited, Loo et alReference Loo, Bourgault and Poirier26 found that the mean age of C. diff colonization on admission was lower than for those with an infection. Those aged 35–64 years are of interest given that such individuals are of working age, are raising children, and often have parents or other elder relatives to care for.27 Exposure to C. difficile from young children, as well as from frequent interactions with the healthcare system for both these children and aging parents/relatives, and the strain of caregiving itself, may augment the risk that an individual becomes colonized, with or without symptoms.Reference McDonald, Gerding and Johnson1, Reference Delate, Albrecht, Won and Jackson8, 27 This subsequently confers the risk of further transmission among and between individuals of all ages.
Most successful primary interventions to date have focused on protecting against symptomatic CDI and reducing transmission through enhanced antibiotic stewardship and infection prevention and control procedures in hospitals—the highest-risk setting.Reference McDonald, Gerding and Johnson1 Durham et alReference Durham, Olsen, Dubberke, Galvani and Townsend28 recently demonstrated that C. difficile transmission between healthcare settings and the community are interconnected, and the effects of community-based and hospital-based transmission on hospital-onset CDI are comparable.Reference Durham, Olsen, Dubberke, Galvani and Townsend28 The findings from the present study and developments in our understanding of the interconnectedness of transmission underscore the need to account for such dynamics within and beyond healthcare settings when evaluating intervention and control strategies.Reference Kumar, Miyajima and He29 Targeting C. diff in high-risk, high-contact settings, including community or ambulatory care facilities, such as through initiatives launched by CDC and United Hospital Fund to reduce outpatient antibiotic use, might serve as an effective approach to not only decrease primary CDI cases, but, perhaps just as importantly, reduce transmission of this pathogen to individuals with the greatest vulnerability and for whom outcomes tend to be more severe: those aged 65 years and older.30, 31
Several potential limiting factors should be considered when interpreting the results of our study. As is common among retrospective cohort studies, misclassification bias may impact the design. Here, the definitions employed for episode identification have not been thoroughly validated and our data sources may be incomplete, such as for prescriptions filled outside VHA or laboratory results not recorded in structured data fields. Although diagnostic coding for CDI has been shown to have sufficient sensitivity and specificity, we attempted to reduce the potential for misclassification by requiring that treatment accompany a diagnosis within a 14-day window, an approach established by prior studies, but that nevertheless may have resulted in overestimation of the incidence.Reference Olsen, Young-Xu and Stwalley2, Reference Goto, Ohl, Schweizer and Perencevich32 Episodes identified by laboratory test result criteria align with widely accepted methods; however, by including PCR tests among those searched, misclassification of colonization rather than an episode of infection may have also resulted in overestimation of the incidence.Reference McDonald, Gerding and Johnson1, 19
Additionally, our data lack information about care received outside of the VHA system. To account for this, we included only patients with evidence of recent VHA healthcare utilization and a healthcare priority group rating of 1 in order to select for those that we assume are more likely to use its services and, therefore, have a more complete EMR. Although we found the included study population had, on average, 3 more outpatient visits per year than the VHA population meeting all criteria but with any healthcare priority group rating (Supplementary Table A online), non-VHA heathcare utilization has been shown to account for 15% of healthcare costs borne by individuals <65 years of age with a rating of 1, a proportion likely to have risen slightly towards the end of study period with the implementation of the Veterans Choice Program in 2014.Reference Petersen, Byrne, Daw, Hasche, Reis and Pietz33 Furthermore, the estimates of overall incidence and our classification of HCF-associated CDI may be underestimated due to the potential for fewer face-to-face encounters with the healthcare system and, subsequently, diagnostic testing.Reference Kuntz and Polgreen34
Finally, that we found slight differences in disease burden for the study population as compared to the VHA population meeting all criteria but with any healthcare priority group rating is not surprising given that comorbidity information is captured by diagnostic coding during healthcare visits, for which those with a rating of 1 experienced 33% more in the outpatient setting. Nevertheless, the US VHA study population and healthcare system differ from other populations and systems in size, growth over time, age, sex, and health status thereby limiting the generalizability of our findings to other settings.Reference Rogers and Kazis35
Our findings indicate that CDI incidence, driven primarily by community-associated infection, is rising among the Veteran population aged 18–64 years with high service-related disability. The increasing burden of community-associated CDI in this vulnerable population warrants attention. Future studies quantifying the economic and societal burden of CDI will inform decisions surrounding prevention strategies.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/ice.2019.160.
Author ORCIDs
Ellyn M. Russo, 0000-0002-6935-5569
Acknowledgments
The authors would like to thank Leah Eickhoff, Loretta Grikis, Melissa Lewis and Nabin Neupane for their assistance with preparing content for and editing this manuscript.
Financial support
The study was supported with funding from an unrestricted research grant from Pfizer, Inc., Collegeville, Pennsylvania, USA. This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Medical Center, White River Junction, Vermont, USA. The content is solely the responsibility of the authors and does not necessarily represent the views of the US Department of Veterans Affairs or the US Government.
Conflicts of Interest
H.Y. is an employee of Pfizer, Inc., Collegeville, PA. E.R., J.K., J.S., Y.H., and Y.Y.X. received research funding from Pfizer.









