INTRODUCTION
Shigellosis is a potentially severe infectious disease caused by four species of Shigella: S. boydii, S. dysenteriae, S. flexneri and S. sonnei, with different virulence and geographical distribution. A small infective dose (10–200 organisms) can cause disease. Watery diarrhoea, fever and abdominal cramps are common 12–96 h after infection. Humans transmit the disease directly via the faecal–oral route, or indirectly by faecal contamination of food or water. Elimination through faeces can persist for up to 1 month and asymptomatic carriers can transmit the disease [Reference Heymann1]. Worldwide, an estimated 164·7 million cases occur annually, of which only 1·5 million are in developed countries [Reference Kotloff2]. In Western Europe, most cases are imported [3–Reference Müller8], but person-to-person transmission often occurs in children [Reference Suspiro and Menezes9–Reference Mohle-Boetani11]; outbreaks in men who have sex with men (MSM) have also been described [Reference Marcus12]. The European Food Safety Authority (EFSA) reported nine foodborne outbreaks of shigellosis, affecting a total of 325 people in 2007. S. sonnei caused five of these outbreaks affecting 223 persons [13]. However, in Belgium, reported foodborne shigellosis is very uncommon.
Belgium performs notifiable diseases surveillance at a regional level (Brussels, Flanders, Wallonia). At the national level, a network of sentinel laboratories (SLN) documents trends in Shigella detection and the National Reference Centre for Salmonella and Shigella (NRCSS) characterizes Shigella isolates. Between 1990 and 2007, this laboratory analysed 7307 specimens, of which 68% corresponded to S. sonnei [Reference Vrints14]. Travel abroad was mentioned in 12–25% of the records between 2000 and 2008 [Reference Bertrand15]. The SLN detected a yearly average of 52 cases (range 39–60) of shigellosis between 1998 and 2007. Forty-six percent of the cases were men, 63% were aged 25–64 years and 60% occurred in Flanders [Reference Ducoffre16]. Thus far two outbreaks of S. sonnei in Flanders were linked to person-to-person transmission; one, after Jewish Easter ceremonies (42 cases) [Reference De Schrijver17] and the other, after an accidental infection of a laboratory worker (four cases) [Reference De Schrijver18]. Shigellosis was a notifiable disease in Flanders until June 2009 [19].
THE ALERT
On 13 November 2009, a general practitioner (GP) triggered an alert after a patient diagnosed with S. sonnei informed about the existence of more people with gastrointestinal problems, at her workplace, during the previous month.
From 1 October to 12 November 2009, the number of notifications of cases of shigellosis (n=11) had increased in the province of Flemish Brabant compared to the same period (n=6) in 2008. Cases had been routinely interviewed and, in the early phase, no common link had been established.
The Infectious Disease Control Unit in Flemish Brabant (ToVo) re-contacted the 11 notified cases. Six out of 11 cases were working at the same public institution. On 16 November 2009, ToVo in collaboration with the Federal Agency for Safety of the Food Chain (FASFC) visited the institution, inspected hygiene and procedures in the canteen and provided advice on hygiene to all workers. On 20 November, ToVo, together with the Scientific Institute of Public Health (IPH), started a coordinated outbreak investigation to assess the outbreak's extent, discover the source and to implement further control measures.
METHODS
Descriptive epidemiology
Setting
The outbreak alert indicated a public institution with more than 700 employees. The institution comprised of 35 different departments in two adjacent buildings. A canteen operated by an external company was located in one of the buildings. This canteen employed three full-time workers and occasionally engaged additional staff from another restaurant owned by the same company. Drinking water at the workplace is from the municipal supply and is already chlorinated. It is dispensed through cooling machines that were installed during the week of 14 September 2009.
Separate toilets for men and women are located on every floor. Additionally there are common toilets for physically handicapped people.
Case definition
We identified cases using the case definitions shown in Table 1.
Table 1. Case definitions for shigellosis in the outbreak at a public institution in Flemish Brabant, Belgium 2009

Case finding
In order to identify probable cases in the affected institution ToVo sent an email questionnaire to all employees on 17 November, hereafter referred to as the ‘employee survey’. They were informed about the outbreak and asked to complete the questionnaire. The questionnaire included questions on demography, working department, symptoms, visit to the doctor, and exposure to canteen and to canteen-food items during October.
The three canteen employees were asked about previous travel history, tasks they performed in the canteen, holiday and/or sick leave, symptoms of disease, visit to a doctor, antibiotic consumption, and the presence of affected people in their households.
Analytical study design
The exposure information from the employee survey allowed us to estimate the frequency of different exposures. Subsequently, we conducted a matched case-control study with a 1:2 design in order to assess the association between becoming a case of shigellosis and having eaten canteen food during the exposure period. Each case was matched to two controls by sex and working department, first, to be able to assess the same period of exposure for the case and for the case's controls, and second, to control possible confounding regarding water consumption and toilet use. We included probable and confirmed cases in the case-control study.
Definition of exposures
Employee survey
We asked about exposure to the canteen food and type of food consumed during October.
Matched case-control study
The distribution of cases over time made us suspect continuous or recurrent exposure. For this reason we defined exposure period as the maximum incubation for S. sonnei (4 days). Thus, for each matched set of case and controls, we defined exposure to canteen food as having eaten food items from the canteen up to 4 days before the date when the case first began to experience symptoms.
We asked about specific canteen-food items during this period. Exposure to other restaurants during the incubation period and travel abroad 1 week before onset of symptoms were also registered.
Statistical analysis
In both studies, data were collected in an email-based questionnaire and stored in Epidata®. Double data entry was performed. We employed Stata v. 10 software (StataCorp, USA).
Employee survey
We calculated the prevalence (as percentage) of exposure to different factors in cases and non-cases and then, for each exposure, the difference between the prevalence (percentage) of cases and non-cases and the 95% confidence interval (CIs).
Matched case-control
We used conditional logistic regression analysis to calculate matched odds ratios (mORs) and 95% CIs.
Microbiological investigation of human faecal samples
Isolates from confirmed Shigella cases were sent to the NRCSS for characterization by pulsed-field gel electrophoresis (PFGE). Genomic DNA suitable for PFGE was prepared according to the PulseNet method and digested with the restriction endonuclease XbaI (New England Biolabs, The Netherlands) [Reference Litwin20]. Salmonella enterica serovar Braenderup H9812 was used as a size marker. Additionally, unrelated Shigella strains originating from national collections were used as internal reference.
Fingerprinting II Informatix™ software (Bio-Rad, USA) was used to compare the PFGE profiles. The bands generated were analysed using the Dice coefficient and the unweighted pair-group method with averages (UPGMA) using a tolerance of 0·5%.
Employees of the private catering (permanent or occasional) company provided faecal samples which were tested in the laboratory of Brussels University Hospital.
Environmental investigation
The authorities visited the institution to assess environmental aspects and kitchen conditions and procedures. There were no food leftovers available. A private laboratory analysed 25-ml water samples from three cooling machines, including the canteen. ISO norm 21567 was used for Shigella detection.
RESULTS
Descriptive findings
Persons
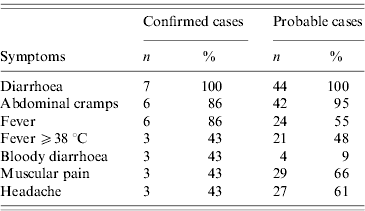
From a total of 708 employees 374 (52·8%) responded to the employee survey. We identified seven confirmed and 44 probable cases. Of the total number of cases, 21 (41%) were men and 30 (59%) women. The median age of respondents was 40 years (range 19–62). The median age of cases was 34 years (range 22–60). The attack rate in respondents was 14% (17% for men and 12% for women). The main symptoms of confirmed and probable cases are described in Table 2.
Table 2. Frequency of symptoms in probable (n=44) and confirmed (n=7) cases of shigellosis, by Shigella sonnei, in a public institution, Flemish Brabant, Belgium, October 2009
Time
The first probable case presented symptoms on 25 September 2009 and the last probable case on 22 November 2009. The outbreak lasted 2 months. Confirmed and probable cases presented a median duration of disease of 8 days (range 7–12) and 6 days (range 2–25), respectively. The epidemic curve (Fig. 1) shows the dates of symptom onset of the cases.
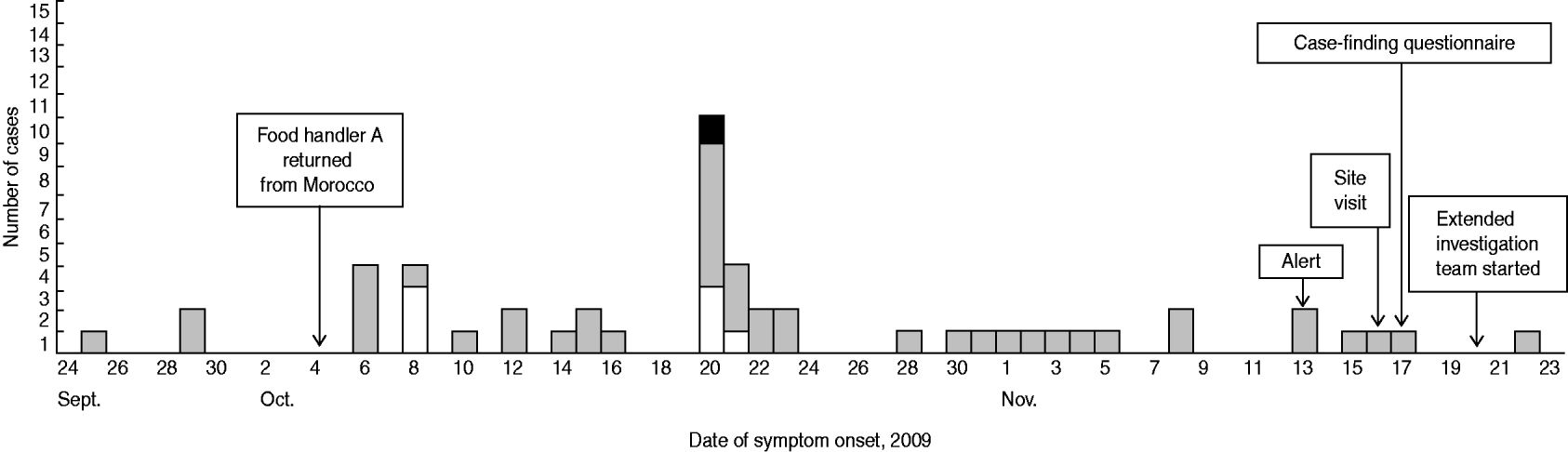
Fig. 1. Cases of shigellosis, by date of onset of symptoms and confirmation status, in a public institution, Flemish Brabant, Belgium, September–November 2009 (n=52). The total of 52 includes a suspected case (food handler A) found in food handlers via their specific questionnaire. □, Confirmed cases; ![]() , probable cases; ▪, probable case (food handler B).
, probable cases; ▪, probable case (food handler B).
Many probable and confirmed cases had an onset of disease between 6 and 8 October and between 20 and 23 October. After 28 October, many employees reported symptoms, but their treating GPs did not request a laboratory diagnosis.
No further cases have been detected in the institution since 22 November 2009.
Place
All confirmed cases (n=7) worked in different departments. There were probable or confirmed cases in 25 out of 35 departments.
Interview of food handlers
The three food handlers working permanently in the canteen responded to the questionnaire. Food handler A travelled to Turkey from 23 September to 4 October 2009. She started working on 7 October. She prepared sandwiches, washed dishes and served food. She fell ill on 20 October, and had been exposed to canteen food during the 4 days prior to disease onset. Food handler B travelled to Morocco from 23 September to 1 October. This person started working on 4 October and was involved in vegetable washing, preparation of hot meals, sandwiches, cold dishes involving vegetables and cleaning the canteen. He did not declare having fallen sick. Food handler C was also involved in all activities except in hot meal preparation. He had not travelled, been absent or fallen sick.
Employee survey
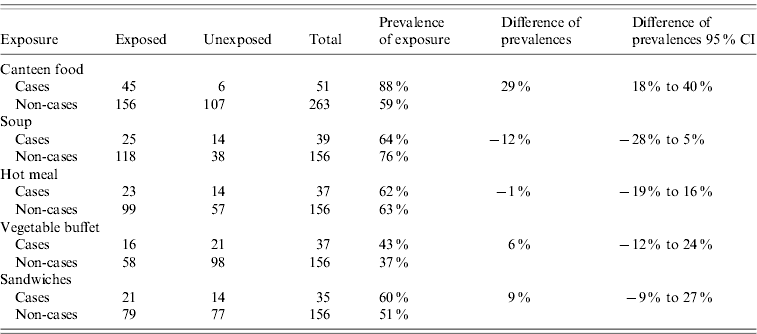
Of the cases, 88% had eaten canteen food in October while only 59% of the non-cases had. Thus the difference of prevalences of exposure to canteen food between cases and non-cases was 29% (95% CI 18–40). The prevalence of exposure to other food items, the differences in prevalence in cases and non-cases and their 95% CIs are shown in Table 3. Nobody declared affected persons at the household level.
Table 3. Prevalence of different exposures and differences of prevalence, in cases and non-cases, according to the employee survey, Flemish Brabant, Belgium, October 2009
Matched case-control study
We sent a questionnaire to 40 cases and 80 controls. We were able to recruit 33 (82·5%) cases and 41 (51·3%) controls and could analyse 20 matched sets: 11 sets of one case and two controls and nine sets of one case and one control. None of the 33 cases had travelled abroad during the week prior to the onset of symptoms. Cases had a mOR of 3·84 (95% CI 1·02–14·44) for having eaten canteen food during the 4 days before the onset of symptoms compared to controls. Other exposures did not show a significant association (see Table 4).
Table 4. Exposures and matched odds ratios found in the case-control study during an outbreak of shigellosis in a public institution in Flemish Brabant, Belgium, September–November 2009Footnote *
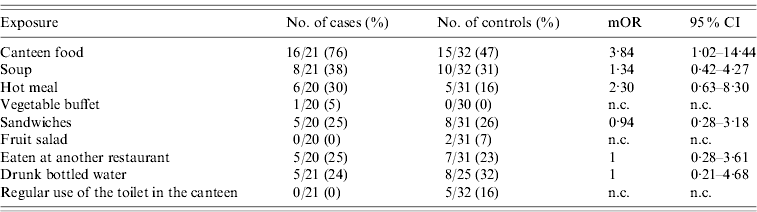
mOR, Matched odds ratio; CI, confidence interval; n.c., not calculable.
* Information for 33 cases and 41 controls was available but only 20 sets could be included in the analysis.
Faecal samples
Faecal samples were provided by 13 patients. From them, seven were positive for S. sonnei. Five positive samples were characterized at the NRCSS. Three very similar PFGE profiles (XbaI, XbaIa, XbaIb), were observed to differ by only one additional band (Fig. 2).
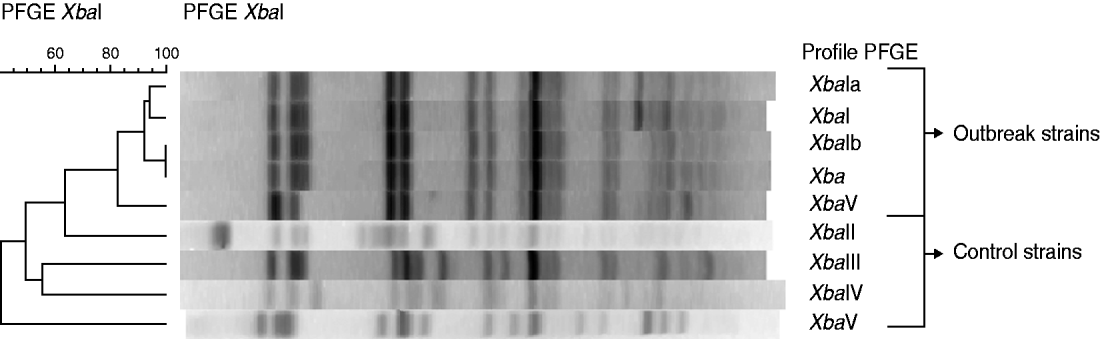
Fig. 2. Dendrogram generated by BioNumerics showing the results of cluster analysis on the basis of PFGE fingerprinting. Similarity analysis was performed using the Dice coefficient, and clustering was by UPGMA. The five PFGE profiles of the strains of the outbreak in Flemish Brabant were compared to different profiles of strains isolated from patients without travel information or having travelled to Egypt. Dendrogram showing the results of cluster analysis on the basis of PFGE fingerprinting of five Shigella sonnei isolates from the outbreak in Flemish Brabant and three controls taken from patients with no travel information or travel to Egypt.
These highly related patterns were compared with 19 reference strains. Eleven of these 19 strains were isolated from patients returning from Morocco between 2008 and 2009. The outbreak strains closely resembled those from 10 of the 11 isolates of patients returning from Morocco (Fig. 3). Other internal controls (taken from specimens with no travel information or returning from countries other than Morocco) presented completely different PFGE profiles (Fig. 2).

Fig. 3. Representative PFGE of XbaI-digested genomic DNA from Shigella sonnei isolates. M, XbaI-digested DNA from S. enterica serotype Braenderup H9812 used as molecular size marker; lanes 1, 3–13, internal reference strains isolated from patients having travelled to Morocco; lane 2, internal reference strain isolated from a patient without travel information; lanes 14–16, three of the five strains from the outbreak in Flemish Brabant.
The faecal cultures from the three permanent food handlers were negative.
Additional cultures from seven other occasional food handlers (taken from 17 November 2009 onwards) also tested negative for Shigella.
Environmental samples
The three water samples were negative for Shigella.
DISCUSSION
Fifty-two cases of shigellosis were found in 708 employees of a public institution in Flemish Brabant province, Belgium, between September and November 2009. Seven cases were confirmed as S. sonnei. There was a common PFGE profile which resembled those from archived specimens from Morocco. Cases of shigellosis were associated with canteen-food consumption.
Regarding the route of transmission, we worked with three hypotheses: (i) waterborne transmission through a contaminated water dispenser, (ii) person-to-person transmission or via surfaces (toilets), or (iii) foodborne transmission (through previously contaminated food or during the preparation process by a contaminated food handler).
Waterborne transmission through water machines would have led to a clustered distribution of cases by department.
Person-to-person transmission, in developed countries, usually occurs in young children and their contacts [Reference Suspiro and Menezes9–Reference Mohle-Boetani11] or through practices leading to faecal–oral exposure, e.g. in MSM [Reference Marcus12]. Neither of these situations corresponded with our scenario, composed of an adult working population.
The epidemic curve shows a pattern of intermittent point source (with confirmed cases appearing in both peaks) combined with residual person-to-person transmission towards the end of the outbreak. This pattern resembles an outbreak associated with canteen food and person-to-person spread in a school in the UK [Reference Maguire21]. Foodborne transmission through canteen food is supported by the results of the employee survey and by the matched case-control study.
This led us to think that a food handler might have been the source of the outbreak. Food handler B returned from Morocco shortly before the appearance of the first confirmed cases. He did not report any symptoms and worked continuously since his return. Foodborne transmission might have happened had he been an asymptomatic case. Healthy carriers can shed 102 Shigella c.f.u./g of faeces during 1 month [Reference Sansonetti22]. Thus, food handler B could have unintentionally acted as an intermittent source of food contamination during the period of faecal shedding. Conversely, food handler A, who had travelled to Turkey, could not be the source of the outbreak, since her onset of disease happened after the onset of symptoms of some confirmed cases.
We did not isolate Shigella in any of the food handlers' faecal samples. However, samples were tested around 6 weeks after food handler B returned from Morocco and around 1 month after the onset of symptoms of the last confirmed cases. This delay could have led to the negative results obtained.
All the five subtyped S. sonnei isolates from this outbreak demonstrated closely related PFGE patterns. These pulsotypes were considered identical according to the criteria of Tenover et al. [Reference Tenover23] because only one band difference was found. Our study has also shown that these PFGE patterns were common in the strains isolated from patients returning from Morocco. These results indicate an imported strain and strengthen the hypothesis of a common source.
Our study has some limitations. First, recall bias may be present. In order to minimize recall bias we sent the detailed weekly menus for the period studied. Second, misclassification could have occurred due to the lack of a definition for diarrhoea within the employee survey.
Another concern is the inclusion of duration within the case definition. This probably diminished sensitivity leading to a potential underestimation of the number of cases in respondents. Moreover, the inclusion of abdominal pain might have diminished specificity, and this could have led to some false positives. This might partly explain the detection of cases before the suggested source started contaminating people.
Due to the response rate of around 50%, response bias cannot be ruled out.
Employees with symptoms were probably more likely to respond than those without. Thus the attack rate of 14%, if extrapolated to the whole institution, could be overestimated. This is supported by the findings of an investigation of a shigellosis outbreak associated with canteen food in a school in the UK, where the attack rate of diarrhoea and fever was 8% in children aged 8–12 years [Reference Maguire21].
Moreover, attack rates found in shigellosis waterborne outbreaks were 4% in adolescents and adults in Crete and between 5% and 7% for people aged 15–64 years in Spain [Reference Samonis24, Reference Arias25].
By matching by sex and department we aimed to control for confounding and to analyse the exposure to canteen food. Nevertheless, overmatching could have led to very similar cases and controls and thus have diminished our statistical efficiency; moreover, we could only analyse 20 matched sets from 40 potential ones, and this might account for the wide CIs and the borderline significance (mOR 3·8, 95% CI 1·02–14·44) for the association between canteen-food consumption and becoming a case of shigellosis.
Since we could not link any specific food item to the disease, it is more likely that a food handler contaminated not one but several food items during the preparation of food. Food contamination during food production or any other process of the food chain is less likely, and would also have led to an increase in cases outside the institution.
To conclude, this outbreak caused by a single strain of S. sonnei, very similar to strains imported from Morocco, affected a number of employees of a public institution in Flemish Brabant. The consumption of canteen food was a significant risk factor. It is possible that a food handler with history of travel to Morocco acted as an intermittent source. Asking notified cases about their workplace during routine interviews would have led to an earlier detection of the outbreak.
Our recommendations included: (i) washing hands with soap and water before eating and after defecation for employees and food handlers; (ii) preventing sick food handlers from working until full recovery or until negative faecal culture in the case of laboratory confirmation, (iii) maintaining surveillance of further possible cases of shigellosis through the institution's prevention service, and (iv) collecting information on the workplace when interviewing notifiable cases in order to detect infectious disease clusters early.
ACKNOWLEDGEMENTS
The authors thank all employees at the affected public institution. We thank Dr Viviane Bremer for helpful comments on the questionnaires used and on the manuscript. We thank Christiane Banckaerts, from the Infectious Disease Control Unit, Department of Public Health Surveillance, Flemish Agency for Care and Health, for entering the data. We thank the Federal Agency for Safety of the Food Chain for technical assistance.
DECLARATION OF INTEREST
None.








