Introduction
Over the last decade, direct oral challenge (DOC) without prior skin-testing has been increasingly utilized as the standard of care for delabeling patients with a low-risk penicillin allergy. The incorporation of penicillin DOC for low-risk allergy phenotypes into Antimicrobial Stewardship (AMS) interventions has significantly improved antibiotic appropriateness, especially in immunocompromised hosts. Reference Chua, Vogrin and Bury1 This has been enabled by the derivation, validation and implementation of penicillin allergy risk assessment tools and the non-inferiority of DOC compared to traditional skin-testing. Reference Trubiano, Vogrin and Chua2,Reference Copaescu, Vogrin and James3 Similarly, risk assessment and DOC have been successfully applied in the outpatient setting to low-risk sulfa antibiotic allergies. Reference Krantz, Stone, Abreo and Phillips4,Reference Rose, Vogrin and Chua5 In efforts to improve timely access to sulfa antibiotics for hospitalized patients who are more medically complex than a traditional outpatient population, we sought to evaluate the safety, effectiveness and healthcare savings of low-risk sulfa antibiotic allergy delabeling via DOC in the inpatient setting, with a focus on the immunocompromised host.
Methods
Study design, setting and intervention
A prospective cohort study was undertaken at Austin Health, a tertiary referral hospital in Melbourne, Australia. Inpatients on acute wards who reported a sulfa or trimethoprim-sulfamethoxazole allergy were identified by the multidisciplinary AMS-Allergy service and assessed utilizing the Antibiotic Allergy Assessment Tool (AAAT) Reference Devchand, Urbancic and Khumra6 and when derived in 2023, the SULF-FAST clinical decision rule (Figure S1). Reference Waldron, Rose and Vogrin7 The AAAT was used to characterize the sulfa allergy phenotype, and the SULF-FAST clinical decision rule was applied to determine the risk of a positive DOC. Inpatients with a low-risk assessment per the AAAT and/or SULF-FAST score<3 were offered a single-dose trimethoprim-sulfamethoxazole DOC (Appendix S1). Patients who were hemodynamically unstable, unable to provide consent, or concurrently prescribed antihistamines or prednisolone (or equivalent corticosteroid)>25 mg daily were excluded from inpatient DOC. The prednisolone threshold of ≤25 mg daily was chosen as there are limited international consensus recommendations for the maximum corticosteroid threshold at which antibiotic challenge should occur, and this study aimed to improve imminent access to first-line antibiotics, particularly in immunocompromised patients with indeterminate plans for corticosteroid weaning.
Following DOC, the hospital medical record was audited for trimethoprim-sulfamethoxazole utilization within 90-days and relabeling of trimethoprim-sulfamethoxazole allergy was audited at 12-months.
Immunocompromised hosts were defined as i) hemotological or solid organ malignancy, ii) solid organ transplant, or iii) other immunocompromise; autoimmune or connective tissue disorder, human immunodeficiency virus, hemodialysis, end-stage chronic liver disease, splenectomy or receiving prednisolone (or equivalent)>10mg/day for more than one month.
Data were managed and stored using REDCap electronic data capture tools and Ethics approval was obtained from the Austin Health Research Ethics Committee (47585/Austin-2018).
The outcomes were (i) safety; the proportion of inpatients with a low-risk sulfa allergy that were delabeled following DOC, (ii) effectiveness; the proportion of patients who utilized trimethoprim-sulfamethoxazole within 90-days of DOC, and (iii) healthcare savings; the comparative healthcare costs of inpatient DOC and subsequent trimethoprim-sulfamethoxazole use for immunocompromised patients requiring Pneumocystis jirovecii pneumonia (PJP) prophylaxis versus alternative PJP prophylaxis therapy.
Statistical analysis
Characteristics were compared between immunocompromised and non-immunocompromised groups using Fisher’s Exact or rank sum test. Logistic regression was used to evaluate utilization of trimethoprim-sulfamethoxazole post-DOC between immunocompromised and non-immunocompromised groups.
Results
Between 23 March 2022 and 14 May 2025, 221 inpatients with a reported trimethoprim-sulfamethoxazole or sulfa antibiotic allergy were assessed, of which 118 (53%) were low-risk on the AAAT and 140 (63%) had a SULF-FAST score<3.
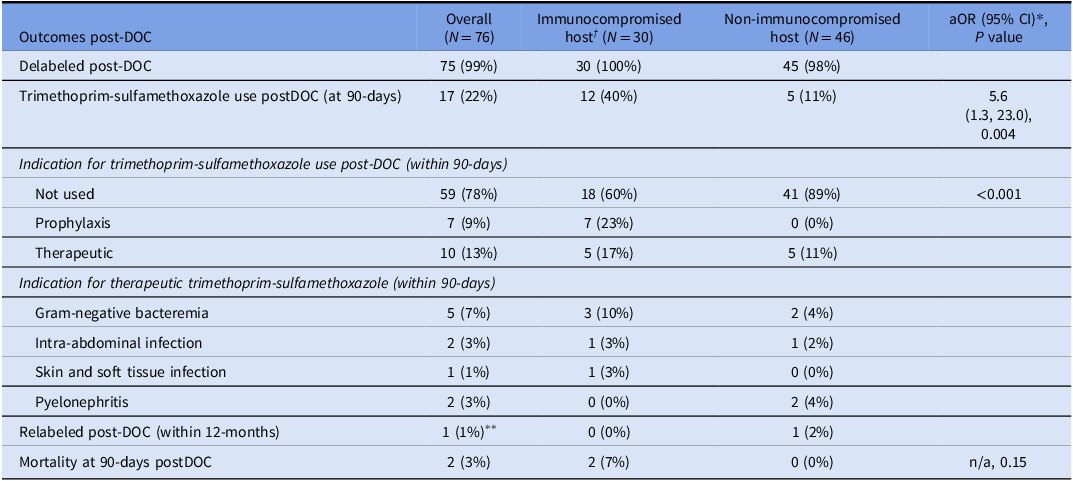
In total, 76 (34%) inpatients underwent DOC, of which 30 (40%) were immunocompromised hosts (Table 1). Of 30 inpatients in the immunocompromised host group, 18 (60%) had a hematological or solid organ malignancy, six (20%) were solid organ transplant recipients, and six (20%) were immunocompromised for other reasons (Table S1). Of 76 inpatient trimethoprim-sulfamethoxazole DOC performed, 75 (99%) were delabeled. One non-immunocompromised patient experienced a delayed exanthem that was managed in the outpatient setting with an antihistamine.
Table 1. Cohort and allergy phenotype characteristics of immunocompromised host vs non-immunocompromised host inpatients undergoing trimethoprim-sulfamethoxazole direct oral challenge

IQR: interquartile range
† Immunocompromised hosts—solid organ transplant recipient, hematological or solid organ malignancy, autoimmune or connective tissue disorder, human immunodeficiency virus, hemodialysis, end-stage chronic liver disease, splenectomy or prednisolone (or equivalent) use>10mg/day for 1 month.
‡ Medical Immunocompromised host: Oncology, Hematology, Liver transplant.
§ As per previously published AAAT.
* Patients may report more than one reaction description.
Seventeen (22%) patients utilized trimethoprim-sulfamethoxazole within 90-days of DOC; 10 (13%) for therapeutic indications and 7 (9%) for PJP prophylaxis. There was greater use of subsequent trimethoprim-sulfamethoxazole (prophylaxis or treatment) in the immunocompromised host cohort (40% vs 11%, P = 0.004). After adjusting for age, sex, and Charlson Comorbidity Index, immunocompromise status was a predictor of increased use of trimethoprim-sulfamethoxazole post-DOC (adjusted OR 5.6 95% CI 1.3, 23.0) (Table 2). The outcomes of 30 immunocompromised hosts who underwent inpatient DOC, stratified by sub-group, are in Table S2.
Table 2. Outcomes of immunocompromised host vs non-immunocompromised host inpatients undergoing trimethoprim-sulfamethoxazole direct oral challenge

DOC: direct oral challenge, aOR: adjusted odds ratio.
†Immunocompromised hosts - transplant recipient, hematological or solid organ malignancy, autoimmune or connective tissue disorder, human immunodeficiency virus, hemodialysis, end-stage chronic liver disease, splenectomy or prednisolone (or equivalent) use>10mg/day for 1 month.
*Univariable logistic regression or Fisher’s Exact.
**Delayed positive direct oral challenge, as described.
Aside from the single positive challenge event, no patients had their allergy label reinstated in the medical record at 12-months post-DOC. Mortality, unrelated to DOC, occurred in 2 (3%) patients within 90-days of evaluation (Table 2). Of note, four (5%) inpatients also underwent penicillin allergy delabeling via DOC during the same hospital admission.
Of 145 patients who did not undergo inpatient DOC, 22 (15%) were directly delabeled based on history alone, 35 (24%) were medically unstable precluding inpatient DOC, and 10 (7%) were discharged prior to DOC. In total, 26 (18%) patients declined testing in the inpatient setting, most with a preference to defer testing to post-discharge, and a smaller proportion declining any future evaluation. Further, 52 (36%) patients were assessed as moderate-high risk phenotypes and inappropriate for inpatient DOC, however were offered evaluation in the outpatient antibiotic allergy clinic.
In the Australian context, for immunocompromised inpatients requiring PJP prophylaxis, use of DOC to delabel low-risk sulfa antibiotic allergy, and enable first-line trimethoprim-sulfamethoxazole therapy in lieu of inhaled pentamidine, the projected healthcare savings were estimated at $416.94 AUD per delabeled patient for 6-months of PJP prophylaxis (Table S3).
Discussion
This study is one of the first to examine the utility of trimethoprim-sulfamethoxazole DOC for inpatients with a low-risk sulfa antibiotic allergy, facilitating access to sulfa antibiotics where an acute therapeutic need exists, and eliminating delays to accessing outpatient testing. The approach appears safe and led to appropriate trimethoprim-sulfamethoxazole utilization for both prophylaxis and treatment, especially in immunocompromised patients. Although limited by its single regional experience, small study numbers, and predominance of patients with a SULF-FAST score of 0–1, the findings are supported by the prior success of a similar penicillin allergy delabeling program, Reference Chua, Vogrin and Bury1 external international validation of the SULF-FAST clinical decision rule, Reference Stehlin, Vogrin, Mitri, Isabwe, Trubiano and Copaescu8 and known similar immunological patterns showing loss of immunogenicity over time for both penicillin and sulfa antibiotic allergies. Reference Krantz, Stone, Abreo and Phillips4 The use of inpatient trimethoprim-sulfamethoxazole DOC to evaluate low-risk sulfa antibiotic allergy, particularly in patients with remote (>5 years) non-anaphylactic, benign cutaneous reactions, negates the requirement for time- and resource-intensive desensitization protocols, and further supports sulfonamide allergy testing recommendations from the American Academy of Asthma Allergy and Immunology. Reference Khan, Banerji and Blumenthal9 In addition to the healthcare savings described, further economic benefits may be achieved by implementing DOC for low-risk sulfa antibiotic allergy and reserving the use of desensitization for patients with a convincing history of anaphylaxis. Reference Khan, Banerji and Blumenthal9 However, unlike penicillin allergy, trimethoprim-sulfamethoxazole is a drug more commonly associated with severe cutaneous adverse reactions, and education around risk assessment, including identifying non-cutaneous features of severe cutaneous adverse reactions such as organ dysfunction, is required to ensure only low-risk phenotypes undergo DOC. Nonetheless, this study highlights the promise of inpatient sulfa antibiotic allergy delabeling via DOC for low-risk phenotypes, addressing an identified need in immunocompromised hosts where trimethoprim-sulfamethoxazole is a preferred prophylaxis and treatment strategy, and its avoidance is associated with inferior infection outcomes. Reference Al-Shaikhly, Al-Obaydi, Craig and Henao10 Further studies demonstrating the safety, effectiveness, and implementation of inpatient trimethoprim-sulfamethoxazole DOC, especially for immunocompromised hosts, will help confirm this strategy as a novel AMS tool.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/ice.2026.10400
Acknowledgements
None.
Author contribution
EAM: Writing—review and editing, Writing—original draft, Formal analysis, Data curation, Conceptualization. SV: Writing—review and editing, Formal analysis, Data curation, Conceptualization. RH: Writing—review and editing. RM: Writing—review and editing. GKR: Writing—review and editing. JAT: Writing—review and editing, Writing—original draft, Formal analysis, Data curation, Conceptualization.
Financial support
This work was supported by a PhD Scholarship from the Australian Government funded National Allergy Centre of Excellence (NACE), hosted by the Murdoch Children’s Research Institute (MCRI) to EAM, and their work was supported by the Victorian Government’s Operational Infrastructure Support Program. GKR receives NHMRC PhD scholarship #2013970. JAT is supported by a National Health and Medical Research Council Emerging Leadership Fellowship (1139902).
Competing interests
The authors declare no conflicts of interest in relation to this work.