Introduction
Leptospirosis is a neglected zoonotic disease that can cause a wide range of symptoms from mild flu-like illness to death [Reference Adler and de la Pena Moctezuma1]. Globally, approximately 1 million cases and 60 000 deaths occur annually [Reference Costa2], with a loss of 2.9 million disability-adjusted life years [Reference Torgerson3]. At-risk populations vary by location and include people who are in direct contact with infected animal urine or are indirectly exposed through contaminated environments, for example, contact with livestock (farmers and meat workers), contact with rodents (sewage workers, rice paddy workers, subsistence farmers, and urban slum dwellers), and contact with recreational and flood waters [Reference Haake and Levett4]. The highest burden of leptospirosis occurs in resource-poor tropical countries with incidence greater than 10/100 000 compared to high-income temperate countries where incidences range between 0.1 and 10/100 000 [Reference Dunay, Bass and Stremick5]. Aotearoa has one of the highest leptospirosis morbidities for a high-income temperate country [Reference Victoriano6], even though the estimates for incidence [Reference Mansell and Benschop7] and burden are under-ascertained [Reference Sanhueza8] (human notification is compulsory under the New Zealand Health Act 1956 [9]).
Since the early 1970s, leptospirosis has been considered an occupational disease in Aotearoa when the incidence peaked at 30/100 000, with abattoir workers, dairy farmers, and pig farmers identified as high-risk occupations [Reference Marshall and Manktelow10]. From the 1980s, cattle and pig vaccines were developed, and a combination of animal vaccination programmes [Reference Marshall and Chereshsky11, Reference Fairly12], guidelines on dairy farm operations [13], and personal protective equipment (PPE) among agricultural workers were implemented to prevent human infections. While incidence has decreased to 2.0/100 000 over time (1999–2017), these interventions have been only partially effective. Analysis of human notification data revealed infections from serovars not included in current animal vaccines [Reference Nisa14, Reference Thornley15] and, the use of protective equipment did not necessarily prevent infection [Reference Dreyfus16]. Furthermore, from 2017 to 2019, there was an 89% increase in cases compared with the previous 5 years (2012–2016), and there has been an increase in cases from occupations traditionally not considered high-risk [Reference Nisa14, Reference Benschop, Nisa and Spencer17]. These emerging trends suggest knowledge gaps on current risk factors.
We hypothesize that the shifting disease patterns indicate a transition towards a more tropical, epidemic-style transmission of leptospirosis in Aotearoa, with rodent and environmental pathways becoming increasingly important. The overall aim of this study was to identify modifiable risk factors for human leptospirosis in Aotearoa to inform effective policies and practices to lower the incidence and the associated social and economic burden.
Methods
Study design
This was a nationwide case–control study frequency-matched by sex and rurality, with a 2:1 ratio of controls to cases, with the aim of recruiting 300 controls and 150 cases in Aotearoa. For common exposures (prevalence 30%–70%) [Reference Fang18], this would provide >80% power for odds ratios (ORs) as low as 1.8, while for less common exposures (15%) [Reference Sanhueza19], this would provide 80% power to detect ORs as low as 2.1. The matching frequency was based on the distribution of sex and rurality of leptospirosis notifications in Aotearoa from 1 January 2014 to 31 December 2018, which included 90% males and 65% living in rural areas [20]. Rurality was determined by home address according to the most recent urban:rural classification method by Statistics New Zealand [21]. Participants were recruited between 22 July 2019 and 31 January 2022. Full details of the study design and methods, including case and control definitions, recruitment strategy, data collection, and questionnaire development, have been published in a protocol paper [Reference Nisa22].
Study participants
The initial case definition included individuals who met the New Zealand Ministry of Health definition for a confirmed or probable leptospirosis case [23]. The case definition was expanded twice during the study based on data from one of the two diagnostic laboratories that perform serological tests. These data revealed that many cases were under-ascertained between 2019 and 2020 as 72% (540/747) of patients suspected of leptospirosis did not return to provide the required second sample for leptospirosis serological testing and were therefore never diagnosed. Thus, the case definition was expanded to include patients who tested positive: (a) on any one sample, including an Immunoglobulin M screening test in diagnostic laboratories from 15 October 2020 or( b) by polymerase chain reaction in the research laboratory (Molecular Epidemiology and Public Health Laboratory) from 28 January 2021.
Controls consisted of participants from the 2016/2017 and 2017/2018 New Zealand Health Surveys (NZHS) [24] that had previously agreed to be approached for future surveys. Potential controls were excluded if they reported experiencing an influenza-like illness in the 4 weeks preceding the control survey to reduce the chances of enrolling individuals who may have had undiagnosed leptospirosis.
All cases and controls under the age of 16 were excluded.
Participant recruitment and data collection
Cases were identified as described earlier [Reference Nisa22] to ensure all eligible cases were approached. Eligible cases were contacted and invited either by Public Health Units (if they tested positive in diagnostic laboratories) or the research team (if they tested positive in the research laboratory). All cases who agreed to participate were subsequently contacted by the research team via telephone to obtain verbal consent (Appendix 1 of the Supplementary Material) and to complete the survey involving the cases’ support network when necessary. Cases were asked about their exposures in the month before they became ill.
Contact details of the NZHS cohorts were acquired from the Ministry of Health and sent to two market research companies [Reference Nisa22] who conducted the control surveys over the telephone. For logistical reasons, controls were contacted, consented, and interviewed in batches of 50 at six time periods during the study. Controls were asked about their exposures in the month preceding the interview [Reference Nisa22].
Study variables
The development of the study questionnaire is described previously [Reference Nisa22]. The variables for this study were grouped in seven main categories, including sociodemographic factors; contact and activities with livestock; contact and activities with pets; contact and activities with wild mammals; contact or activities with any animals or their products; water and environmental exposures; and health status (Appendix 2 of the Supplementary Material).
Data handling
Variables were either analysed individually or aggregated to reduce complexity and multicollinearity in multivariable models if deemed necessary. For example, the aggregate variable ‘any livestock contact’ was created by combining all variables related to contact with different livestock species. Activities with livestock were grouped by animal production type (dairy cattle, beef cattle, or sheep) or by activities (assisting calving and assisting lambing were included in an aggregate variable ‘assisting birth’). All activities involved in slaughtering (slaughtering, skinning, dressing carcasses, home killing, and killing for welfare reasons) were included in an aggregate variable called ‘any slaughter activities’. Any slaughter activities were also assessed by wearing gloves as PPE. Seeing evidence of rats and mice was aggregated as ‘seeing evidence of rodents’, as the distinction between rat and mouse ‘gnawings’ and droppings was considered difficult. Exposures to recreational water from creeks, lakes, streams, and dams were aggregated as ‘rivers’. Reactional activities such as swimming, boating, and fishing were aggregated as ‘any recreational water activities’.
When more than one answer was given for ethnicity, the New Zealand prioritized ethnicity was used in order of priority: Māori, Pacific Peoples, European, Asian, MELAA (Middle Eastern, Latin American, and African), and other ethnicities [25].
Occupations were grouped into six broad sectors: dairy, dry stock, meat works, mixed stock, not working (includes unemployed and retired), and other occupations (occupations that did not fall under the previous five sectors).
Statistical analysis
All data were analysed using R version 4.3.1 [26] with packages listed in Supplementary Table S1. Statistical significance was set at p ≤ 0.05 for all analyses.
Descriptive statistics were used to compare the sociodemographic characteristics of cases and controls. Fisher’s exact test was used to test for associations between exposures and case status.
Individual associations were initially analysed with unconditional logistic regression that was adjusted for the matched variables to calculate partially adjusted odds ratios (paORs) and 95% confidence intervals (CIs) to inform final variable inclusion in the multivariable logistic regression (MLR) models, which were used to calculate the adjusted odds ratios (aORs).
MLR models were constructed using a bidirectional stepwise variable selection approach. Three separate regression models were used to show the impact of adjustments on the association between exposures and leptospirosis risk. Model A was a priori adjusted for only the matching variables to establish a baseline; model B included additional adjustments for age and season; and model C further included the occupational sector due to the significant association of leptospirosis with livestock occupations. Age was adjusted for because controls were significantly older than cases (Wilcoxon p < 0.001), and age is frequently associated with exposures and health outcomes [Reference Grimes and Schulz27]. Season was adjusted for because 50% of control interviews were conducted during summer (21 December to 20 March), compared to only 21% of cases (Table 1). Individual associations were included in the preliminary MLR model if they had paORs of ≥3 or ≤0.3, or a p ≤0.2 (threshold). Variables with a Pearson correlation coefficient of ≥0.5 were subsequently removed from the model by running the model with each correlated variable separately (e.g., contact with dairy cattle and activities with dairy cattle), and the variable with the lowest p-value was kept. The inclusion of two-level interaction terms was considered but not included due to the complexity of the model and the abundance of potential interactions. Final variable selection was performed with stepwise regression until the lowest Akaike Information Criterion was achieved. Least absolute shrinkage and selection operator (LASSO) regression [Reference Friedman, Hastie and Tibshirani28] was used on the final variables to prioritize the importance of the risk factors while excluding those that were not strongly supported. Model fit was evaluated with five metrics: sensitivity, specificity, Area Under the Receiver Operating Characteristic curve, Cox and Snell’s Pseudo-R2, and Hosmer and Lemeshow test (Supplementary Table S2).
Table 1. Sociodemographic characteristics, season of exposure, and District Health Boards of 95 cases and 300 controls in a case–control study to identify risk factors for leptospirosis in Aotearoa New Zealand
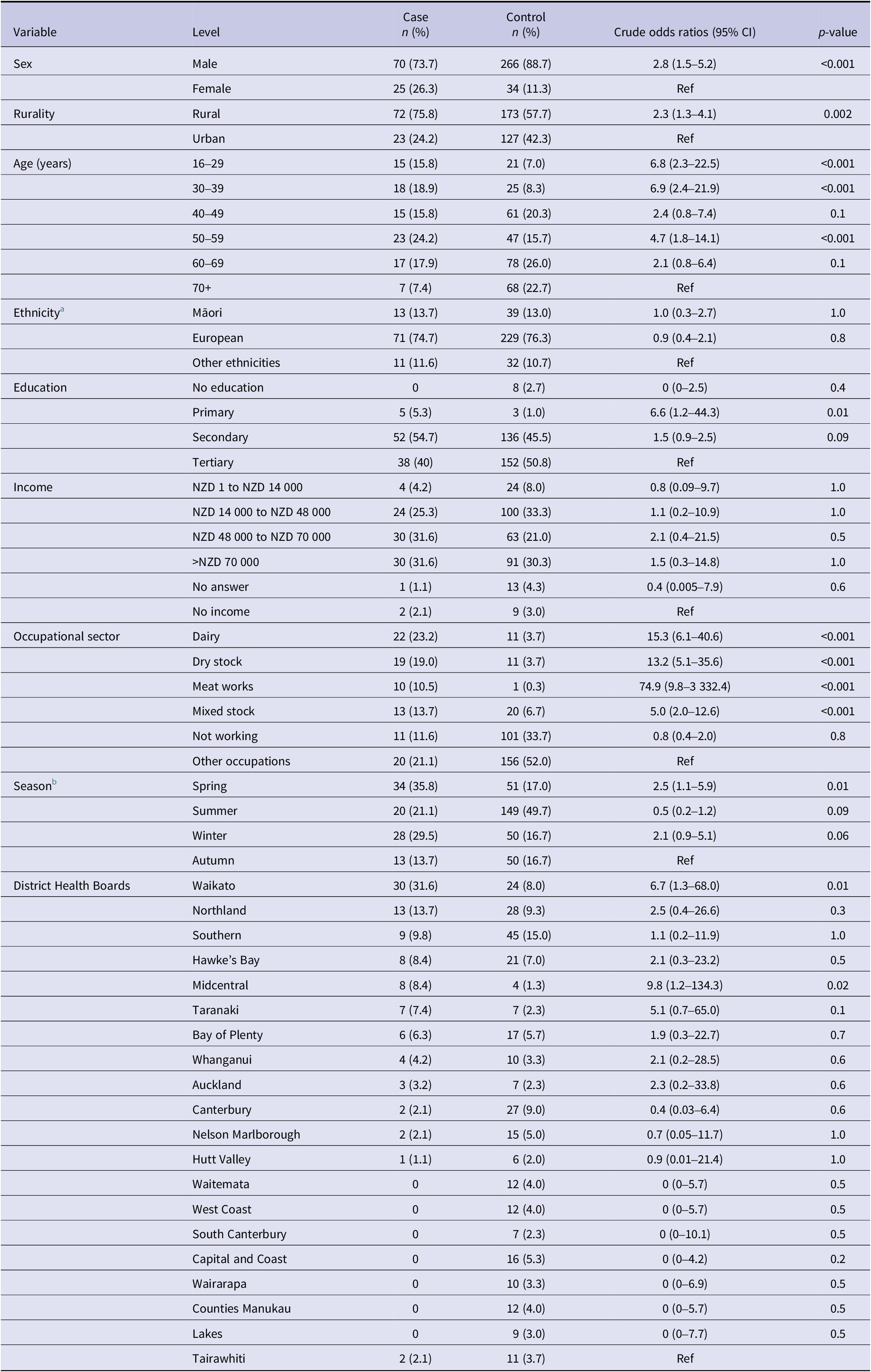
a There were no Pacific Peoples or MELAA cases; one Asian case was included in Other.
b Season was calculated from the date of disease onset for cases and the date of interview for controls, which corresponds to the date used to inquire about exposures in the month preceding the occurrence.
Population-attributable fractions (PAFs) were calculated with CIs computed via bootstrapping from 1 000 simulations [Reference Kooperberg and Petitti29].
Ethical approval
This study received human ethics approval from the Health and Disability Ethics Committee, reference number 19/STH/80 and locality agreements, together with local Māori consultation from the 20 District Health Boards (DHBs) in Aotearoa.
Results
Sociodemographic characteristics of participants
Between July 2019 and January 2022, 220 cases were notified to EpiSurv [20]; of these, 139 agreed to be contacted by the research team, 131 were able to be contacted and invited to participate, 12 declined, and 24 did not meet the case definition. Thus, 43% (95/220) of cases approached over the study period met the case definition and agreed to participate in the study. A total of 1 340 controls were called; 238 had no active telephone connection, 539 could not be contacted (no answer/engaged), 61 were not eligible, and 202 did not participate (162 refused, 35 had language issues, and 5 abandoned/stopped the interview). Thus, 53% (300/563) of controls approached over the study period met the control definition and agreed to participate in the study. In total, 395 participants (95 cases and 300 controls) were interviewed between 25 July 2019 and 13 April 2022.
The percentage of males among controls (89%) was significantly higher compared with cases (74%, p < 0.001). A higher percentage of cases lived in rural areas (76%) compared with controls (58%, p = 0.002). Cases were younger (median: 49 years; interquartile range (IQR): 35.5–59.5) than controls (median: 59 years; IQR: 46–57.8) and 35% of controls were >65 years of age compared to 13% of cases (p < 0.001). The percentage of cases (67%) employed in the livestock industry (dairy, dry stock, mixed stock, and meat works) was significantly higher compared with controls (14.4%, p < 0.001). Controls were predominantly engaged in occupations that were not in the livestock industry (52%) or were not working (retired/unemployed = 33.7%, p < 0.001). No significant differences in ethnicity were observed between cases and controls. The sex, age, and ethnicity of cases in this study (Table 1) are reflective of all notified cases that met the Ministry of Health case definition during the study period (Supplementary Table S3).
Table 1 also shows seasonality, which corresponds to the date used to inquire about exposures in the month preceding the occurrence and the distribution of cases from the 20 DHBs. Waikato DHB had the highest proportion of cases (32%), which is consistent with notification data [30].
Individual associations
Tables 2–5 present individual associations between potential risk factors and leptospirosis (p ≤ 0.2), while associations with p >0.2 are in Supplementary Tables S4–S9.
Table 2. Individual association between livestock factors and leptospirosis in Aotearoa New Zealand, adjusted for sex and rurality in logistic regression analysis (p-value ≤ 0.2)

Table 3. Individual association between pets, mammalian wildlife, and contact/activities with any animals or their products, and leptospirosis in Aotearoa New Zealand, adjusted for sex and rurality in logistic regression analysis (p-value ≤ 0.2)
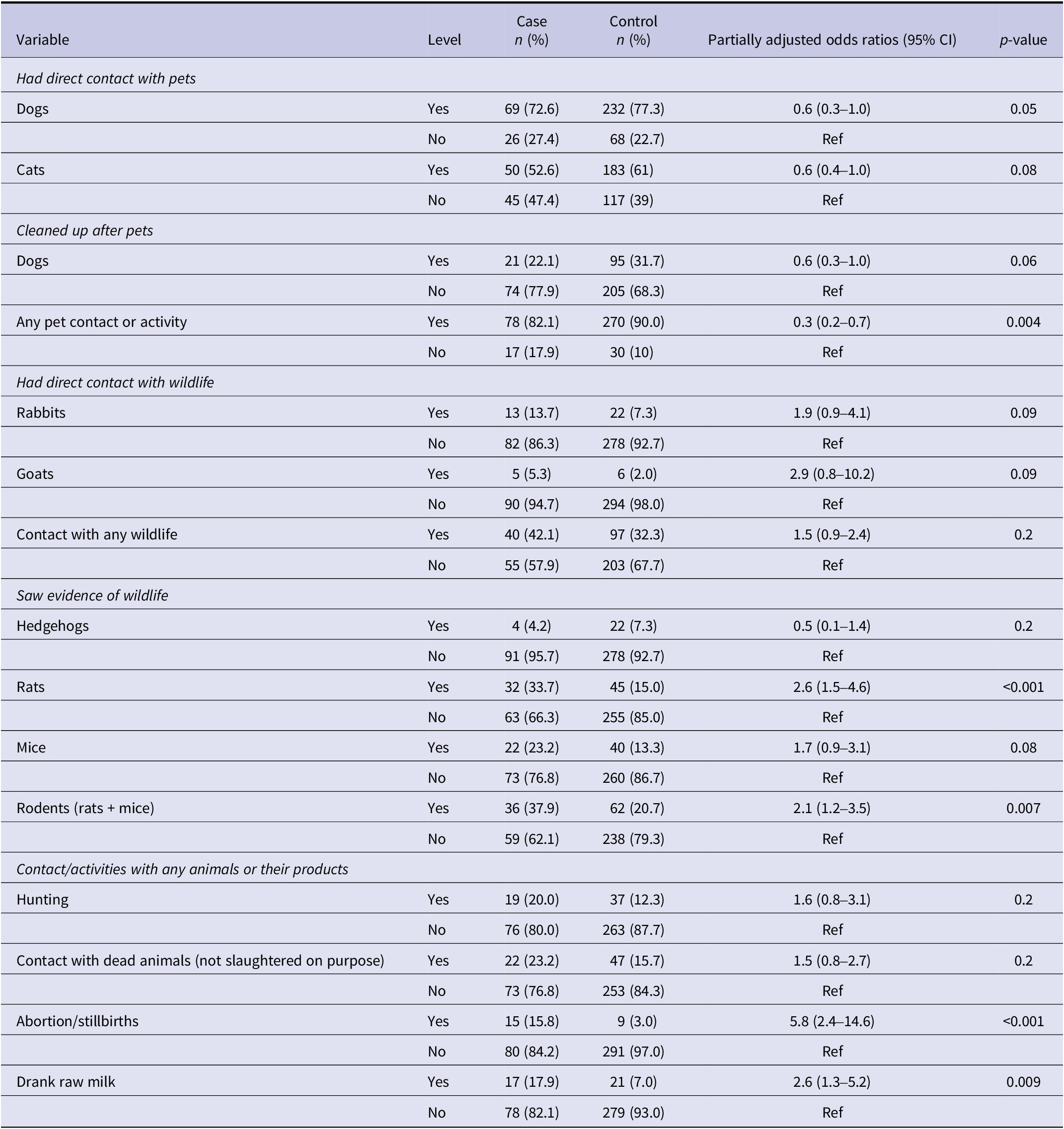
Table 4. Individual association between water/environmental exposures and leptospirosis in Aotearoa New Zealand, adjusted for sex and rurality in logistic regression analysis (p-value ≤ 0.2)
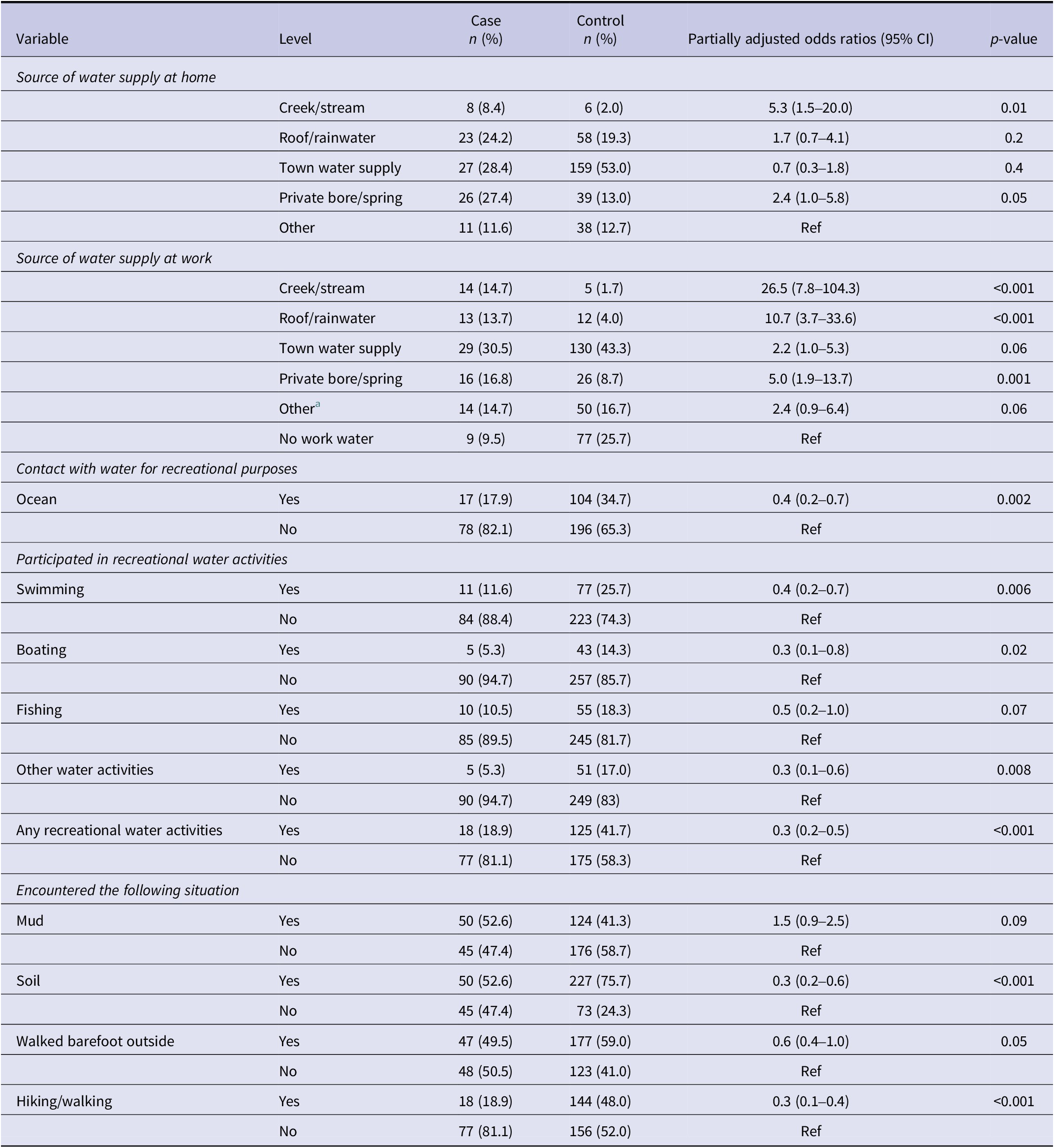
a Unsure was added to other.
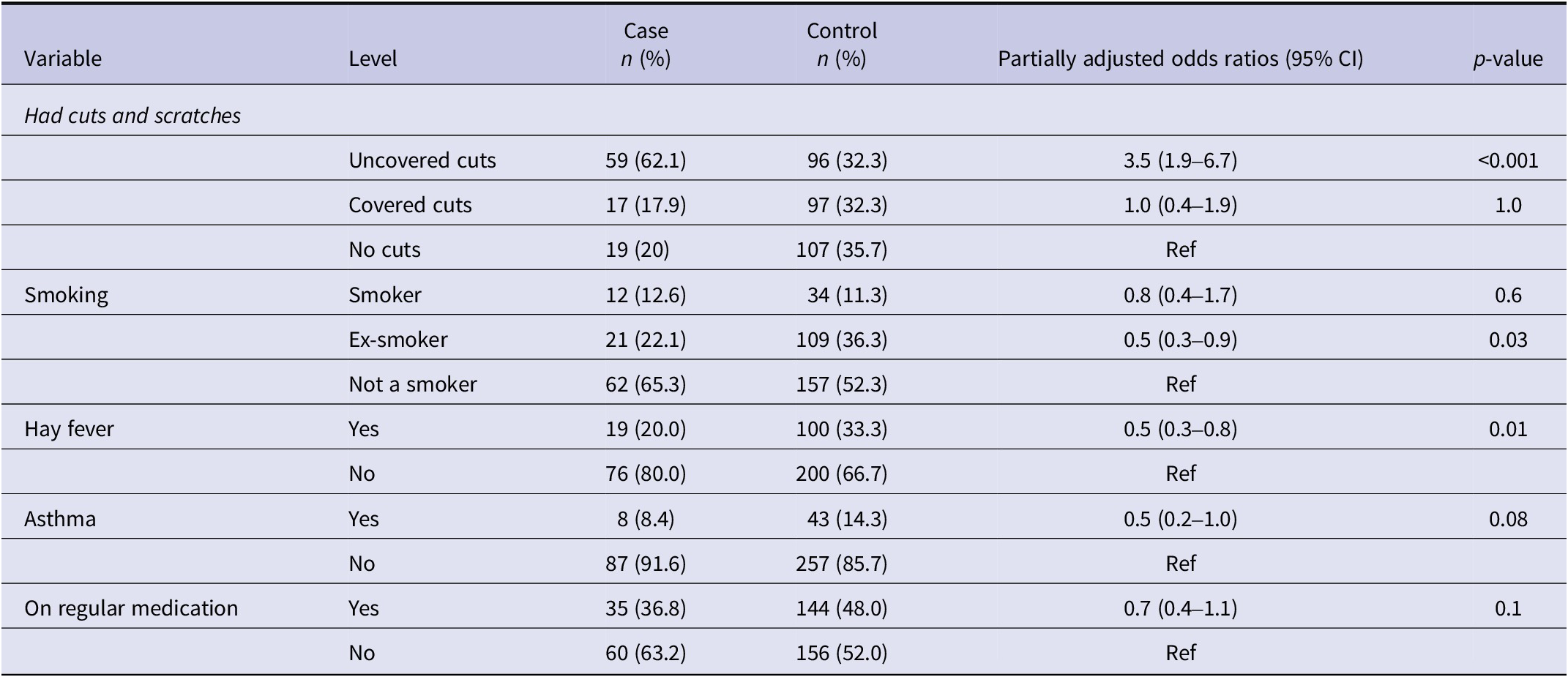
Table 5. Individual association between health status and leptospirosis in Aotearoa New Zealand, adjusted for sex and rurality in logistic regression analysis (p-value ≤ 0.2)
Significant risk factors included any livestock contact, specifically contact with dairy and beef cattle, sheep, and pigs, as well as activities like assisting birth, milking, castrating, drenching, cleaning urine/faeces, and slaughtering with or without gloves (Table 2). Contact with goats, deer, alpacas, horses, or poultry, and activities like shearing or trimming soiled wool (crutching) were not significantly associated (Supplementary Table S4).
Contact with pet dogs was inversely associated with leptospirosis (Table 3), while most cases had no contact with other pets (Supplementary Table S5). Seeing evidence of rats and rodents was a significant risk factor, but direct wildlife contact was not (Table 3 and Supplementary Table S6). Handling aborted materials, stillbirths, and drinking raw milk were significant risks, whereas hunting and handling animal feed/manure were not (Table 3 and Supplementary Table S6).
Work water supply from creeks/streams, rainwater, private bore/spring, and town water was significantly associated with leptospirosis, while home water supply was only significant for creeks/streams and private bore/spring (Table 4). Ocean contact, recreational water activities, soil exposure, and walking barefoot were inversely associated (Table 4), with other environmental factors non-significant (Supplementary Table S7).
Uncovered cuts/scratches were a significant risk factor, while hay fever was inversely associated (Table 5). Other common health issues were not significantly associated with leptospirosis (Supplementary Table S8).
Multivariable risk factor analysis
Table 6 presents the aORs of three models, showing minimal variation between the baseline model A and model B, which was adjusted for confounders. Risk factors significantly associated with leptospirosis in model B included contact with dairy cattle, activities with beef cattle, cleaning urine/faeces from yard surfaces, work water supply from either creeks/streams or roof-collected rainwater, and uncovered cuts/scratches. Seeing evidence of rodents was identified as a risk factor but was excluded with LASSO regression. Significant factors inversely associated with leptospirosis included any recreational water activities, exposure to soil, and being an ex-smoker. When additionally adjusted for the occupational sector, significant risk factors retained included activities with beef cattle, cleaning urine/faeces from yard surfaces, work water supply from creeks/streams, uncovered cuts/scratches, and seeing evidence of rodents. Other risk factors identified when adjusted for occupation but not reaching statistical significance included work water supply from roof-collected rainwater and slaughtering without gloves; the latter was excluded with LASSO regression. Significant factors inversely associated with leptospirosis remained similar with any recreational water activities and exposure to soil. Pet cats, smoking, hay fever, and activities with sheep were also inversely associated with leptospirosis, all of which were excluded by LASSO regression.
Table 6. Multivariable association between risk factors and leptospirosis in Aotearoa New Zealand
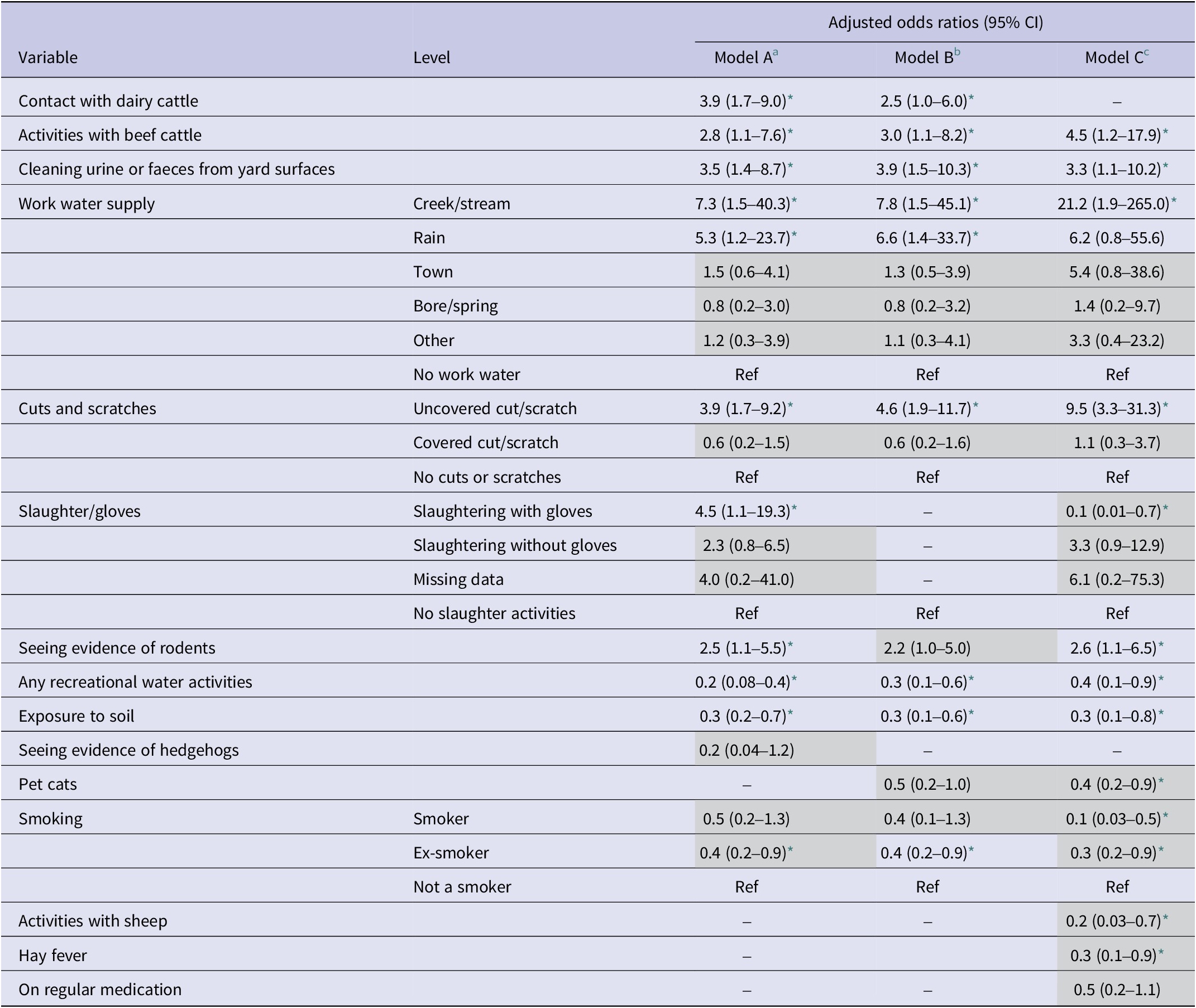
a Adjusted for sex and rurality.
b Additionally adjusted for confounders’ age and season.
c Additionally adjusted for the occupational sector.
* p-value ≤ 0.05; greyed-out adjusted odds ratios represent variables that were excluded by least absolute shrinkage and selection operator regression.
Table 7 presents the PAFs of the three models. The highest PAFs in models B and C were for uncovered cuts, followed by seeing evidence of rodents, cleaning urine/faeces from yard surfaces, and activities with beef cattle. The PAF for different sources of work water supply was higher in model C than in model B (Table 7).
Table 7. Population-attributable fractions for risk factors associated with leptospirosis in Aotearoa New Zealand
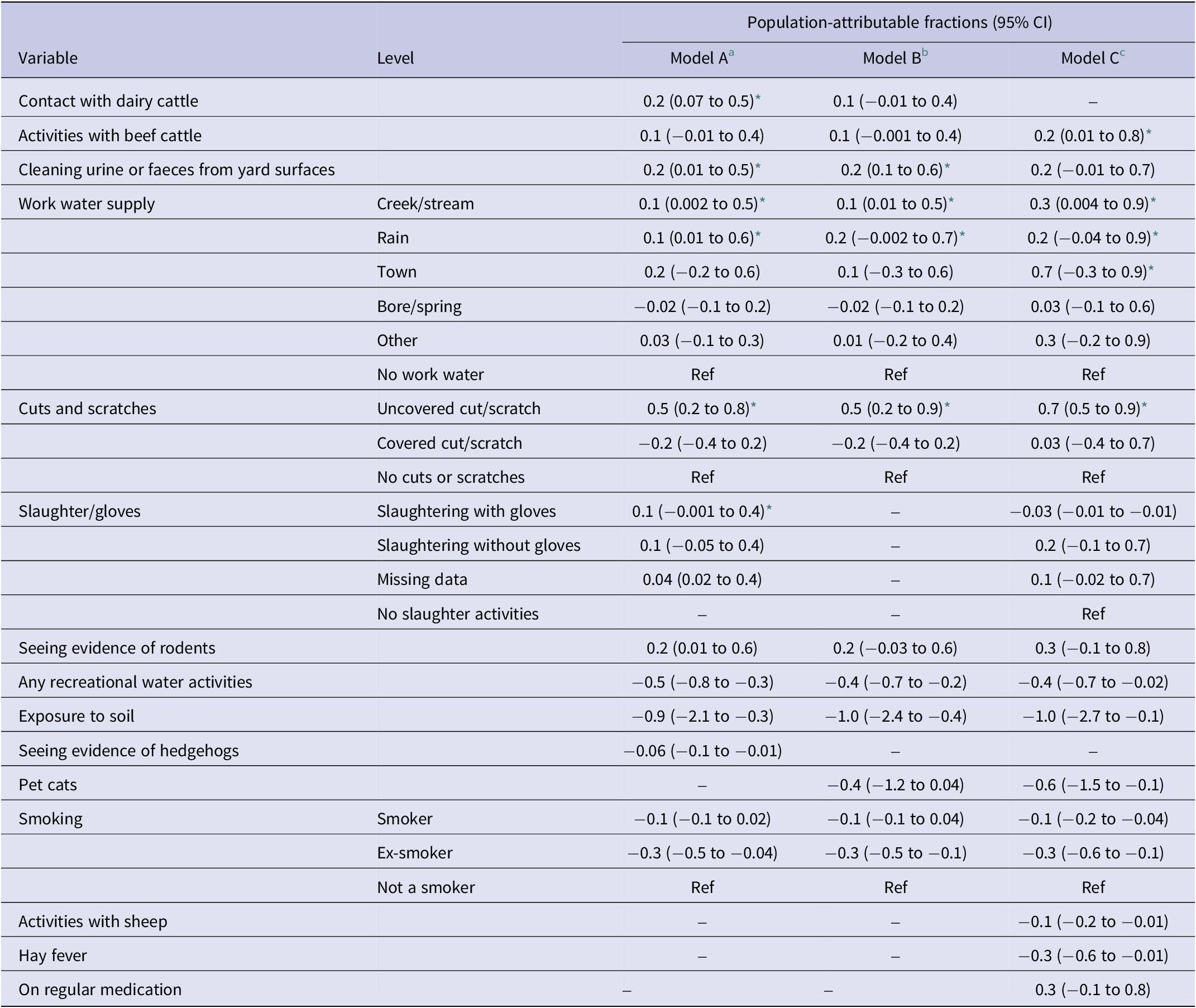
a Adjusted for sex and rurality.
b Additionally adjusted for confounders’ age and season.
c Additionally adjusted for the occupational sector.
* p-value ≤ 0.05.
Discussion
This study identified modifiable risk factors for human leptospirosis in Aotearoa, including novel behavioural and environmental factors, and confirmed its continued association with livestock occupational factors.
Uncovered cuts/scratches, the behavioural factor with the largest PAF (Table 7), increased leptospirosis risk when adjusted for the occupational sector (Table 6). This is a global risk factor for leptospirosis [Reference Mwachui31], and common in rural, manual labour-intensive sectors such as agriculture, forestry, and fishing, which have high injury rates in Aotearoa [Reference Kool32]. An investigation of a subset of occupational cases from this study found that, despite awareness of this risk, wound care was inconsistently practised – often due to time constraints and impracticality in work environments – and major wounds were typically addressed, while minor cuts were often neglected [Reference Prinsen33].
The lack of glove usage during slaughter activities emerged as a risk factor when adjusted for the occupational sector, while glove usage was inversely associated with leptospirosis (Table 6). Slaughtering in an abattoir involves experienced butchers, following strict protocols for using PPE [34], which may not be as rigorously adhered to in farm settings. In this study, 52% (17/33) of mixed stock workers engaged in slaughtering activities with only 24% (8/33) wearing gloves, while 82% (9/11) of meat workers were involved in slaughtering activities, with 72% (8/11) of these wearing gloves while performing these tasks. Other PPE usage was not assessed as they correlated with variables such as contact with dairy cattle (apron) or any farm activities (boots).
Contact with dairy cattle was identified as a risk factor and was heavily confounded by the occupational sector, despite high vaccination rates (99% of dairy farms) [Reference Yupiana35]. During this study period, animal vaccines in New Zealand only targeted selected serovars (Pomona, Hardjo, and Copenhageni) and excluded others (Tarassovi and Ballum), which continued to pose risks, especially to dairy farmers as most dairy herds are milked twice daily [Reference Nisa14]. Activities with beef cattle were also confounded by occupation, likely contributing to exposure due to lower vaccination rates (beef: 18%–25%; deer: 5%–9%; sheep: <1%) [Reference Sanhueza19]. Assisting calving [Reference Sanhueza19] and handling cattle have been linked to increased risk in both local and international studies [Reference Agampodi36, Reference Schonning37], highlighting exposure to infected urine as a common pathway.
Livestock vaccination status was collected from participants with livestock contact (Supplementary Table S9), but their knowledge was not always accurate. For instance, one case mentioned a 5-in-1 vaccine, which is not available in Aotearoa. Reported vaccination practices also differed from previous studies [Reference Sanhueza19, Reference Fang38]. This inconsistency is unsurprising, as meat workers typically lack herd vaccination data, and farm workers may not know specific vaccine details. As a result, vaccination status was excluded from MLR models, preventing analysis of its association with leptospirosis risk.
Rodent exposure was identified as a leptospirosis risk, a novel finding for Aotearoa. Rodents, primary hosts for serovars Ballum and Copenhageni [Reference Moinet39–Reference Hathaway and Blackmore41], play a key role in transmission globally, particularly in areas with poor sanitation [Reference Goarant42]. A 2016–2017 study found high rodent densities on Aotearoa farms, with 41% shedding Ballum [Reference Moinet39]. While most dairy cattle are vaccinated against Hardjo and Pomona (99%), only 27% are vaccinated against Copenhageni, suggesting that rodents may infect cattle with non-vaccine serovars, potentially creating a bridging host to humans [Reference Yupiana35, Reference Moinet43]. However, human cases associated with serovar Ballum often occur in non-livestock occupations (49%) [Reference Nisa14], indicating that direct rodent exposure, such as farm rodent control or other outdoor, plays a larger role. While leptospirosis is historically termed ‘dairy farm fever’ in Aotearoa, it is known as ‘rat fever’ elsewhere [Reference Masali44], suggesting underdiagnosis among individuals with rodent exposure in non-agricultural roles [Reference Thornley15].
Work water supply, particularly from creeks/streams, or roof-collected rainwater, was identified as a risk factor. In Aotearoa, over 10% of rural dwellings rely on untreated rainwater, with studies showing 50% of samples exceeding contamination standards and 41% heavily contaminated, often by faecal matter from animals and insects [Reference Abbott, Caughley and Douwes45, Reference Simmons46]. Rainwater shortages during dry months lead residents to supplement with water from bores, springs, or streams, often for non-potable uses like livestock and gardening [47]. This risk likely extends to rural work water supplies, particularly on farms where home and workplace overlap. The increased risk linked to work water when adjusted for occupation suggests that infection is more likely tied to contamination during work activities, such as cleaning faeces or urine from surfaces – a known risk factor. Similar risks from natural water sources have been identified globally, with variations by region [Reference Meny48, Reference Sohail49].
Exposure to soil and recreational water activities was inversely associated with leptospirosis, suggesting that these were unlikely sources of infection in this study. While floods are a known risk factor globally [Reference Mwachui31, Reference Baharom50], it was not identified as a risk here due to limited exposure during the study period (Supplementary Table S7). However, mud was associated with leptospirosis (Table 4), and there was a 140% increase in leptospirosis cases in 2023 following floods. This suggests that rainfall may be a key driver for risk, leading to pathogen mobilization either through runoffs from farms or by Leptospira resurfacing from different soil layers during erosion [Reference Thibeaux51]. Evidence of this was seen at a lake close to Auckland where leptospirosis cases were linked to swimming in the lake following flooding [52, 53]. Subsequent lake water testing detected pathogenic Leptospira; however, follow-up testing a month later was negative. Environmental Leptospira remains underexplored in Aotearoa, with only one study conducted in a farm setting [Reference Wilkinson54]. While robust surveillance exists for human leptospirosis and reservoir animals [Reference Wilkinson40], environmental data are lacking. Further research is needed to assess whether the environmental persistence of virulent Leptospira in soil and water significantly contributes to transmission, given their ability to survive in such conditions for extended periods [Reference Bierque55].
Limitations
During 2020–2021, leptospirosis cases (n = 74) averaged half of the previous 3 years (n = 140) [20] due to the impact of the COVID-19 pandemic. Diagnostic resources were redirected to COVID-19 testing, and overlapping symptoms with leptospirosis resulted in COVID-19 being prioritized, likely contributing to leptospirosis underdiagnosis. Telephone consultations replaced face-to-face care, potentially increasing the underreporting of milder cases. Thus, the study may have captured mostly severe cases, introducing selection bias. Furthermore, movement restrictions during the pandemic also limited recreational activities, likely reducing environmental exposure, and increased PPE use in meat processing may have lowered transmission risk in that sector, though farm risks likely remained unchanged. Lastly, the study’s PAF is most applicable to rural males, reflecting the demographic distribution of leptospirosis cases. While residual confounding may remain, these findings are crucial for shaping public health interventions for high-risk rural populations.
Implications
One seemingly simple yet effective measure to reduce leptospirosis risk would be covering wounds, though it can be challenging in wet and dirty environments where regular plasters may not adhere. Promoting surgical or industrial-grade waterproof plasters could improve adherence in such settings [Reference Prinsen33]. This study identified contact with dairy and beef cattle as significant risk factors, with unvaccinated livestock likely posing a greater risk, as dry stock farmers and meat workers are often infected with vaccine-covered strains, while dairy farmers are infected with non-vaccine strains [Reference Nisa14, Reference Benschop, Nisa and Spencer17], highlighting the importance of livestock vaccination. Rodent control is also crucial, with recommended measures, including baits, traps, and good hygiene practices [56]. Lastly, education and awareness campaigns are essential [Reference Pasquier57], as one-third of leptospirosis cases occurr outside the agricultural sector who are generally not aware of the disease. Collaborating with workplace experts to integrate scientific and stakeholder knowledge to help co-create practical intervention strategies for leptospirosis in Aotearoa is recommended [Reference Prinsen33].
Conclusions
This population-based case–control study identified risk factors for human leptospirosis in Aotearoa and highlighted the complex interplay of behavioural, occupational, and environmental factors. Risk factors were associated with reservoir host animals (rodents, beef, and dairy cattle), lack of protective measures (such as gloves and wound covering), and untreated work water supply. These findings not only emphasize the need for targeted prevention efforts in Aotearoa but also offer globally relevant insights, particularly for regions where similar agricultural practices, water management challenges, and animal reservoirs drive leptospirosis risk.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0950268825100071.
Data availability statement
All relevant data are provided in the tables within the main manuscript and the Supplementary Material.
Acknowledgements
The investigators thank the following for their assistance and support with this study: study participants; medical officers of health from the DHBs who worked with us to obtain locality agreement and Māori consultations; Public Health Officers, clinicians, nurses, and practice managers who assisted with case recruitment; the Ministry of Health and the Institute of Environmental Science and Research for providing monthly surveillance of cases from EpiSurv; the Ministry of Health for providing access to the NZHS participant database; Ann Bouma and Pete Morgan (Morlands Farm, Pokuru, South Waikato) for accommodation and discussions on exposures for farmers; and our Māori health advisor, Dr. Maureen Holdaway, for her experience and support in guiding the team to appropriately protect the rights and interests of Māori and in developing partnerships with local iwi.
Author contribution
Conceptualization: J.B., J.D., M.G.B., J.C.-E., J.M., E.V., G.P., J.W., S.N.; Data curation: S.N., A.F.; Formal analysis: S.N., E.O., J.B.; Funding acquisition: J.B., J.D., M.G.B., J.C.-E., J.M., E.V., G.P., J.W., S.N.; Investigation: S.N., S.L.; Methodology: J.M., E.O., J.B., S.N.; Project administration: J.B., S.N.; Resources: J.W., T.Q., P.Y.; Software: E.O., A.F.; Supervision: J.B., P.Y.; Validation: J.M.; Writing – original draft preparation: S.N.; Writing – review and editing: E.O., E.V., J.M., J.C.-E., G.P., P.Y., J.D., M.G.B., J.W., A.F., T.Q., S.L., J.B.
Funding statement
This study was funded by the Health Research Council of New Zealand (Reference ID 18/239). The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests
The authors declare no competing interests.









