INTRODUCTION
Leishmaniasis and dirofilariasis are both vector-borne diseases. Leishmania (Trypanosomatida, Trypanosomatidae) uses sand flies (Phlebotomus spp.) (WHO, 1997) and Dirofilaria (Spirurida, Onchocercidae) uses mosquitoes (species of Aedes, Culex, Anopheles and Mansonia) for their transmissions to mammalian hosts (Otto and Jachowski, Reference Otto, Jachowski and Otto1981). During a blood meal, an infected sand fly introduces promastigotes to the warm-blooded host, whilst the infected mosquito introduces third-stage filarial larvae (L3) of Dirofilaria. In the mammalian host, promastigotes enter macrophages and inside the cell they replicate as amastigotes, whilst the L3 larvae undergo two moults to become adults who reside in pulmonary arteries and the heart. For 5–10 years the female worms produce microfilariae (L1), which enter into the peripheral blood (McCall et al. Reference McCall, Genchi, Kramer, Guerrero and Venco2008). Infected macrophages and free L1 are then taken up by their vectors to complete their life cycles. In areas where hosts, infected by these parasites, and competent insect vectors exist, coinfections may arise (Aresu et al. Reference Aresu, Valenza, Ferroglio, Pregel, Uslenghi, Tarducci and Zanatta2007; Maia et al. Reference Maia, Coimbra, Ramos, Cristóvão, Cardoso and Campino2015). It appears that changes in climate and human activities aid in the geographical spread of these zoonoses and human, as well as dog and feline; cases are increasing in numbers even in areas where the problems were not reported before (Orihel and Eberhard, Reference Orihel and Eberhard1998; Pampiglione and Rivasi, Reference Pampiglione and Rivasi2000; Dujardin, Reference Dujardin2006; Maroli et al. Reference Maroli, Rossi, Baldelli, Capelli, Ferroglio, Genchi, Gramiccia, Mortarino, Pietrobelli and Gradoni2008; Tanczos et al. Reference Tanczos, Balogh, Kiraly, Biksi, Szeredi, Gyurkovsky, Scalone, Fiorentino, Gramiccia and Farkas2012).
Five species of filarial nematodes: Dirofilaria immitis, Dirofilaria repens, Dipetalonema reconditum, Dipetalonema grassii and Acanthocheilonema reconditum have being recovered from dogs (Genchi et al. Reference Genchi, Kramer and Rivasi2011); and Leishmania infantum (synonym: Leishmania chagasi in the New World) is the most common Leishmania species causing canine leishmaniasis (CanL), worldwide (Alvar et al. Reference Alvar, Velez, Bern, Herrero, Desjeux, Cano, Jannin and den Boer2012). In Greece, D. immitis has been reported in dogs (Polizopoulou et al. Reference Polizopoulou, Koutinas, Saridomichelakis, Patsikas, Leontidis, Roubies and Desiris2000; Sinanis et al. Reference Sinanis, Koutinas, Diakou and Papadopoulou2012) and D. repens (D. conjunctivae) in humans (Vakalis and Himonas, Reference Vakalis and Himonas1997), whilst L. infantum comprises one of the most important parasitic diseases in dogs in most parts of Greece, and an important zoonosis for public health (Antoniou et al. Reference Antoniou, Messaritakis, Christodoulou, Ascoksilaki, Kanavakis, Sutton, Carson and Courtenay2009; Christodoulou et al. Reference Christodoulou, Antoniou, Ntais, Messaritakis, Ivovic, Dedet, Pratlong, Dvorak and Tselentis2012; Ntais et al. Reference Ntais, Sifaki-Pistola, Christodoulou, Messaritakis, Pratlong, Poupalos and Antoniou2013).
The aim of this work was to evaluate the geographical distribution of coinfected dogs in Greece, to isolate the two parasites from the blood of dogs, using a simple method, and to assess their survival and interaction in vitro.
MATERIALS AND METHODS
Study area and animal sampling
The study was conducted in all (54) prefectures of Greece where 63 veterinarians, collaborating with our laboratory, provided dog samples from 5772 animals visiting their clinic for any reason: vaccination, haircut, nail cut, deworming, general check-up, treatments and other purposes, without discrimination (Ntais et al. Reference Ntais, Sifaki-Pistola, Christodoulou, Messaritakis, Pratlong, Poupalos and Antoniou2013). The animals were examined clinically and biological material was collected, after the written consent of the owner, and a questionnaire with personal, epidemiological and clinical data for each dog was completed. Biological samples, including peripheral blood without anticoagulants from all dogs taking part in the study, were collected and stored in sterile tubes at 4 °C until transfer to the laboratory for processing. The maximum number of samples reached 300 per prefecture and depended on the size of the prefecture and dog population (Ntais et al. Reference Ntais, Sifaki-Pistola, Christodoulou, Messaritakis, Pratlong, Poupalos and Antoniou2013).
Serology
Dog sera were tested serologically using anti-dog, anti-immunoglobulin G antibodies by an indirect immunofluorescent antibody test (IFAT, Leishmania SPOT IF, BioMerieux, France). A series of 2-fold serum dilutions, starting from 1/40 were performed and a cut-off titre of ≥1/160 was regarded positive for dog sera since most animals lived in endemic areas (Ferroglio et al. Reference Ferroglio, Trisciuoglio, Gastaldo, Mignone and Delle Piane2002).
Polymerase chain reaction (PCR) and PCR-restriction fragment length polymorphism (PCR-RFLP)
The PCR for the detection of Leishmania DNA was carried out on whole blood, lymph node and/or spleen tissue from dogs, according to availability. The QIAamp DNA Blood Mini kit (QIAGEN, Hilden, Germany) and DNeasy Tissue kit (QIAGEN) were used for DNA extraction from blood and tissue, respectively. Primers T2 and B4 were used as described previously with few modifications (Minodier et al. Reference Minodier, Piarroux, Gambarelli, Joblet and Dumon1997; Christodoulou et al. Reference Christodoulou, Antoniou, Ntais, Messaritakis, Ivovic, Dedet, Pratlong, Dvorak and Tselentis2012). The Leishmania ITS1 region followed by a HaeIII restriction endonuclease digestion of the positive PCR products (Schönian et al. Reference Schönian, Nasereddin, Dinse, Schweynoch, Schallig, Presber and Jaffe2003) was amplified in order to identify the parasite species infecting the dogs. All samples were tested in duplicates. Positive controls were used in all PCR assays, which consisted of DNA extracted from L. infantum (MCAN/GR/2009/GD70) and Leishmania tropica (MCAN/GR/2009/GD52), the two Leishmania spp. found in Greece, which had derived from Greek dogs and humans, respectively, and typed by enzyme electrophoresis (Ntais et al. Reference Ntais, Sifaki-Pistola, Christodoulou, Messaritakis, Pratlong, Poupalos and Antoniou2013; Karayiannis et al. Reference Karayiannis, Ntais, Messaritakis, Tsirigotakis, Dokianakis and Antoniou2015). Negative controls included samples from healthy dogs born and lived in non-endemic areas in Crete (at >1000 m altitude), that had being tested for Leishmania serologically, by PCR and culture and proved negative.
Parasite culture
Parasite culture was performed using blood, without anticoagulants, of 1275 animals, which were positive both by IFAT and PCR. Sterile clotted blood (0·5 cm3 obtained from the core of the sample) was suspended in 3 mL RPMI 1640 culture medium containing 25 mm Hepes buffer, supplemented with 2 mm glutamine (GIBCO Invitrogen, Grand Island, NY), 10% heat inactivated fetal bovine serum (FBS – GIBCO Invitrogen), 100 IU mL−1 penicillin, 100 mg mL−1 streptomycin (Roche Diagnostics, Indianapolis, IN), and 5% filtered human urine, and incubated, in culture flasks, at 26 °C (±1° C) (Howard et al. Reference Howard, Pharoah, Ashall and Miles1991; WHO, 1991).
Parasite identification
Leishmania was identified using PCR-RFLP, as described above, and Dirofilaria was identified after microscopic examination of the microfilariae using morphometric characters (body size and shape) (Magnis et al. Reference Magnis, Lorentz, Guardone, Grimm, Magi, Naucke and Deplazes2013).
RESULTS
Of the 5772 randomly selected dogs examined for leishmaniasis by IFAT, and PCR, 1275, (22·09%) were positive both by serology and PCR (Ntais et al. Reference Ntais, Sifaki-Pistola, Christodoulou, Messaritakis, Pratlong, Poupalos and Antoniou2013). PCR-RFLP showed the protozoan parasite to be L. infantum and morphometric characters of the microfilariae (length, 290–332 µm; width, 6–7 µm; straight body and tail; tapered anterior end) showed the Nematode parasite to be D. immitis (Magnis et al. Reference Magnis, Lorentz, Guardone, Grimm, Magi, Naucke and Deplazes2013).
Culture, performed using clotted blood of only the 1275 PCR and IFAT-positive animals, resulted in 165 positive for Leishmania (12·94%) and 26 positive for Dirofilaria (2·04%) culture media. In two of these cultures (both from the island of Corfu, with animal IFAT titres against Leishmania 1/640 and 1/5120), both Leishmania and Dirofilaria emerged (0·16%).
The 26 dogs, from the blood of which Dirofilaria L1 was isolated, were 16 males and 10 females; none was a stray animal. Their age was: 6 > 3 years, 12 between 4 and 6 years, 4 between 7 and 9 years, and 4 > 10 years. Their weight was: 4 > 10 kg, 10 between 11 and 20 kg, 10 between 21 and 30 kg, and 2 > 30 kg. All 26 dogs presented antibodies against Leishmania: IFAT titres 1/320 to 1/5120 and had symptoms common to both diseases: unusual tiredness, ocular lesions, weight loss. Twenty-four of these cultures contained 5–6 L1 mL−1 live and active Dirofilaria, which appeared on the first day and stayed alive until over 30 days at 26 ± 1 °C. From the other two L1 positive cultures, 1 and 2 L1 mL−1 emerged on day 1. In the culture containing 1 microfilariae mL−1, the microfilariae died on day 4 of the culture and on day 7, Leishmania promastigotes appeared, grew in numbers and propagated successfully. In the second culture containing 2 L1 mL−1, a small number of Leishmania promastigotes emerged on day 8, but disappeared after about 1 week, whilst the L1 survived for 30 days.
In the 165 cultures which yielded Leishmania promastigotes, the parasites propagated indefinitely in the RPMI medium at 26 ± 2 °C until the culture was terminated after the second passage to be frozen in liquid nitrogen for future work.
The Dirofilaria and Leishmania coinfected dogs originated from five prefectures (nine dogs from Corfu, seven from Drama, five from Fthiotida, two from Arta, two from Ioannina, one from Rodopi) in which CanL is endemic (Fig. 1).
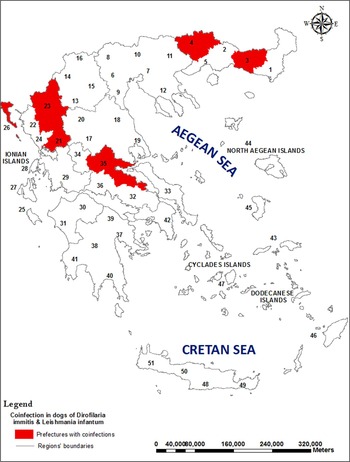
Fig. 1. Leishmania infantum and Dirofilaria immitis coinfections in dogs in Greece. L1 of D. immitis were isolated from clotted blood of dogs which were positive for L. infantum by serology and PCR from prefectures: Rodopi (indicated by number 3), Drama (4), Arta (21), Ioannina (23), Corfu (26), Fthiotida (35).
DISCUSSION
Leishmaniasis is recognized as a very important disease for public health in the whole of the Mediterranean Basin, including Greece where it is notifiable. It appears that it has spread geographically nearly in the whole of the country (Antoniou et al. Reference Antoniou, Messaritakis, Christodoulou, Ascoksilaki, Kanavakis, Sutton, Carson and Courtenay2009; Ntais et al. Reference Ntais, Sifaki-Pistola, Christodoulou, Messaritakis, Pratlong, Poupalos and Antoniou2013) implying that competent sand fly vectors are present and active. The problem of dirofilariasis in Greece, on the other hand, is not considered very important for public health since a few sporadic human cases have being reported which, however, are increasing in number (Vakalis and Himonas, Reference Vakalis and Himonas1997). The presence of microfilaraemic dogs in an area, and possibly of other domestic and wild reservoirs, such as the red foxes (Karayiannis et al. Reference Karayiannis, Ntais, Messaritakis, Tsirigotakis, Dokianakis and Antoniou2015), allows the circulation of the parasite via the mosquito vectors, which explains the escalation of human cases and poses a thread for the future. The spread of both zoonoses is further favoured by the movement of people and pets and the spread of the vectors in space and time due to climatic changes, as well as human activities.
Measures for safeguarding unaffected areas from the introduction of these pathogens (through infected humans or animals) and their vectors must be undertaken. The introduction of Leishmania donovani MON-37 in Cyprus (Antoniou et al. Reference Antoniou, Haralambous, Mazeris, Pratlong, Dedet and Soteriadou2008) and the arrival of the aggressive anthropophilic mosquito Aedes albopictus in Europe, which can play the role of the vector for D. immitis and could increase the risk of transmission of this parasite from animals to humans (Cancrini et al. Reference Cancrini, Frangipane di Regalbono, Ricci, Tessarin, Gabrielli and Pietrobelli2003), is an indication of the enhanced movement of pathogens by globalization.
The culture method used is fast, simple and inexpensive and allows the isolation of both parasites from a big number of samples during an epidemiological study for monitoring risk areas. In this study, 12·94% of the dogs studied were positive for Leishmania and 2·04% were positive for Leishmania and Dirofilaria. Coinfections were found in the prefectures of Corfu, Drama, Fthiotida, Arta, Ioannina and Rodopi (Fig. 1). Coinfected red foxes have also being reported in Fthiotida prefecture by Karayiannis et al. (Reference Karayiannis, Ntais, Messaritakis, Tsirigotakis, Dokianakis and Antoniou2015).
Although filarial worms, including Dirofilaria, have being maintained in vitro (Silverman and Hansen, Reference Silverman and Hansen1971), as far as we know, this is the first record of isolation of live Dirofilaria L1 from the blood of a host and their maintenance for at least 1 month in the RPMI medium at 26 °C. The method can also be used for the isolation of Leishmania promastigotes from biological samples (blood without anticoagulants, spleen tissue and lymph node), by placing a small segment of the sterile tissue in the RPMI culture medium. This method can be used to isolate L. infantum and L. tropica from biological samples without the need of a density gradient cell separation medium for the acquisition of lymphocytes to be used for the culture.
It is interesting to note that only in two out of the 26 IFAT and PCR positive for Leishmania samples in which Dirofilaria L1 emerged, Leishmania promastigotes appeared. In one of these two cases, promastigotes proliferated successfully after the three L1 (in the 3 mL culture medium) died, but the weak Leishmania culture which appeared on day 8 of the second culture died out after 1 week in the presence of seven L1 in the culture medium. It would be interesting to investigate whether the presence of microfilariae do not favour promastigote proliferation and survival for long in the culture medium and possibly in the host as well. The microfilariae may feed on the promastigotes or produce metabolic substances that do not allow the protozoan parasite to exist. This could allow the development of potential new substances in combating leishmaniasis in dogs and humans.
ACKNOWLEDGEMENTS
The authors wish to thank all veterinarians who helped in obtaining the dog biological samples and data.
FINANCIAL SUPPORT
We wish to thank EDENext for funding the work (EU grant FP7-261504 to M. A.). The manuscript was catalogued by the EDENext Steering Committee as EDENext388 (http://www.edenext.eu). The contents of this publication are the sole responsibility of the authors and don't necessarily reflect the views of the European Commission.


