Introduction
Streptococcus pyogenes, also known as group A Streptococcus (GAS), is an opportunistic pathogen capable of asymptomatic colonization of skin and mucosa or causing diseases ranging from cellulitis to life-threatening invasive infections (iGAS).Reference Brouwer, Rivera-Hernandez and Curren1 Beyond its global health burden, GAS have been linked to outbreaks and substantial morbidity and mortality among military personnel, partly due to physical training and living in close quarters.Reference Sanchez, Cooper and Myers2 A United States (U.S.) military review documented 17 GAS outbreaks involving thousands of cases over a single decade, highlighting the need for effective surveillance.Reference Sanchez, Cooper and Myers2
GAS type emm92, as defined by molecular typing of the M protein, is among the most prevalent U.S. emm types causing iGAS, primarily in community-associated infections.3,Reference Valenciano, Onukwube and Spiller4 Here, we describe a GAS emm92/ST82 outbreak detected by genomic surveillance at a military treatment facility (MTF) by the Multidrug-Resistant Organism Repository and Surveillance Network (MRSN). Genomic and epidemiological analyses revealed high clonality among isolates and a link to grappling-sports training at multiple gyms surrounding a U.S. military base.
Methods
Bacterial isolates, whole genome sequencing (WGS), and bioinformatics analysis
GAS isolated colonies were identified in the MTF microbiology laboratory using a Bruker (MALDI-TOF) instrument and sent to the MRSN for molecular analysis. DNA extraction and library preparation followed published protocols.Reference Stribling, Hall and Powell5 Whole-genome sequencing was performed on Illumina MiSeq or NextSeq-2000 instruments (2 × 300 bp or 2 × 150 bp). Reads were trimmed with Bbduk v38.96, classified and screened for contamination using Kraken2, and assembled de novo with Shovill v1.0.9 (≥200 bp contigs, ≥50x coverage).
Genome annotation and in silico typing used AMRFinderPlus v3.12.8 for AMR gene detection, emmtyper v0.1.0 for emm typing, and mlst v2.22 for sequence typing (ST). Core-genome MLST (cgMLST) was conducted in SeqSphere+ v7.7.2 using the S. pyogenes scheme applying a < 5 allele threshold to define potential clusters and was visualized in iTOL.Reference Toorop, Kraakman and Hoogendijk6,Reference Letunic and Bork7 SNP analysis was performed with Snippy v4.4.5 (earliest isolate as reference) and Pilon v1.23 for error correction.
Epidemiological analysis and infection and prevention control (IPC) response
Epidemiological investigation combined patient interviews and retrospective chart review to identify potential exposures and links among confirmed cases. Data were used to assess associations with grappling sports, training sites, and household contacts. Intervals between positive culture and interview ranged from 27 to 36 days for 2025 cases, and could extend to 384 days for initial cases. Infection prevention efforts, led by the Department of Public Health (DPH), included community outreach, environmental inspections of gyms, and staff education on hygiene and disinfection. Rapid genomic data supported timely interventions and refinement of control strategies. While recall bias may affect the accuracy of individual risk factor assessment, consistent associations with grappling sports indicated an overall epidemiological trend.
Results
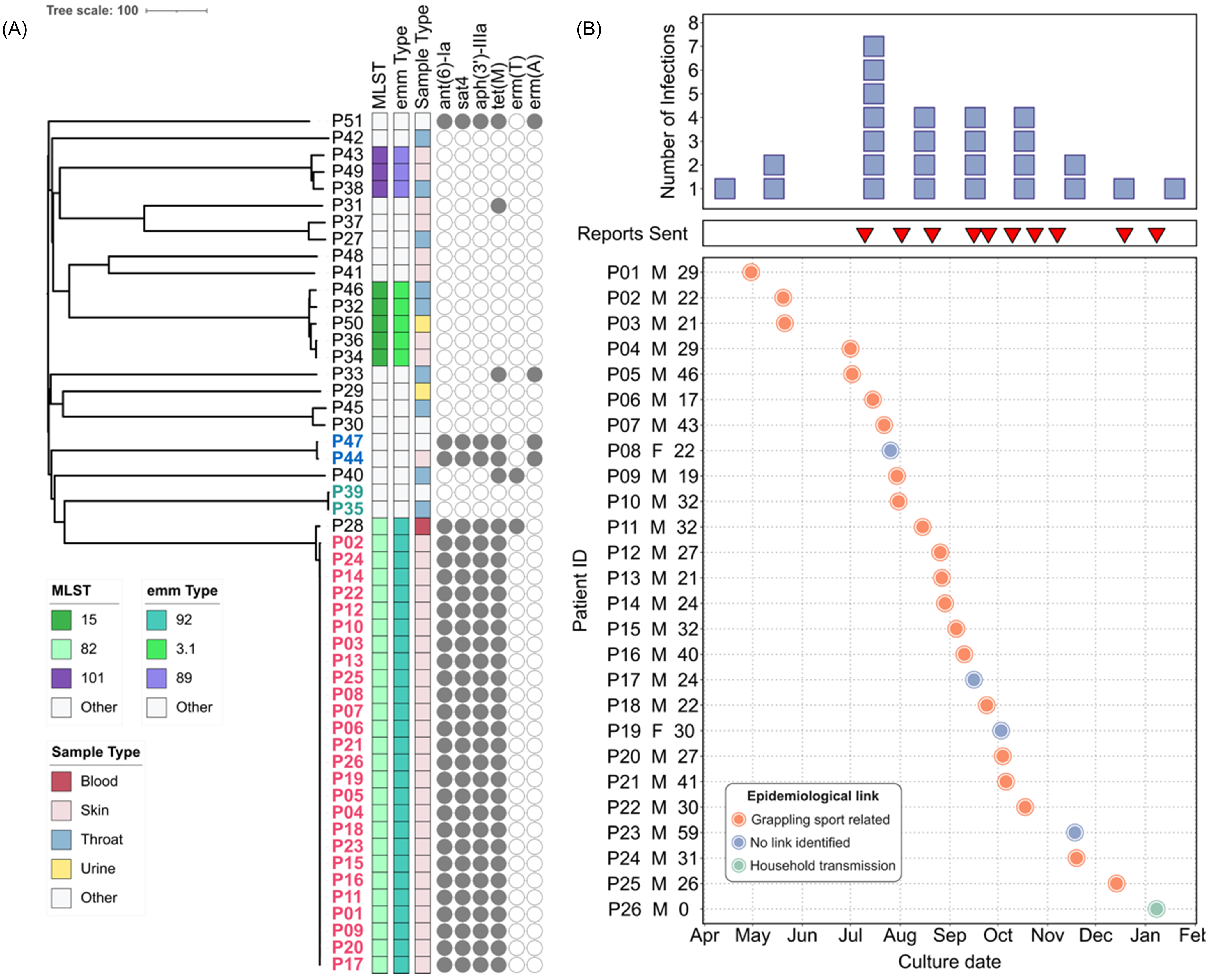
Starting in 2024, because of its relevance to military medicine, the MRSN expanded its genomic surveillance programReference Stribling, Hall and Powell5 to include GAS. Consequently, all GAS isolates cultured from all types of clinical specimens from both inpatients and outpatients were collected for genomic analyses. Between March 2024 and February 2025, 51 GAS isolates representing 15 distinct STs were recovered from 51 patients at a single MTF (Table S1). ST-82 (n = 27), ST-15 (n = 5), and ST-101 (n = 3) were the most represented. Retrospective cgMLST analysis revealed three clusters of high genetic relatedness (Figure 1A), two of which involved two isolates from two outpatients each: P35 and P39 (ST-28, 1 SNP apart); and P44 and P47 (ST-433 isolates, 2 SNPs apart). At the time of detection, reports were sent to the hospital initiating infection prevention and control (IPC) investigations that, in the absence of patient/staff overlap, ruled against a likely nosocomial transmission. The third cluster grouped isolates collected from 26 outpatients and is hereby described as the ST-82 outbreak (Table 1).

Figure 1. ST-82 outbreak S. pyogenes (GAS) linked to grappling-based sports. (A) Neighbor-joining tree based on cgMLST of 51 GAS isolates from one MTF (March 2024 –February 2025). (B) Number of ST-82 outbreak infections over 9 months (top panel), alert reports sent (middle panel), and patient chart (bottom panel). Orange, grappling sport related; green, suspected household transmission.
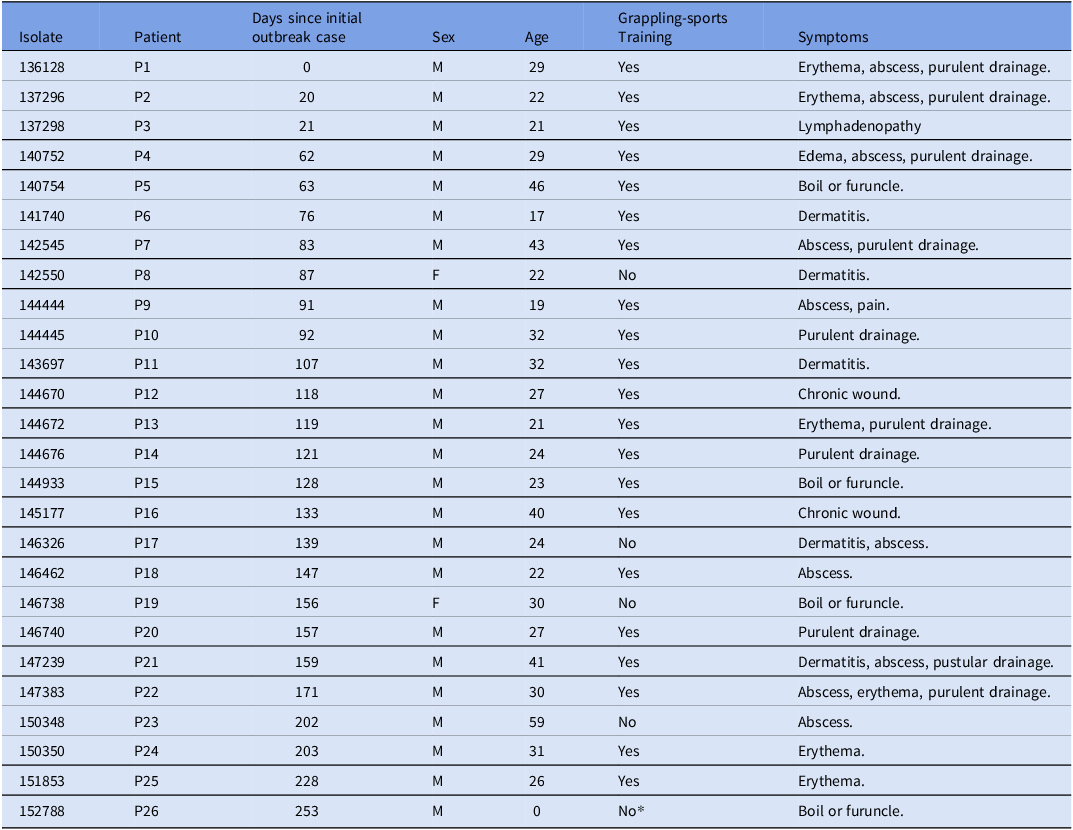
Table 1. Metadata of patients associated with S. pyogenes (GAS) ST-82 outbreak

* Suspected household transmission event.
All 26 ST-82 outbreak isolates belonged to emm type 92/ST-82 and carried the resistance determinants ant(6) and aph(3’)-III, sat4, and tetM, conferring resistance to aminoglycoside, streptothricin, and tetracycline, respectively (Figure 1A). Variant analysis confirmed the genetic relatedness between these isolates (0–2 SNPs). An additional emm type 92/ST-82 isolate (outpatient P28) was confirmed by SNP analysis to be a distinct strain (31 SNPs apart).
Because sequencing and genomic analysis were performed prospectively, multiple alerts were sent to the IPC staff as new cases were detected. In July 2024, the initial ST-82 outbreak cluster involving 3 patients (P1–P3) was identified (Figure 1B). Subsequent reports describing 23 additional cases (P4–P26) were sent to the MTF, with the base DPH notified in September 2024. The base DPH epidemiological analysis revealed that 21 cases (81%) had direct epidemiological links to grappling sports (ie Army Combatives training, jiu jitsu, or wrestling). One case involving an infant was linked to household transmission likely from a sibling who participated in jiu jitsu at one of the previously identified facilities (Figure 1B). Cases spanned nine different training facilities, four grappling-focused, with eleven cases associated with a single facility (Table S2). Environmental swabs (n = 19) of mats and equipment at this facility were negative for GAS. When compared with the rest of GAS cases within the same MTF and period, the 26 outbreak-associated patients displayed a significantly higher male to female ratio (M:F ratios = 12 vs. 1.8, respectively; Fisher’s exact test p-value = 0.0207), while median ages were comparable (ST-82 outbreak cases median age: 27 years, IQR: 9.5; other GAS cases median age: 28 years; IQR: 10) (Table S1 and Figure. 1). No additional epidemiological links were identified.
Discussion
We report a GAS community outbreak linked to grappling sports. Twenty-six outpatients over 9 months presented with skin and soft tissue infection, a condition common in grappling sports involving skin-to-skin contact and abrasions.Reference Williams, Wells and Klein8 Individuals were largely male, likely reflecting the bias in populations performing combative sports and active-duty service members.Reference Bueno, Faro and Lenetsky9,10 Twenty-one individuals had direct links to training facilities, and one infant patient was indirectly related through a family member; however, the suspected household transmission remains unconfirmed due to lack of samples from subjects involved.
All 26 ST-82 isolates were nearly identical (0–2 SNPs) despite been associated with multiple training facilities (42% form a single facility). Swabbing of high-contact surfaces at this facility ruled out an environmental reservoir, suggesting skin-to-skin transmission during combat as the most likely route of transmission. Participation in regional competitions (reported during interviews) may have facilitated the spread across multiple training facilities, but further investigations are needed for corroboration. The association of this outbreak with nine grappling training facilities underscores the need for proactive prevention measures, including routine skin assessments, strict enforcement of hygiene protocols, regular disinfection of equipment and mats, and educational efforts.
Although epidemiological investigations revealed community-driven transmission, detection relied on routine genomic surveillance at the MTF by the MRSN. Non-DoD beneficiaries or mildly affected individuals who did not seek medical care were likely missed, indicating this report likely underestimates the true case count linked to this outbreak. Surveillance at this location continues with six additional closely related cases (1–2 SNPs apart from outbreak isolates) detected between April and August 2025. Four of these patients participated in grappling-sports training, reinforcing the link between grappling-based activities and this GAS outbreak.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ice.2025.10367.
Data availability statement
Genomes described herein have been deposited at GenBank under BioProject PRJNA1257432.
Acknowledgments
The authors are thankful to all the staff of the MRSN. The manuscript has been reviewed by the Walter Reed Army Institute of Research, and there is no objection to its presentation. The views expressed herein are those of the author(s) and do not necessarily reflect the official policy or position of the Defense Health Agency, the Department of War, nor any agencies under the U.S. Government.
Author contributions
J.W.B., P.T.M., and F.L. designed research; G.V.C., W.S., S.G., R.W., A.N., L.P., and Y.I.K. performed research. G.V.C., W.S., M.J.M, S.G., J.W.B., P.T.M., and F.L. analyzed data; J.W.B., P.T.M., S.G., R.W., and A.N. contributed clinical metadata and isolates. G.V.C., M.J.M, S.G., F.L., and J.W.B. wrote the paper with input from all authors.
Financial support
This study was funded by Defense Health Program (DHP) Operation and Maintenance (O&M). In addition, this study was partly funded by the Armed Forces Health Surveillance Division (AFHSD), Global Emerging Infections Surveillance (GEIS) Branch ProMIS ID P0094_25_WR.
Competing interests
None.
Ethical standard
The isolates and clinical information were collected as part of the public health surveillance activities of the MRSN, as determined by the WRAIR branch director and Human Subjects Protection Branch (HSPB) which granted ethical approval. Informed patient consent was waived as samples were taken under a hospital surveillance framework for routine sampling.