Introduction
Water is essential for life and yet there are organisms that have phases of their life cycle during which they can withstand dehydration to 10% water content on a dry weight basis (0.1 g H2O g–1 dry mass; Alpert, Reference Alpert2005). This phenomenon, known as ‘anhydrobiosis’ or ‘life without water’ (actually with little water), is an attribute of many bacteria (Mattimore and Battista, Reference Mattimore and Battista1996; Billi and Potts, Reference Billi and Potts2002), fungi (Mtwisha et al., Reference Mtwisha, Brandt, McCready and Lindsey1998), lichens (Kranner et al., Reference Kranner, Cram, Zorn, Wornik, Yoshimura, Stabentheiner and Pfeifhofer2005), and some animals (Browne et al., Reference Browne, Tunnacliffe and Burnell2002; Tunnacliffe and Lapinski, Reference Tunnacliffe and Lapinski2003; Browne et al., Reference Browne, Dolan, Tyson, Goyal, Tunnacliffe and Burnell2004; Hengherr et al., Reference Hengherr, Heyer, Kohler and Schill2008; Menze et al., Reference Menze, Boswell, Toner and Hand2009). In the kingdom Plantae, certain algae and mosses exhibit vegetative anhydrobiosis (Clegg, Reference Clegg2001; Oliver et al., Reference Oliver, Tuba and Mishler2000) as do some ferns (Stuart, Reference Stuart1968; Muslin and Homann, Reference Muslin and Homann1992) and those higher plant species constituting the ‘resurrection plants’ (Moore et al., Reference Moore, Le, Brandt, Driouich and Farrant2009; Costa et al., Reference Costa, Cooper, Hilhorst and Farrant2017). The result of sexual reproduction for many of the gymno- and angiosperm species worldwide are orthodox seeds (Roberts, Reference Roberts1973) which are capable of desiccation. Largely due to the capacity to dehydrate, these seeds remain viable in extremes of temperature (Ellis et al., Reference Ellis, Hong and Roberts1988; Vertucci, Reference Vertucci1989) and, in some instances, beyond a millennium (Shen-Miller et al., Reference Shen-Miller, Mudgett, Schopf, Clarke and Berger1995; Sallon et al., Reference Sallon, Solowey, Cohen, Korchinsky, Egli, Woodhatch, Simchoni and Kislev2008), or even unprotected in space (Tepfer et al., Reference Tepfer, Zalar and Leach2012). A continuing, fascinating quandary is how this anhydrobiosis, leading to such resilience, is possible, prompting attempts to gain insight into the molecular mechanisms underlying the attribute (Potts et al., Reference Potts, Slaughter, Hunneke, Garst and Helm2005; Nambara and Nonogaki, Reference Nambara and Nonogaki2012). On a practical level, understanding the components of anhydrobiosis would lead to various uses in plasma- and pharmaceutical-preservation at ambient temperatures, reductions in transportation costs, and long-term stasis of complex cell assemblages without freezing.
Anhydrobiosis and the natural protection and repair mechanism
Orthodox seeds (Roberts, Reference Roberts1973) must weather a host of detrimental events when becoming, or while, dehydrated allowing the vigour of the seed, and the seedlings established from them, to remain uncompromised (Fig. 1). The natural protection and repair mechanism (Fernandez-Marin et al., Reference Fernandez-Marin, Kranner, San Sebastian, Artetxe, Laza, Vilas, Pritchard, Nadajaran, Miguez, Becerril and Garcia-Plazaola2013) is a vital but understudied facet potentiating desiccation tolerance and is linked to seed/seedling vigour by preventing or counteracting damage sustained while entering or during anhydrobiosis. It is, consequently, the foundation of crop field performance (Li et al., Reference Li, Zhang, Wang, Liu, Dirk, Goodman, Downie, Wang, Wang and Zhao2017b). The fundamental importance of the orthodox seed as the cornerstone of agriculture (Li and Pritchard, Reference Li and Pritchard2009) is in stark contrast to our lack of a basic understanding of the molecular mechanism of action for many presumptive protective molecules in shielding the cellular milieu from dehydration stress (Battaglia et al., Reference Battaglia, Olvera-Carrillo, Garciarrubio, Campos and Covarrubias2008; ElSayed et al., Reference ElSayed, Rafudeen and Golldack2014; Kester et al., Reference Kester, Geneve and Houtz1997; Van den Ende, Reference Van den Ende2013).
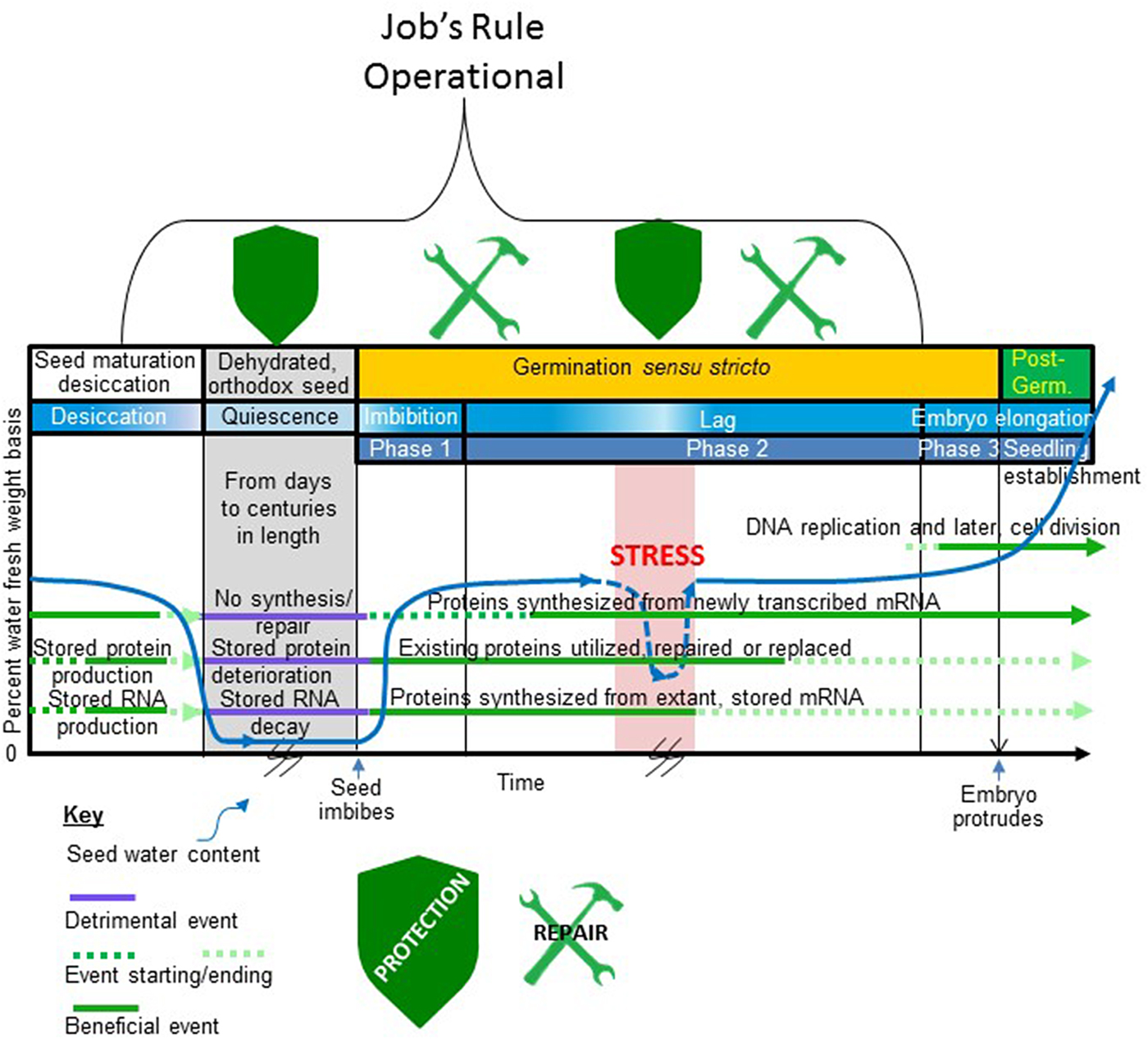
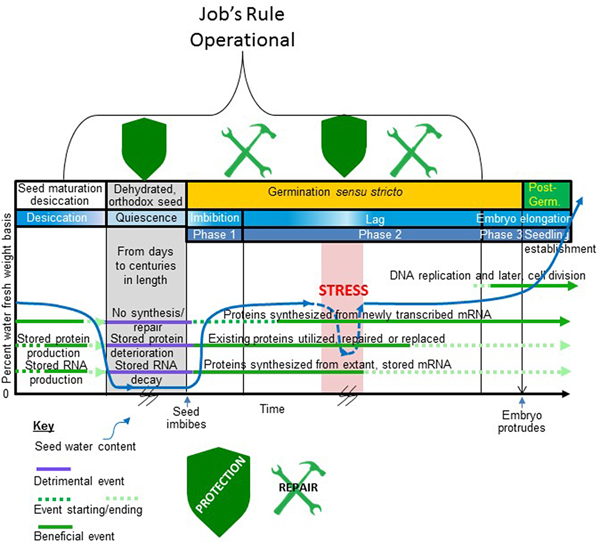
Figure 1. When in the seed life cycle Job's rule is operational. Depicted is that part of an orthodox seed's existence from late embryogenesis to the completion of germination and major events in this period. Time is on the x-axis and seed water content (per cent fresh weight basis) is on the y-axis. A typical profile of water loss during seed maturation desiccation, low water content during quiescence, rehydration during imbibition (Phase 1), a lag period (Phase 2) of stable seed water content, and a period of water uptake prior to the protrusion of the embryo from the covers (Phase 3) is shown. A stressful event occurs during the lag phase that drastically reduces the water content of the germinating seed before water stress is alleviated and the seed rehydrates. This stressful period is also coloured red to indicate that the stress may involve supra- or sub-optimal temperature. The time axis is broken during quiescence and during the stressful dehydration during germination to indicate uncertainty in the duration of these events. The period of germination is indicated on the figure bracketed by seed imbibition and embryo protrusion from the covers as the start and end point of this process, respectively, indicated on the x-axis. Horizontal lines running through the figure depict periods when (1) proteins are synthesized from de novo transcribed mRNA; (2) proteins that are stored in the seed for use following imbibition are produced and used (this group includes but is not limited to the storage proteins); (3) the stored transcriptome (structural and mRNA) is produced and used; and (4) DNA replication and cell division commence. A key describing how these various periods (1–4) are influenced by seed water content is provided beneath the figure. The stylized shields located at the top of the timeline indicate periods when protective mechanisms are synthesized/utilized to maintain the proteome functional following imbibition/rehydration. The tools at the top of the timeline indicate when protein repair processes are likely to be active in recovering the activity of some of the stored proteome following imbibition/rehydration. The bracket outlines the period when Job's rule is functioning. Note that the natural protection and repair system is dynamic as stress during germination re-invokes the protective mechanisms (shield reappears) while repair mechanisms remain functional after rehydration.
Important for anhydrobiosis and the focus of research activities worldwide (Berjak, Reference Berjak2006), protective mechanisms include reduction of reactive molecular species (Bailly, Reference Bailly2004) particularly in the prevention of lipid peroxidation (Debeaujon et al., Reference Debeaujon, Leon-Kloosterziel and Koornneef2000; Sattler et al., Reference Sattler, Gilliland, Magallanes-Lundback, Pollard and DellaPenna2004) and vitrification of the cytoplasm upon water removal (Buitink et al., Reference Buitink, Claessens, Hemminga and Hoekstra1998, Reference Buitink, Hemminga and Hoekstra2000; Sun and Leopold, Reference Sun and Leopold1997; Wolkers et al., Reference Wolkers, Alberda, Koornneef, Leon-Kloosterziel and Hoekstra1998a,Reference Wolkers, Oldenhof, Alberda and Hoekstrab). The cytoplasmic phase transitions from liquid-to-viscous-to-glass, are thought to increasingly impede deleterious biochemical reactions while progressively dampening respiration (Leprince et al., Reference Leprince, Harren, Buitink, Alberda and Hoekstra2000). Those cellular components, dependent on water to maintain their structure/function, are thought to be protected using so-called ‘water replacement’ by specific, non-reducing oligosaccharides (Crowe et al., Reference Crowe, Carpenter and Crowe1998; Clerkx et al., Reference Clerkx, El-Lithy, Vierling, Ruys, Blankestijn-De Vries, Groot, Vreugdenhil and Koornneef2004; Li et al., Reference Li, Zhang, Wang, Liu, Dirk, Goodman, Downie, Wang, Wang and Zhao2017b). It is thought that, in conjunction with highly hydrophilic proteins, these oligosaccharides can also enhance the quality and persistence of the glassy state (Buitink and Leprince, Reference Buitink and Leprince2004; Wolkers et al., Reference Wolkers, McCready, Brandt, Lindsey and Hoekstra2001). The alteration of the soluble carbohydrate profile present in the fully hydrated, active cell which contains reducing sugars, to a profile in the desiccating cell where non-reducing disaccharides (e.g. sucrose) predominate has also long been interpreted to be a protective mechanism preventing Maillard- (Maillard, Reference Maillard1912; Hodge, Reference Hodge1953) and subsequent Amadori-reactions (Isbell and Frush, Reference Isbell and Frush1958), in addition to the capacity of oligosaccharides to form a glass. Additional protection is thought to be mediated by intrinsically disordered proteins, abundant late during embryogenesis, through the prevention of aggregation of cellular constituents as water is withdrawn and the distance between macromolecules diminishes (Goyal et al., Reference Goyal, Walton and Tunnacliffe2005; Boucher et al., Reference Boucher, Buitink, Lin, Boudet, Hoekstra, Hundertmark, Renard and Leprince2010; Chakrabortee et al., Reference Chakrabortee, Tripathi, Watson, Schierle, Kurniawan, Kaminski, Wise and Tunnacliffe2012).
Some cellular constituents suffering damage while dehydrated can be repaired upon rehydration. These repairs include recognition, excision and ligation of damaged bases through apurinic or apyrimidinic intermediates (Dendoy et al., Reference Dendoy, Schyns, Deltour and Verly1987) and ligation of outright DNA strand breaks (Huang et al., Reference Huang, Boubriak, Osborne, Dong and Gutterman2008; Waterworth et al., Reference Waterworth, Masnavi, Bhardwaj, Jiang, Bray and West2010). Many proteins partially damaged or denatured during desiccation or upon rehydration, can be retrieved from aggregation (Boucher et al., Reference Boucher, Buitink, Lin, Boudet, Hoekstra, Hundertmark, Renard and Leprince2010), refolded (Tonsor et al., Reference Tonsor, Scott, Boumaza, Liss, Brodsky and Vierling2008), and repaired (Grimaud et al., Reference Grimaud, Ezraty, Mitchell, Lafitte, Briand, Derrick and Barras2001; Holmgren et al., Reference Holmgren, Johansson, Berndt, Lonn, Hudemann and Lillig2005; Smyczynski et al., Reference Smyczynski, Roudier, Gissot, Vaillant, Grandjean, Morin, Masson, Bellec, Geelen and Faure2006; Oge et al., Reference Oge, Bourdais, Bove, Collet, Godin, Granier, Boutin, Job, Jullien and Grappin2008; Nayak et al., Reference Nayak, Putnam, Addepalli, Lowenson, Chen, Jankowsky, Perry, Dinkins, Limbach, Clarke and Downie2013; Verma et al., Reference Verma, Kaur, Petla, Rao, Saxena and Majee2013).
Ten years ago, while comparing proteomic alterations in Arabidopsis thaliana seeds during natural and artificial ageing (controlled deterioration), Rajjou et al. (Reference Rajjou, Lovigny, Groot, Belghazi, Job and Job2008) postulated that the proteins involved in translation, although desiccated, must be maintained during quiescence in a functional state for the maintenance of seed vigour. They reasoned that if sufficient numbers of seed cells lose translational competence, neither the stored – nor de novo – synthesized transcriptome (Nakabayashi et al., Reference Nakabayashi, Okamoto, Koshiba, Kamiya and Nambara2005) can rescue translational capability and the seed will die (Fig. 2). This postulate (named Job's rule in honour of Dominique Job from whose laboratory this concept arose) has been supported in intervening years when proteins involved in translation have been shown to be among the preferred client proteins of presumptive desiccation protective mechanisms as well as particular targets of protein repair processes. This paper will present the existing documentation for Job's rule acquired by examining the proteins identified as preferred client proteins for protection and repair mechanisms.
Job's rule: The proteins critical for translation must endure desiccation, quiescence, and rehydration in a functional state if the seed is to survive to complete germination.
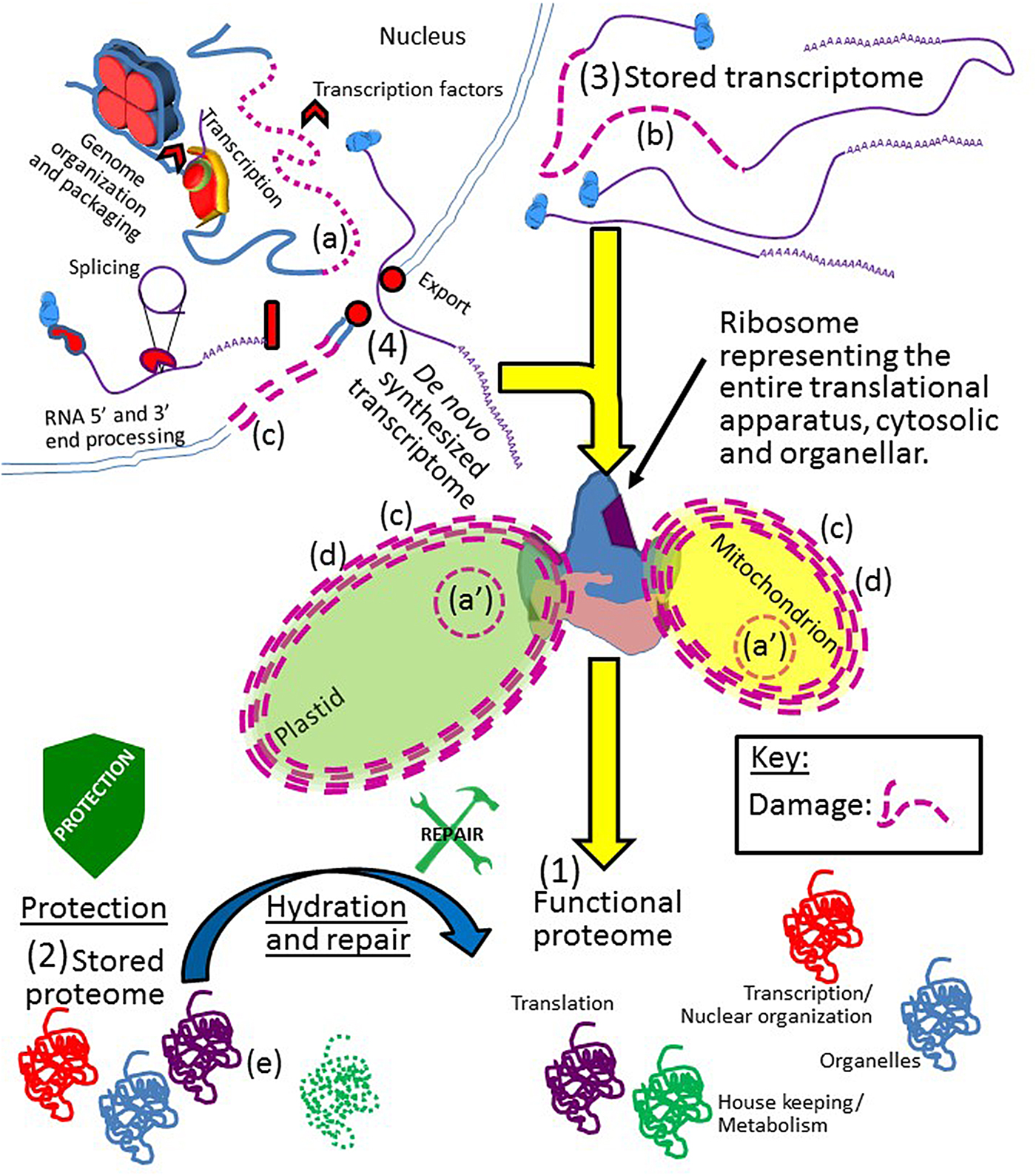
Figure 2. A depiction of Job's rule. All cellular components of the orthodox seed must be protected from, and/or repaired after, damage inflicted upon them throughout drying, while desiccated, and during rehydration. The integrity of the genomic DNA [nuclear (a) or organellar (a′)]; stored transcriptome (b); cell membrane system (c); organelles (d); and stored proteome (e) must all be protected/repaired/replaced to produce a (1) functional proteome. With regards to functional proteins, all proteins involved in all cellular processes, if they are not present in the (2) stored proteome or, if present, cannot recover a functional state after hydration and repair, may still be synthesized from the (3) stored or (4) de novo-synthesized transcriptome. However, of all the proteins in a seed, the function of those proteins crucial to translation cannot fall below a threshold of functionality because replacement of these proteins is impossible from either transcriptome if translational capacity is lost. Note that although only the plastid, mitochondria and nucleus are depicted, ‘organelle’ above encompasses all such bodies and compartments within the cell. Due to the fact that each mitochondrion and plastid in the cell has a genome, and the organelle is capable of fission, these two genomes are more redundantly backed up than the nuclear genome and might be considered less ‘at risk’ for permanent integrity loss. Key: damage to any cellular component is indicated by the use of dashed lines and/or the colour purple. Stylized shields and tools are as in Fig. 1.
Death by other means
Maintaining the translational apparatus functional is not a panacea preventing seed death. The variety of cellular components that must be protected from extensive dysfunction so that organisms might survive desiccation are considerable and the list of issues impacting survival of desiccation continues to grow as our sophistication concerning the variety of tissues, organelles and molecules involved in longevity increases (Cheah and Osborne, Reference Cheah and Osborne1978; Oliver et al., Reference Oliver, Velten and Mishler2005; Potts et al., Reference Potts, Slaughter, Hunneke, Garst and Helm2005; Berjak, Reference Berjak2006; Rebecchi et al., Reference Rebecchi, Altiero and Guidetti2007; Moore et al., Reference Moore, Le, Brandt, Driouich and Farrant2009; Sano et al., Reference Sano, Rajjou, North, Debeaujon, Marion-Poll and Seo2016; Costa et al., Reference Costa, Cooper, Hilhorst and Farrant2017; Pereira Lima et al., Reference Pereira Lima, Buitink, Lalanne, Rossi, Pelletier, da Silva and Leprince2017). An example of a recent overturn of a previously widely held tenet is that de novo transcription appears to be crucial for the completion of seed germination, at least in Arabidopsis (Fig. 1). Using Cordycephin, Bai et al. (Reference Bai, Novak, Ljung, Hanson and Bentsink2018) determined that blocking transcription reduced the completion of seed germination to near zero. At least in Arabidopsis, the capacity for de novo transcription, divorced from the maintenance of an intact genome (i.e. DNA integrity in these studies was unaffected and therefore, not the cause of poor transcription), must be safeguarded to maintain seed longevity. The integrity of the genome in the dehydrated state is critical to seed longevity (Waterworth et al., Reference Waterworth, Bray and West2015) as is the integrity of the stored transcriptome (Fleming et al., Reference Fleming, Richards and Walters2017) (Fig. 1). The protection of membranes from peroxidation both drastically and positively impacts seed viability (Sattler et al., Reference Sattler, Gilliland, Magallanes-Lundback, Pollard and DellaPenna2004; Mene-Saffrane et al., Reference Mene-Saffrane, Jones and DellaPenna2010; Xu et al., Reference Xu, Wei, Zhu, Lian, Xie, Cai, Chen, Lin, Wang, Xie and Zhang2015). Even the proper size and integrity of oilbodies is crucial for the survival of desiccated soybean cotyledons (Schmidt and Herman, Reference Schmidt and Herman2008).
The above pathways, the dysfunction of which can lead to seed death, places the fate of the stored proteome, and its influence on seed longevity (the focus of Job's rule), in context. The proteome is only one subset of a larger assemblage of molecular entities that must retain biological function if the seed is to survive. Among the proteins comprising the stored proteome, those directly and critically involved in translation occupy a special status and must be protected from, or repaired after, dysfunction if the seed is to survive.
What proteins encompass those ‘critical for translation’?
It is imperative to realize that the focus of Job's rule on ‘proteins critical for translation’ includes all proteins that are indispensable for production of active proteins from mRNA. But what are these? An attempt at defining a Minimal Protein Synthesis Machinery (MPSM) has been undertaken in some prokaryotes (Grosjean et al., Reference Grosjean, Breton, Sirand-Pugnet, Tardy, Thiaucourt, Citti, Barre, Yoshizawa, Fourmy, de Crecy-Lagard and Blanchard2014). Pared down, this assemblage still incorporates 104 core proteins with an additional 25 persistent proteins in seven different protein categories: (1) ribosomal proteins; (2) rRNA modification; (3) ribosome assembly and protein maturation; (4) RNA processing; (5) tRNA modification; (6) tRNA aminoacylation; and (7) translation factors (Grosjean et al., Reference Grosjean, Breton, Sirand-Pugnet, Tardy, Thiaucourt, Citti, Barre, Yoshizawa, Fourmy, de Crecy-Lagard and Blanchard2014). In eukaryotes this list may be expanded to include an eighth category of proteins responsible for transporting nuclear encoded proteins critical for translation into the organelles. Using this list as a guide, we sought evidence that proteins in these categories were inherently resilient or preferential client proteins of the natural protection and repair mechanism to provide support for Job's rule. Where no evidence has been published for a particular category, we hope that this review will spur future research efforts in this direction. Rather than attempt to list evidence under these eight categories, we have found it to be less repetitive to mention the Category when a report has been published pertaining to its resilience, protection or repair.
Proteins involved in translation that are not associated with Job's rule include those that peripherally influence or modulate translation but are not critical for it. These may include the proteins of the TOR protein complex (e.g. TARGET OF RAPAMYCIN; TOR and conserved TOR associated proteins; REGULATORY ASSOCIATED PROTEIN OF TOR 1B (RAPTOR1B) and LETHAL WITH SEC-THIRTEEN PROTEIN 8 (LST8)) the complex of which is known to influence the efficiency of translation but is not critical for it (Kravchenko et al., Reference Kravchenko, Citerne, Jehanno, Bersimbaev, Veit, Meyer and Leprince2015). The WD40-repeat protein GIGANTUS1 (GTS1) is also capable of altering ribosomal morphology, is co-expressed with several r-proteins, and is predicted to interact with at least two r-proteins, potentially influencing translation, but GTS1 is deemed non-essential for translation (Gachomo et al., Reference Gachomo, Jimenez-Lopez, Baptiste and Kotchoni2014). One can imagine that plasmodesmatal proteins, crucial to protein trafficking throughout discrete, symplastically continuous cell assemblages in the mature embryo (Kim et al., Reference Kim, Hempel, Sha, Pfluger and Zambryski2002; Stadler et al., Reference Stadler, Lauterbach and Sauer2005), may revitalize cells in which translational competence has declined below a critical threshold that the surrounding cells can complement by sharing translational components, predicated on mechanisms to facilitate passage through the size exclusion limit of their plasmodesmata. These plasmodesmatal proteins are, however, not directly involved in translation and are, therefore, excluded from Job's rule.
Support for Job's rule
The proteins of the translational apparatus possess a remarkable resiliency
In a survey of protein half-lives in barley leaves, Nelson et al. (Reference Nelson, Alexova, Jacoby and Millar2014) found those proteins associated with the translational apparatus (no specific Category) to be particularly long-lived, suggesting that these proteins might somehow be inherently resilient or that they are a class of the proteome to which partiality is shown by the protective and/or repair mechanisms of the cell. Indeed, the ribosomal proteins (r-proteins; Category 1) from diverse species, once assembled into ribosomes, are particularly long-lived, at least in the cytoplasm (Boisvert et al., Reference Boisvert, Ahmad, Gierlinski, Charriere, Lamont, Scott, Barton and Lamond2012; Christiano et al., Reference Christiano, Nagaraj, Frohlich and Walther2014; Li et al., Reference Li, Nelson, Trosch, Castleden, Huang and Millar2017a).
The r-proteins of the large- and small-ribosomal subunits are intimately involved in translation. Some insight into the long-lived nature of some of the r-proteins has been linked to their high positive net charge at physiological pH, with some species’ large subunit r-proteins all possessing a net charge at physiological pH greater than 7 (Fedyukina et al., Reference Fedyukina, Jennaro and Cavagnero2014). This has relevance for protein and ribosomal stability because Lawrence et al. (Reference Lawrence, Phillips and Liu2007) demonstrated a surprising capacity of protein variants, engineered to impart to them either a positive or a negative ‘supercharge’, to re-gain structure and function following stressful events (in this case supra-optimal temperatures). They demonstrated that supercharging prevents protein aggregation upon denaturation and yet does not dramatically inhibit subsequent refolding into an active protein. Inherent r-protein positive supercharging is not strictly due to functional demands on proteins that must associate with negatively charged r-, t- and mRNA. Intriguingly, Fedyukina et al. (Reference Fedyukina, Jennaro and Cavagnero2014) have recorded a surprising plasticity in the net charge of r-proteins of the large subunit with those from halophiles considerably less positively charged than those from non-halophilic organisms. Upon further investigation, it was evident that r-proteins of the large subunit are generally segregated into positively and negatively charged protein moieties, a characteristic that becomes more pronounced in the halophile. The halophile negatively charged amino acids were found to be placed in solvent exposed positions, potentially better competing with salt for water for protein hydration while the positively charged portions of the protein tended to be buried within the protein-ribosomal RNA core (Fedyukina et al., Reference Fedyukina, Jennaro and Cavagnero2014). In keeping with Job's rule, such durability is a useful attribute if the proteins comprising the translational apparatus must remain in an active form throughout quiescence and are of such vital importance to the longevity of seeds following imbibition.
In some organisms, including plants, ribosomal protein (r-protein) families have numerous paralogous members (Barakat et al., Reference Barakat, Szick-Miranda, Chang, Guyot, Blanc, Cooke, Delseny and Bailey-Serres2001; Carroll et al., Reference Carroll, Heazlewood, Ito and Millar2008; Carroll, Reference Carroll2013; Hummel et al., Reference Hummel, Dobrenel, Cordewener, Davanture, Meyer, Smeekens, Bailey-Serres, America and Hanson2015). One hypothesis concerning this r-protein diversity is that the r-protein paralogues can impart to the translational machinery selectivity regarding which mRNAs are preferentially translated (Hummel et al., Reference Hummel, Dobrenel, Cordewener, Davanture, Meyer, Smeekens, Bailey-Serres, America and Hanson2015), constituting a so-called ‘ribosome filter’ (Mauro and Edelman, Reference Mauro and Edelman2002). Indeed, such are the subtleties of r-protein paralogue alterations to the ribosome, influencing the preference of the ribosome for translating specific mRNAs, that, in yeast, paralogues of the LARGE SUBUNIT RIBOSOMAL PROTEIN1 (RPL1; a or b), although identical in amino acid sequence, somehow dramatically bias the mRNA species translated by the ribosomes containing one or the other RPL1 paralogue (Segev and Gerst, Reference Segev and Gerst2018). Despite this redundancy, many genes encoding r-proteins belonging to cytoplasmic, mitochondrial (Tzafrir et al., Reference Tzafrir, Pena-Muralla, Dickerman, Berg, Rogers, Hutchens, Sweeney, McElver, Aux, Patton and Meinke2004; Zhang et al., Reference Zhang, Luo, Day, Talbot, Ivanova, Ashton, Chaudhury, Macknight, Hrmova and Koltunow2015; Robles and Quesada, Reference Robles and Quesada2017), as well as plastidal (Tsugeki et al., Reference Tsugeki, Kochieva and Fedoroff1996; Gong et al., Reference Gong, Jiang, Xu, Zhang, Teng, Lin and Dong2013) ribosomes are known to be lethal when mutated (Lloyd and Meinke, Reference Lloyd and Meinke2012). As our sophistication regarding translation during germination increases, the identities of r-protein paralogues, and their post-translational modifications (Carroll, Reference Carroll2013; Sanchez-de-Jimenez et al., Reference Sanchez-de-Jimenez, Aguilar and Dinkova1997), that are crucial to selective translation at various stages of germination, may be revealed to be absolutely required for the recovery from quiescence and the completion of seed germination (Galland et al., Reference Galland, Huguet, Arc, Cueff, Job and Rajjou2014; Basbouss-Serhal et al., Reference Basbouss-Serhal, Soubigou-Taconnat, Bailly and Leymarie2015; Galland and Rajjou, Reference Galland and Rajjou2015; Bai et al., Reference Bai, Peviani, van der Horst, Gamm, Snel, Bentsink and Hanson2017). In this vein, it should be noted that some of the first transcripts to be translated following imbibition, are those in the stored transcriptome encoding ribosomal proteins (Beltran-Pena et al., Reference Beltran-Pena, Ortiz-Lopez and Sanchez de Jimenez1995; Tatematsu et al., Reference Tatematsu, Kamiya and Nambara2008; Weitbrecht et al., Reference Weitbrecht, Muller and Leubner-Metzger2011) prioritizing the replacement of critical translational capacity with what might be the last functional translation of which the rehydrated, aged ribosome is capable! This is entirely consistent with the tenets of Job's rule.
The r-proteins are only one of seven different categories listed as crucial for translation (Grosjean et al., Reference Grosjean, Breton, Sirand-Pugnet, Tardy, Thiaucourt, Citti, Barre, Yoshizawa, Fourmy, de Crecy-Lagard and Blanchard2014). In studies of aged seeds, certain members of Categories 3 and 7, (translation factors; initiation and elongation factors, chaperonins) have been documented to decline in abundance as ageing progresses (Min et al., Reference Min, Lee, Cheon, Han, Ko, Kang, Kim, Agrawal, Rakwal, Gupta and Kim2017; Wang et al., Reference Wang, Ma, Song, Shu and Gu2012). The distinction to be made here is that the studies indicating that Category 3 and 7 proteins declined in abundance were conducted using hydrated seeds exposed to high temperatures (accelerated ageing conditions) which may or may not reflect the dynamics of protein destabilization under natural ageing conditions (Schwember and Bradford, Reference Schwember and Bradford2010).
Protection: Proteins involved in translation may be protected from oxidation in the quiescent seed
Oxidation is a stress assailing the components of cells of the seed to which lipids, nucleic acids and proteins are all susceptible (El-Maarouf-Bouteau et al., Reference El-Maarouf-Bouteau, Meimoun, Job, Job and Bailly2013). The reactive oxygen species (ROS) are also potent signalling molecules. Hence the hydrated cell must permit sufficient ROS generation to fulfil relevant signalling while dampening ROS quantities below a threshold where cellular constituents are damaged by them (Bailly et al., Reference Bailly, El-Maarouf-Bouteau and Corbineau2008) and the hydrated cell employs a plethora of mechanisms to establish and maintain redox balance (Apel and Hirt, Reference Apel and Hirt2004). The sulfur-containing amino acids (AAs; cysteine and methionine) are prone to oxidation (Levine et al., Reference Levine, Moskovitz and Stadtman2000). Both of these oxidations, proceeding no further than cysteine sulfenic acid or methionine sulfoxide, respectively, are repairable by enzymatic reduction (Tarrago et al., Reference Tarrago, Laugier and Rey2009; Meyer et al., Reference Meyer, Belin, Delorme-Hinoux, Reichheld and Riondet2012; Akter et al., Reference Akter, Huang, Waszczak, Jacques, Gevaert, Van Breusegem and Messens2015; Waszczak et al., Reference Waszczak, Akter, Jacques, Huang, Messens and Van Breusegem2015) and even cysteine sulfinic acid is reversible in some cellular compartments (Rey et al., Reference Rey, Becuwe, Barrault, Rumeau, Havaux, Biteau and Toledano2007). Other AAs (proline, histidine, lysine, arginine, tyrosine and tryptophan) are also subject to oxidation but, to date, no reductive repair mechanism for these has been identified (Rinalducci et al., Reference Rinalducci, Murgiano and Zolla2008; Sweetlove and Moller, Reference Sweetlove and Moller2009). Some proteins in cell-free extracts from desiccated seeds appear to be preferentially oxidized; this damage occurring to these specific proteins at frequencies far surpasses their proportionate abundance in the cell (Oracz et al., Reference Oracz, El-Maarouf Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007; El-Maarouf-Bouteau et al., Reference El-Maarouf-Bouteau, Meimoun, Job, Job and Bailly2013). This oxidation is presumed to have occurred during the sojourn of the seed in the dehydrated state (Gao et al., Reference Gao, Rampitsch, Chitnis, Humphreys, Jordan and Ayele2013) although it is impossible to emphatically state that the oxidation does not take place immediately upon addition of aqueous buffer used to extract the proteins from the desiccated seed, an ubiquitous caveat dogging most assays of dehydrated tissues. Nevertheless, in support of Job's rule, of those proteins identified as preferentially oxidized from desiccated seed, only one is involved in translation (eukaryotic ELONGATION FACTOR2 [eEF2]; Category 7; Job et al., Reference Job, Rajjou, Lovigny, Belghazi and Job2005; Oracz et al., Reference Oracz, El-Maarouf Bouteau, Farrant, Cooper, Belghazi, Job, Job, Corbineau and Bailly2007) and its oxidation in dry seeds is associated with dormancy alleviation in Helianthus annuus L. (sunflower), stimulating the completion of germination upon subsequent hydration, constituting a time-dependent signal eliciting a specific event (dormancy alleviation), rather than random protein damage.
In contrast, hydration of the seeds prior to protein extraction results in the oxidation of many proteins directly involved in translation (Job et al., Reference Job, Rajjou, Lovigny, Belghazi and Job2005) so these translation-associated proteins, present in the desiccated cells of the seed, are not impervious to oxidation but seem, rather, to be specifically protected from it while dehydrated. The means by which the proteins that are prone to oxidation are rendered susceptible to it remains relatively unknown, as does the means by which proteins involved in translation are seemingly protected from oxidation while desiccated. One intriguing example of the former is the use, in animal mitochondria, of AA substitution through alternative codon translation, resulting in methionine (or N-formylmethionine) residues for both codons AUG and AUA (usually isoleucine). In this instance, solvent-exposed Met residues are thought to act as oxidation decoys, sacrificing their integrity to protect other mitochondrial constituents from ROS while, due to their peripheral placement in the protein they act like a ROS sponge, minimally influencing the ROS sponge protein's structural integrity and hence, function (Bender et al., Reference Bender, Hajieva and Moosmann2008). A repair pathway capable of reducing methionine sulfoxide also exists in the cell, which can render this damage temporary (Achilli et al., Reference Achilli, Ciana and Minetti2015). The seed storage proteins (one class of seed stored proteins) which are degraded to supply energy, nitrogen and carbon for the establishing seedling, are prone to all manner of damage during quiescence. Oxidative alterations have been suggested to be evidence of a role for the abundant storage proteins (Nguyen et al., Reference Nguyen, Cueff, Hegedus, Rajjou and Bentsink2015) in seeds to soak up ROS, reducing the titre of the damaging ROS during quiescence. An alternative opinion is that the storage proteins, destined for degradation and present in a compartment bereft of some repair mechanisms, are simply unworthy of repair (Dinkins et al., Reference Dinkins, Majee, Nayak, Martin, Xu, Belcastro, Houtz, Beach and Downie2008), slated as they are for destruction. There is even a role for phytic acid (frequently associated with seed storage proteins as inclusion bodies) as an anti-oxidant contributing to the seed longevity of maize (Zea mays) seeds (Doria et al., Reference Doria, Galleschi, Calucci, Pinzino, Pilu, Cassani and Nielsen2009).
Protection: LEA protein association with proteins involved in translation
The LATE EMBRYOGENESIS ABUNDANT proteins (LEA proteins) are thought to protect intracellular membranes and proteins of seeds while drying and during their sojourn in the desiccated state (Hundertmark et al., Reference Hundertmark, Buitink, Leprince and Hincha2011). LEA proteins have been suggested to act as molecular spacers in the increasingly crowded intracellular milieu during dehydration (Goyal et al., Reference Goyal, Walton and Tunnacliffe2005) and some have been shown to possess remarkable anti-aggregation properties (Boucher et al., Reference Boucher, Buitink, Lin, Boudet, Hoekstra, Hundertmark, Renard and Leprince2010). Some of the LEA proteins have been demonstrated to exert a protective function for membranes of particular composition (Thalhammer et al., Reference Thalhammer, Hundertmark, Popova, Seckler and Hincha2010; Tolleter et al., Reference Tolleter, Hincha and Macherel2010; Eriksson et al., Reference Eriksson, Kutzer, Procek, Grobner and Harryson2011; Thalhammer et al., Reference Thalhammer, Bryant, Sulpice and Hincha2014; Eriksson et al., Reference Eriksson, Eremina, Barth, Danielsson and Harryson2016) and some to stabilize sugar glasses (Wolkers et al., Reference Wolkers, McCready, Brandt, Lindsey and Hoekstra2001; Shimizu et al., Reference Shimizu, Kanamori, Furuki, Kikawada, Okuda, Takahashi, Mihara and Sakurai2010). Certainly the cosmopolitan distribution of usually two or more members of the LEA proteins within compartments of the plant cell (Candat et al., Reference Candat, Paszkiewicz, Neveu, Gautier, Logan, Avelange-Macherel and Macherel2014) augurs well for a general, shielding mechanism, redundantly backed up (Chakrabortee et al., Reference Chakrabortee, Tripathi, Watson, Schierle, Kurniawan, Kaminski, Wise and Tunnacliffe2012). Recently, this view is being altered due to the recovery of a diversity of specific phenotypes upon mutation of a single LEA (Manfre et al., Reference Manfre, Lanni and Marcotte2006; Chen et al., Reference Chen, Nayak, Majee, Lowenson, Schafermeyer, Eliopoulos, Lloyd, Dinkins, Perry, Forsthoefel, Clarke, Vernon, Zhou, Rejtar and Downie2010; Olvera-Carrillo et al., Reference Olvera-Carrillo, Campos, Reyes, Garciarrubio and Covarrubias2010; Salleh et al., Reference Salleh, Evans, Goodall, Machin, Mowla, Mur, Runions, Theodoulou, Foyer and Rogers2012), scarcely possible if LEAs all acted as general shield molecules with multiple members present in cellular compartments. While demonstrations of certain of the LEA proteins safeguarding, to some degree, the function of commercial enzyme preparations abound (e.g. Hara et al., Reference Hara, Fujinaga and Kuboi2004; Reyes et al., Reference Reyes, Rodrigo, Colmenero-Flores, Gil, Garay-Arroyo, Campos, Salamini, Bartels and Covarrubias2005; Kovacs et al., Reference Kovacs, Kalmar, Torok and Tompa2008; Liu et al., Reference Liu, Zheng, Zhang, Wang and Li2010; Zhang et al., Reference Zhang, Lu, Jiang, Wang, Lv, Shen and Ming2014), efforts to identify particular endogenous proteins that are bound by explicit, conspecific LEA proteins have also been successful (Xie et al., Reference Xie, Zhang, Qu, Miao, Zhang, Shen, Wang and Dong2012; Rivera-Najera et al., Reference Rivera-Najera, Saab-Rincon, Battaglia, Amero, Pulido, Garcia-Hernandez, Solorzano, Reyes and Covarrubias2014; Zhang et al., Reference Zhang, Lu, Jiang, Wang, Lv, Shen and Ming2014; Hernandez-Sanchez et al., Reference Hernandez-Sanchez, Maruri-Lopez, Graether and Jimenez-Bremont2017). Consistent with the tenets of Job's rule, preferred client proteins bound by a SEED MATURATION PROTEIN (SMP) family Arabidopsis LEA protein, and by its soybean orthologue, belonged to Categories 1, 4 and 7 of translation (Kushwaha et al., Reference Kushwaha, Lloyd, Schafermeyer, Kumar and Downie2012) providing evidence that at least some of the LEA proteins can bind (and presumably protect) proteins of the translational apparatus (Kushwaha et al., Reference Kushwaha, Downie and Payne2013). Loss of function of the Arabidopsis SMP LEA, while not lethal to the seeds, does remove the capacity of smp1 seeds to enter thermodormancy in response to thermal insult applied during germination (Chen et al., Reference Chen, Nayak, Majee, Lowenson, Schafermeyer, Eliopoulos, Lloyd, Dinkins, Perry, Forsthoefel, Clarke, Vernon, Zhou, Rejtar and Downie2010), seemingly negating the seed hydration memory of the stress (Fig. 1).
The involvement of the LEA protein SMP1 in binding (presumably protecting) proteins involved in translation may be used as a paradigm to suggest that certain LEA proteins may physically shelter oxidation-sensitive AAs of their client proteins from this modification in the quiescent seed. Certainly the region of the client proteins to which both Arabidopsis and soybean orthologues of SMP1 bound was consistently the most, or among the most, hydrophyllic of the client proteins (Kushwaha et al., Reference Kushwaha, Downie and Payne2013), suggesting that these regions are solvent exposed and therefore would be the most susceptible to oxidative damage. Such physical protection from oxidation has been demonstrated for the subunit proteins comprising the 12S cruciferin storage proteins where the β-subunits, thought to be buried within the α-subunits, are 6-fold less oxidized in the desiccated seed (Job et al., Reference Job, Rajjou, Lovigny, Belghazi and Job2005). Following imbibition, at least some LEA proteins, when hydrated, would lose any structural features helping to induce a fit with their client proteins, leaving their client proteins more susceptible to oxidation, which was the case for proteins involved in translation upon seed hydration (Job et al., Reference Job, Rajjou, Lovigny, Belghazi and Job2005).
Using a Tandem Affinity Purification approach repeated four times (twice in the light and twice in the dark) Shaw (Reference Shaw2016) recovered and identified a group of client proteins binding to a TAP-LEA5 protein (At4g02480, which is LEA38 of the LEA3 family; Hundertmark and Hincha, Reference Hundertmark and Hincha2008). Of the five client proteins recovered two or more times using this approach, two were involved in translation and these were recovered most consistently from the screen. The Arabidopsis DEAD-Box RNA Helicase 22 (RH22) (Category 3) was recovered each time the assay was performed. RH22 is required for proper maturation and assembly of plastidial ribosomal RNA crucial for translation in the organelle (Chi et al., Reference Chi, He, Mao, Li, Ma, Ji, Zou and Zhang2012). The next most consistently recovered client protein (three out of four times) was PUMILIO24, an RNA binding protein of the PUF family (Tam et al., Reference Tam, Barrette-Ng, Simon, Tam, Ang and Muench2010), also involved in ribosomal RNA maturation (Category 3) and assembly in the nucleolus (Shanmugam et al., Reference Shanmugam, Abbasi, Kim, Kim, Park, Park, Oh, Park, Muench, Choi, Park and Choi2017; Maekawa et al., Reference Maekawa, Ishida and Yanagisawa2018). Although these efforts to identify legitimate, conspecific LEA protein client proteins have indicated, for two different families of LEAs, that proteins involved in translation are preferred targets, many LEA proteins are known to bind membranes rather than proteins (Thalhammer et al., Reference Thalhammer, Hundertmark, Popova, Seckler and Hincha2010; Tolleter et al., Reference Tolleter, Hincha and Macherel2010; Eriksson et al., Reference Eriksson, Kutzer, Procek, Grobner and Harryson2011). Additionally, for those LEA proteins that do bind proteins, it is still unclear whether proteins involved in translation are an ubiquitous set of client proteins or if the two LEA proteins investigated thus far coincidentally had proteins involved in translation as their targets. Regardless, what is clear is that some proteins of the translational apparatus are preferred client proteins bound (potentially protected) by some members of the LEA family, entirely consistent with Job's rule.
Protection: Difficulty assigning protection of protein associated with translation to altered soluble carbohydrate profiles
The alteration of the sugar profile during dehydration of tissues is a well-documented occurrence in pollen, seeds and resurrection plants (Griffiths et al., Reference Griffiths, Gaff and Neale2014). One of the attributes of increasing sugar concentration concomitant with water loss is the transition to an amorphous state in the cytoplasm, and presumably, the organelles in which the cellular constituents are embedded. The increase in viscosity is sufficient to greatly impede diffusion rates while dampening metabolism to such an extent that it is difficult to measure. Carbohydrate-mediated protection is thought to be a general phenomenon (ElSayed et al., Reference ElSayed, Rafudeen and Golldack2014) encompassing both membranes and proteins that, although not preferentially focused on proteins (let alone proteins involved in translation), by protecting proteins promiscuously, protects those of the translational apparatus as well (Buera et al., Reference Buera, Schebor and Elizalde2004).
There are some cryptic correlations between the abundance of sugars in stressed cells and desiccation tolerance. One of the relationships that has been gaining attention is the importance of the ratio between the raffinose family oligosaccharides (RFO) and sucrose (first identified by Chen and Burris, Reference Chen and Burris1990) in the acquisition of desiccation tolerance (Pereira Lima et al., Reference Pereira Lima, Buitink, Lalanne, Rossi, Pelletier, da Silva and Leprince2017) and/or enhancement of seed longevity (Li et al., Reference Li, Zhang, Wang, Liu, Dirk, Goodman, Downie, Wang, Wang and Zhao2017b). In vitro studies suggest that the mixture of the sugars may not benefit protein longevity or desiccation tolerance, potentially diverting research efforts to examine how the sugar mixture may influence membrane stabilization and/or the propensity of the mixture to remain amorphous (Davidson and Sun, Reference Davidson and Sun2001). However, it is still possible that a favourable RFO/sucrose ratio may protect proteins in vivo due to the complexity of sugar metabolism in the cell being beyond the capacity of in vitro studies to unveil. For instance, trehalose abundance in stressed yeast cells has been linked to the propensity of these cells to reduce protein aggregation generally. Up-regulation of trehalose production has been demonstrated to result in increased heat shock protein (HSP104; a Category 3 chaparonin) abundance, and increased autophagic clearing of protein aggregates (Chaudhary et al., Reference Chaudhary, Kardani, Singh, Banerjee and Roy2014). The beneficial influence in preventing protein aggregation was demonstrated to be, in part, the dual action of trehalose and HSP104 when they were present concurrently and in a strict stoichiometry (Saleh et al., Reference Saleh, Gune, Chaudhary, Turakhiya and Roy2014) that was not entirely dependent on the up-regulation of autophagy by trehalose accumulation. This level of synergy would not be evident with in vitro studies. However, none of these features of RFO/sucrose ratios or trehalose-mediated protection (chaparonin-induction, synergistic action, or autophagy stimulation) has been categorically identified as specifically targeted to proteins of the translational apparatus.
Repair: Protein repair pathways
Certain AAs in a polypeptide are prone to damage through oxidation, isomerization and spontaneous conversion. For some of these forms of damage, cellular repair mechanisms have been identified in most forms of life, testifying to both the ubiquity of the agents of damage (e.g. oxidation; Gracy et al., Reference Gracy, Talent, Kong and Conrad1999) as well as to the importance of rectifying damage, when possible, to maintain a functional proteome (Clarke, Reference Clarke2003). Following the discovery, using radio-labelling and two-dimensional gel electrophoresis (2DGE), that seed proteins involved in translation decreased in abundance 1 day after imbibition in the proteome of aged – relative to unaged – seeds (Rajjou et al., Reference Rajjou, Lovigny, Groot, Belghazi, Job and Job2008), it was noted (again using 2DGE, membrane transfer, and on-blot methylation) that there were certain proteins acting as preferred substrates of the protein repair enzyme PROTEIN ISOASPARTYL METHYLTRANSFERASE (PIMT) (Dinkins et al., Reference Dinkins, Majee, Nayak, Martin, Xu, Belcastro, Houtz, Beach and Downie2008).
Repair: PROTEIN ISOASPARTYL METHYLTRANSFERASE (PIMT)
Isoaspartate (IsoAsp) formation is a common form of damage incurred under physiological conditions (Geiger and Clarke, Reference Geiger and Clarke1987) and its non-enzymatic production (or at least the production of its immediate succinimidyl precursor) is heightened under stressful conditions (Mudgett and Clarke, Reference Mudgett and Clarke1994) and in desiccated, aged tissues (Mudgett et al., Reference Mudgett, Lowenson and Clarke1997). There are numerous studies demonstrating increased seed longevity due to up-regulation of PIMT or a reduction in this seed attribute upon pimt dysfunction (Oge et al., Reference Oge, Bourdais, Bove, Collet, Godin, Granier, Boutin, Job, Jullien and Grappin2008; Verma et al., Reference Verma, Kaur, Petla, Rao, Saxena and Majee2013; Wei et al., Reference Wei, Xu, Diao, Zhu, Xie, Cai, Wu, Wang, Zhang and Xie2015). In an endeavour to identify those proteins in seeds that were particularly prone to isoAsp formation and/or preferentially repaired by the PIMT enzyme, many proteins involved in translation were identified as preferred PIMT targets (or particularly susceptible to isoAsp formation; Chen et al., Reference Chen, Nayak, Majee, Lowenson, Schafermeyer, Eliopoulos, Lloyd, Dinkins, Perry, Forsthoefel, Clarke, Vernon, Zhou, Rejtar and Downie2010). These included proteins responsible for ribosomal maturation (AT3G51270; AT5G62190; Category 3); a component of the mitochondrial inner membrane translocon (AT5G51150; Category 8); rRNA modification (AT5G55920; Category 2); and an r-protein (AT3G22230; Category 1). Moreover, this was in stark contrast with PIMT target proteins identified in organisms incapable of surviving desiccation (Reissner et al., Reference Reissner, Paranandi, Luc, Doyle, Mamula, Lowenson and Aswad2006; Zhu et al., Reference Zhu, Doyle, Mamula and Aswad2006; Dai et al., Reference Dai, Ni, Patananan, Clarke, Karger and Zhou2013). In these desiccation-sensitive organisms, with a large number of PIMT target proteins identified, there is only a single report of a protein (eIF4E-binding protein2; Category 7) intimately associated with the eukaryotic translational complex, being a target of PIMT (Bidinosti et al., Reference Bidinosti, Martineau, Frank and Sonenberg2010).
Of the enzymes involved in translation that were preferentially repaired by PIMT in screens of the seed proteome, one of those involved in the processing of the ribosomal RNAs in the nucleolus (Category 3) was examined further (Galland and Rajjou, Reference Galland and Rajjou2015; Huang et al., Reference Huang, Shen, Huang, Wu, Yeh and Lu2016a; Lorkovic et al., Reference Lorkovic, Herrmann and Oelmuller1997; Nayak et al., Reference Nayak, Putnam, Addepalli, Lowenson, Chen, Jankowsky, Perry, Dinkins, Limbach, Clarke and Downie2013). An orthologue of this Arabidopsis DEAD-box RNA helicase (PLANT RNA HELICASE75, PRH75) had been shown to be the product of one of the few transcripts preferentially up-regulated in imbibed aged seeds of mung bean (Vigna radiata) relative to unaged seeds (Li et al., Reference Li, Chung and Chen2001). This up-regulation of transcript abundance may be a reaction to a decline in active protein abundances, similar to the findings in imbibed, aged Arabidopsis seeds (Rajjou et al., Reference Rajjou, Lovigny, Groot, Belghazi, Job and Job2008) where a DEAD-box RNA helicase, involved in mRNA export (Category 4; Kammel et al., Reference Kammel, Thomaier, Sorensen, Schubert, Langst, Grasser and Grasser2013) declines in aged seeds. Further studies in Arabidopsis determined that most prh75 mutants are embryo lethal and that weakly penetrant prh75 mutations, while viable, produced abnormally shaped (corkscrewed) seeds/embryos (or those with compromised completion of germination) at high frequency (Nayak et al., Reference Nayak, Putnam, Addepalli, Lowenson, Chen, Jankowsky, Perry, Dinkins, Limbach, Clarke and Downie2013; Huang et al., Reference Huang, Sie, Chen, Huang and Lu2016b), underlining how crucial the functionality of PRH75 is during plant development, a functionality safeguarded by PIMT (Nayak et al., Reference Nayak, Putnam, Addepalli, Lowenson, Chen, Jankowsky, Perry, Dinkins, Limbach, Clarke and Downie2013). Indeed, plants possess two different genes encoding PIMT (Xu et al., Reference Xu, Belcastro, Villa, Dinkins, Clarke and Downie2004) and dysfunction of one is sufficient to severely compromise seed longevity (Oge et al., Reference Oge, Bourdais, Bove, Collet, Godin, Granier, Boutin, Job, Jullien and Grappin2008; Verma et al., Reference Verma, Kaur, Petla, Rao, Saxena and Majee2013).
Repair: METHIONINE SULFOXIDE REDUCTASE
If the proteins of the translational apparatus are preferentially repaired (at least by PIMT and Met Sulfoxide Reductase; Caldwell et al., Reference Caldwell, Luk, Weissbach and Brot1978; Chen et al., Reference Chen, Nayak, Majee, Lowenson, Schafermeyer, Eliopoulos, Lloyd, Dinkins, Perry, Forsthoefel, Clarke, Vernon, Zhou, Rejtar and Downie2010; Nayak et al., Reference Nayak, Putnam, Addepalli, Lowenson, Chen, Jankowsky, Perry, Dinkins, Limbach, Clarke and Downie2013), this may be a contributing reason why they are among the more resilient proteins in the cytoplasm (Nelson et al., Reference Nelson, Alexova, Jacoby and Millar2014). At least isoAsp is relegated as problematic to its occurrence in a peptide chain. Oxidation of Met, on the other hand, can occur in a protein as well as to free methionine, or that bound as an aminoacyl-tRNA (Chousterman and Chapeville, Reference Chousterman, Chapeville and Moldave1981). In fact, oxidative stress has been shown to result in the preferential mis-acetylation of methionine to non-methionine tRNAs (Category 6) that, coupled with an oxidation-mediated reduction in the efficiency of AMINOACYL-tRNA SYNTHETASE editing capacity, decreases the fidelity of translation (Ling and Soll, Reference Ling and Soll2010). Such ‘controlled inaccuracy’ (Lee et al., Reference Lee, Kim, Kim, Yang, Hong, Kang, Oh, Kim, Han, Hwang, Kang, Kang, Kim, Kwon and Kim2014) is mediated through an oxidative environment leading to phosphorylation of key residues in the METHIONYL-tRNA SYNTHETASE (Category 5) that render it less discriminating in the tRNAs perceived as cognate (Lee et al., Reference Lee, Kim, Kim, Yang, Hong, Kang, Oh, Kim, Han, Hwang, Kang, Kang, Kim, Kwon and Kim2014). Due to the hypothesized role of solvent-exposed methionines acting as a ROS reductant (Bender et al., Reference Bender, Hajieva and Moosmann2008), it has been postulated that this mis-priming of tRNAs to increase MET incidence in the proteome, is a cryptic means by which cells protect themselves from oxidative stress (Netzer et al., Reference Netzer, Goodenbour, David, Dittmar, Jones, Schneider, Boone, Eves, Rosner, Gibbs, Embry, Dolan, Das, Hickman, Berglund, Bennink, Yewdell and Pan2009). Moreover, free, oxidized methionine can form diastereomers (Met-S-SO and Met-R-SO) for which metazoans and higher plants have not retained the enzyme capable of efficiently reducing Met-S-SO (Le et al., Reference Le, Lee, Marino, Zhang, Fomenko, Kaya, Hacioglu, Kwak, Koc, Kim and Gladyshev2009). Met-SO will not form S-adenosyl methionine (Achilli et al., Reference Achilli, Ciana and Minetti2015), the primary methyl donor in the cell, and the means through which PIMT functions to convert isoAsp to Asp.
While these damages impact translation generally, there is at least one occurrence of an oxidized methionine in an r-protein (Category 1) that is known to inhibit translation specifically but that can be repaired to regain functionality (Caldwell et al., Reference Caldwell, Luk, Weissbach and Brot1978). The reduction of both free methionine-sulfoxide and methionine-sulfoxide present in a polypeptide context is, obviously, an important facet of Job's rule. While METHIONYL-tRNA SYNTHETASE cannot be charged with MET-SO (Category 6) (Lemoine et al., Reference Lemoine, Waller and van Rapenbusch1968), Met can be oxidized once it has formed the aminoacyl tRNAMET-SO (Category 5; Chousterman and Chapeville, Reference Chousterman, Chapeville and Moldave1981), so reduction to tRNAMET is also an important aspect of Job's rule.
Repair: Chaperonins: PEPTIDYL-PROLYL CIS-TRANS ISOMERASE
In addition to being oxidized, proline can isomerize to/from trans-/cis-isomers, important for protein folding (Wedemeyer et al., Reference Wedemeyer, Welker and Scheraga2002). Peptidyl-prolyl cis-trans isomerases (PPIases) are a class of enzyme capable of isomerizing proline between cis- and trans-isomers at the N-terminal amide bond in proteins (Wedemeyer et al., Reference Wedemeyer, Welker and Scheraga2002; Camilloni et al., Reference Camilloni, Sahakyan, Holliday, Isern, Zhang, Eisenmesser and Vendruscolo2014). Proline isomerization has been documented to influence seed vigour through mutation of specific PPIases (Category 7; Bissoli et al., Reference Bissoli, Ninoles, Fresquet, Palombieri, Bueso, Rubio, Garcia-Sanchez, Fernandez, Mulet and Serrano2012). The ribosome inserts all prolines in the trans conformation (Feige et al., Reference Feige, Hendershot and Buchner2010), requiring PPIases to subsequently convert the trans proline to cis, if necessary, to fold the nascent polypeptide. Nevertheless, in yeast, concurrent elimination of 12 cyclophylin and FK506 binding proteins (PPIase enzymes) through mutagenesis was not lethal (Dolinski et al., Reference Dolinski, Muir, Cardenas and Heitman1997), leaving the authors to propose that each PPIase has a unique set of proteins on which it acts in yeast. Later studies recovered specific protein interactors with FK506 binding protein 12 (FKBP12) prolyl isomerase, a cyclophylin known to bind FK506 and cyclosporine, validating the preposition of the authors that PPIases will have specific client proteins with which they react (Dolinski and Heitman, Reference Dolinski and Heitman1999). However, to date, no protein associated with translation has been identified in any organism as specifically interacting with a PPIase in order to be refolded following stress.
In bacteria, the TRIGGER FACTOR chaperonin protein with PPIase activity, is physically associated with the 50S ribosomal-tunnel from which the amino-terminus of the protein being constructed emerges and is the first chaperonin the nascent polypeptide encounters (Kristensen and Gajhede, Reference Kristensen and Gajhede2003). In plants, the plastid is apparently the only compartment supporting translation that has retained a TRIGGER FACTOR chaperonin (Category 7; Ries et al., Reference Ries, Carius, Rohr, Gries, Keller, Lancaster and Willmund2017) which, due to its susceptibility to supraoptimal temperature stress, is thought to confer to plastidial translation some advantage during heat stress (Ries et al., Reference Ries, Carius, Rohr, Gries, Keller, Lancaster and Willmund2017). While none of the above mentioned isomerizations has been considered ‘damage’, or to occur preferentially in proteins involved in translation, in plants it does link PPIase activity tightly to translation, at least in the plastid.
Protection/repair: Chaperonins: heat shock proteins
In developing soybean seeds, transcripts encoding certain HEAT SHOCK PROTEINS (HSPs) accumulate late during embryogenesis and are remarkable as among the most positively correlated with seed longevity (Pereira Lima et al., Reference Pereira Lima, Buitink, Lalanne, Rossi, Pelletier, da Silva and Leprince2017). As with GALACTINOL SYNTHASE over-expression studies, there are numerous examples where over-expression of HSPs or the genes encoding transcription factors (HEAT SHOCK FACTORs), up-regulating the plant HSP arsenal, results in increased seed longevity after artificial ageing (Prieto-Dapena et al., Reference Prieto-Dapena, Castano, Almoguera and Jordano2006; Personat et al., Reference Personat, Tejedor-Cano, Prieto-Dapena, Almoguera and Jordano2014; Kaur et al., Reference Kaur, Petla, Kamble, Singh, Rao, Salvi, Ghosh and Majee2015). The opposite has also been demonstrated where mutated HSF genes, introduced from sunflower into tobacco, have led to the down-regulation of HSPs and a concomitant reduction in seed tolerance of accelerated ageing conditions (Tejedor-Cano et al., Reference Tejedor-Cano, Prieto-Dapena, Almoguera, Carranco, Hiratsu, Ohme-Takagi and Jordano2010). Similarly, reduction of HSP101 though mutagenesis, while producing viable seeds and apparently normal plants in the absence of stress, displayed a reduction in seed resistance to thermal insult during germination (Hong and Vierling, Reference Hong and Vierling2000). While their roles protecting nascent proteins from misfolding and capacity to catalyse proper protein configurations upon folding make them undeniably associated with proteins associated with translation (and justify their title above as protective Chaperonin proteins), are there instances where such chaperonins are known to preferentially assist the refolding of proteins of the translational apparatus following damage (justifying their title as repair Chaperonin proteins)? The HSP90 has been shown to have as substrates other HSPs, as well as cyclophilins (Category 3), translational elongation factor kinases (Category 7), and aminoacyl-t-RNA synthetase complexes (Category 6) (Picard, Reference Picard2002). The bacterium Synechocystis, when subjected to heat stress, up-regulated HSP16.6 that bound an r-protein (Category 1), translation elongation factors (Category 7), a PPIase (Category 7), and tRNA/rRNA modifying enzymes (Categories 2, 6) (Basha et al., Reference Basha, Lee, Breci, Hausrath, Buan, Giese and Vierling2004). The consensus now is that, although promiscuous in binding damaged proteins, a preference for those engaged in translation and for metabolic enzymes exists in bacteria (Haslbeck and Vierling, Reference Haslbeck and Vierling2015). Such was also the case for Arabidopsis thaliana where RNAi (targeting Class I or Class II small HSPs) or over-expression [HSP17.4-OE (Class I) or HSP17.6-OE (Class II)] resulted in reduced versus enhanced tolerance to heat shock in the vegetative stage, respectively (McLoughlin et al., Reference McLoughlin, Basha, Fowler, Kim, Bordowitz, Katiyar-Agarwal and Vierling2016). Proteins that associated with both HSP17.4 and HSP17.6 were identified as the subunits of eEF1B (a, b and g; Category 7) (McLoughlin et al., Reference McLoughlin, Basha, Fowler, Kim, Bordowitz, Katiyar-Agarwal and Vierling2016). Furthermore, these authors stringently examined the capacity of the eEF1B subunits to be: (1) denatured during heat shock with a proportion becoming insoluble; and (2) renatured, re-entering the soluble phase, following recovery from heat shock (McLoughlin et al., Reference McLoughlin, Basha, Fowler, Kim, Bordowitz, Katiyar-Agarwal and Vierling2016). Of these proteins rendered insoluble upon heat shock, eEF1B subunits were preferentially repaired to become resoluble. Such repair is anticipated to be extended to periods of stress during germination (Fig. 1) and potentially to recovery following imbibition, although these investigations remain to be performed. Their conclusion was that these proteins, essential for translation, are preferred client proteins of the two small HSPs (McLoughlin et al., Reference McLoughlin, Basha, Fowler, Kim, Bordowitz, Katiyar-Agarwal and Vierling2016), a view entirely consistent with Job's rule.
Conclusion
We have investigated evidence supporting Job's rule (the proteins involved in translation must endure desiccation, quiescence, and rehydration in a functional state if the seed is to survive), highlighting data indicating that proteins of the translational apparatus are particularly resilient. Studies comparing the identities of proteins oxidized in dry – relative to imbibed – seeds suggest that proteins associated with translation are somewhat protected from oxidation in the dry state, although little is known about how oxidation of proteins of the translational apparatus is mitigated while desiccated, or how they exhibit enhanced resiliency. There is, however, some evidence of innate protein supercharging of r-proteins, involved in translation, at biologically relevant pH that may be relevant to these observations. Furthermore, endeavours to identify preferred client proteins bound by LEA proteins or repaired by PIMT or chaperonin networks, has provided evidence supportive of Job's rule because these systems are seemingly skewed towards serving the proteins of the translational apparatus. The more information obtained on the identities of client proteins preferentially serviced by conspecific protection and repair mechanisms the greater the opportunity to identify: (1) what proteins are susceptible to damage; and (2) what parts of these proteins are particularly at risk of damage. This knowledge is edifying to efforts to understand fundamental constraints to anhydrobiosis and practically, could lead to re-engineering proteins to make them less susceptible to a plethora of damage.
Acknowledgements
The authors wish to thank the USDA Western Section Multistate Research Project Working Group W-3168 ‘Environmental and Genetic Determinants of Seed Quality and Performance’, the International Society for Seed Science (ISSS), and Western Section American Society of Plant Biologists for providing the opportunity to present aspects covered in this manuscript at the ISSS triennial meeting, Monterey, California, USA. Particular thanks are due to Henk Hilhorst, Editor of the ISSS journal, Seed Science Research, for providing space for articles such as this to highlight the ISSS triennial meeting topics. Hatch funds were used to support the project detailed in this communication for which we are grateful.