Introduction
Chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF) are main causes of dyspnea and exercise intolerance, being highly prevalent in the general elderly population (van Mourik et al., Reference van Mourik, Rutten, Moons, Bertens, Hoes and Reitsma2014). The coexistence of both diseases is common but often unrecognized. Considering the overlap in signs and symptoms, one condition frequently pass unnoticed once the another disease has been previously diagnosed. This was showed by previous studies describing a high prevalence of unknown CHF in COPD (McCullough et al., Reference McCullough, Hollander, Nowak, Storrow, Duc, Omland, McCord, Herrmann, Steg, Westheim, Knudsen, Abraham, Lamba, Wu, Perez, Clopton, Krishnaswamy, Kazanegra, Maisel and Investigators2003; Rutten et al., Reference Rutten2005; Beghé et al., Reference Beghé, Verduri, Bottazzi, Stendardo, Fucili, Balduzzi, Leuzzi, Papi, Mantovani, Fabbri, Ceconi and Boschetto2013) and vice versa (Macchia et al., Reference Macchia, Rodriguez Moncalvo, Kleinert, Comignani, Gimeno, Arakaki, Laffaye, Fuselli, Massolin, Gambarte, Romero and Tognoni2012; Boschetto et al., Reference Boschetto, Fucili, Stendardo, Malagù, Parrinello, Casimirri, Potena, Ballerin, Fabbri, Ferrari and Ceconi2013). Accordingly, additional investigational tools, such as spirometry and echocardiography, are required for an adequate diagnosis.
Beyond the diagnostic challenge, the overlap of COPD and CHF has been associated with increased morbidity, poor quality of life and greater utilization of healthcare resources. Moreover, overlap frequently compounds with other systemic co-morbidities contributing to poor prognosis (Rutten et al., Reference Rutten2005; Macchia et al., Reference Macchia, Rodriguez Moncalvo, Kleinert, Comignani, Gimeno, Arakaki, Laffaye, Fuselli, Massolin, Gambarte, Romero and Tognoni2012).
Despite the interest in the interactions between both diseases has recently grown (Fabbri et al., Reference Fabbri, Luppi, Beghé and Rabe2008; Rutten, Reference Rutten, Cramer, Grobbee, Sachs, Kirkels, Lammers and Hoes2013), description of survival predictors are still scarce (Alencar et al., Reference Alencar, Arbex, Souza, Mazzuco, Sperandio, Rocha, Hirai, Mancuso, Berton, Borghi-Silva, Almeida, OʼDonnel and Neder2016). While cardiopulmonary exercise testing (CPET)-derived parameters have demonstrated key prognostic significance in patients with COPD (Oga et al., Reference Oga, Nishimura, Tsukino, Sato and Hajiro2003; Neder et al., Reference Neder, Alharbi, Berton, Alencar, Arbex, Hirai, Webb and O’Donnell2016), CHF (Mancini et al., Reference Mancini, Eisen, Kussmaul, Mull, Edmunds and Wilson1991; Poggio et al., Reference Poggio, Arazi, Giorgi and Miriuka2010) and both diseases (Alencar et al., Reference Alencar, Arbex, Souza, Mazzuco, Sperandio, Rocha, Hirai, Mancuso, Berton, Borghi-Silva, Almeida, OʼDonnel and Neder2016), we must acknowledge that overlap patients are usually extremely frail and frequently never become stable enough to perform an exercise test (Arbex et al., Reference Arbex, Alencar, Souza, Mazzuco, Sperandio, Rocha, Hirai, Mancuso, Berton, Borghi-Silva, Almeida, O’Donnell and Neder2016).
In COPD, the severity of the baseline disease is closely related to the severity of exacerbations, that is patients with severe disease are more likely to be hospitalized due to an exacerbation. In the long term, patients who experience severe exacerbations have an increased risk of more severe exacerbations in the future (Garcia-Aymerich et al., Reference Garcia-Aymerich, Monsó, Marrades, Escarrabill, Félez, Sunyer, Antó and Investigators2001; Donaldson et al., Reference Donaldson, Seemungal, Patel, Lloyd-Owen, Wilkinson and Wedzicha2003). The coexistence with cardiac disease may influence the severity of an exacerbation. In fact, COPD patients with cardiac disease present increased risk of hospitalization due to an exacerbation (Miravitlles et al., Reference Miravitlles, Guerrero, Mayordomo, Sánchez-Agudo, Nicolau and Segú2000) and an increased risk of mortality (Antonelli Incalzi et al., Reference Antonelli Incalzi, Fuso, De Rosa, Forastiere, Rapiti, Nardecchia and Pistelli1997; Macchia et al., Reference Macchia, Rodriguez Moncalvo, Kleinert, Comignani, Gimeno, Arakaki, Laffaye, Fuselli, Massolin, Gambarte, Romero and Tognoni2012).
The main objective of the present study, therefore, was to investigate if markers of disease severity obtained from clinical practice (body composition, dyspnea, functional class, inability to perform a clinical exercise testing and previous hospitalization rate: Boeck et al., Reference Boeck, Soriano, Brusse-Keizer, Blasi, Kostikas, Boersma, Milenkovic, Louis, Lacoma, Djamin, Aerts, Torres, Rohde, Welte, Martinez-Camblor, Rakic, Scherr, Koller, van der Palen, Marin, Alfageme, Almagro, Casanova, Esteban, Soler-Cataluña, de-Torres, Miravitlles, Celli, Tamm and Stolz2016), would be associated with higher risk of mortality beyond cardiac/lung function tests in patients with overlap COPD and CHF. Second, we aim to describe the prevalence and mortality rate of the coexistence of COPD+CHF in outpatient subjects.
Methods
Design
Retrospective cohort study
All patients managed in the COPD and CHF outpatient clinic at our institution presenting both spirometry and echodopplercardiography during the year of 2014 were included. Their vital status was followed-up until May 2016. All data were obtained from an electronic medical record system [AGHweb®; Hospital de Clínicas de Porto Alegre (HCPA), Brazil], which contains the full medical history of the subjects attended at our institution. Functional parameters, number of hospitalizations within the previous year, clinical assessment and other predictors of the outcome were evaluated at baseline (study inclusion). The study was approved by the Research Ethics Committee (No. 14-0513) and, due to its retrospective nature, the obtention of informed consent was waived.
Participants
The main inclusion criteria for COPD were previously established clinical diagnosis, current or previous smoking >10 pack-year, plus spirometric evidence of post-bronchodilator expiratory airflow obstruction [forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC)<0.7] (Vogelmeier et al., Reference Vogelmeier, Criner, Martinez, Anzueto, Barnes, Bourbeau, Celli, Chen, Decramer, Fabbri, Frith, Halpin, López Varela, Nishimura, Roche, Rodriguez-Roisin, Sin, Singh, Stockley, Vestbo, Wedzicha and Agustí2017). CHF diagnosis was based on presence of Framingham criteria (Ho et al., Reference Hsieh1993) plus left ventricular ejection fraction (LVEF) <50% measured by echocardiography. Subjects with both diagnoses (COPD+CHF) comprise the ‘overlap’ group. Charlson comorbidity index was calculated (Charlson et al., Reference Charlson, Pompei, Ales and MacKenzie1987), and pulmonary and cardiovascular medication was recorded.
Measurements
Lung function
Spirometry was obtained (CPF®; Eric Jaeger, GmbH, Wüerzburg, Germany) according to international standards (Miller et al., Reference Miller, Hankinson, Brusasco, Burgos, Casaburi, Coates, Crapo, Enright, van der Grinten, Gustafsson, Jensen, Johnson, MacIntyre, McKay, Navajas, Pedersen, Pellegrino, Viegi, Wanger and Force2005).
Echocardiogram
Bidimensional transthoracic echocardiogram on M-mode (EnVisor C; Philips, Bothell, WA, USA) was performed according to the American Society of Echocardiography guidelines (Lang et al., Reference Lang, Badano, Mor-Avi, Afilalo, Armstrong, Ernande, Flachskampf, Foster, Goldstein, Kuznetsova, Lancellotti, Muraru, Picard, Rietzschel, Rudski, Spencer, Tsang and Voigt2015).
Modified Medical Research Council (mMRC) scale
Patients had to grade their self-perceived dyspnea by using pre-defined statements ranging from dyspnea only with strenuous exercise (0) to dyspnea to leave the house or when dressing or undressing (4) (Bestall et al., Reference Bestall, Paul, Garrod, Garnham, Jones and Wedzicha1999).
Functional capacity
It was evaluated by the New York Heart Association (NYHA) scale (Dolgin et al., Reference Dolgin, Association, Fox, Gorlin and Levin1994), a four-level classification based on a patient’s symptoms to perform graded physical activities.
Hospitalization rate
The number of hospitalizations due to COPD and/or CHF decompensation was recorded from the year preceding each patient inclusion (Müllerova et al., Reference Müllerova, Maselli, Locantore, Vestbo, Hurst, Wedzicha, Bakke, Agusti and Anzueto2015).
Inability to perform a CPET
This parameter was defined by researchers’ agreement (F.P. and D.C.B.) based on the clinical report and related complementary tests at study inclusion considering that the patient would not be able to perform a clinical exercise test for some reason (except social or cognitive).
Statistical analyses
Continuous data are presented as mean±SD, while categorical data as number (%). Overlap patients were contrasted by non-paired t or Mann–Whitney’s test or a χ 2 test for differences in proportions according their vital status at the end of follow-up. Univariate logistic regression analyses were performed to assess parameters at study inclusion [age, body mass index, FEV1 (% of predicted); FVC (% predicted); FEV1/FVC ratio; LVEF; mMRC; NYHA functional class; hospitalization rate; and inability to exercise] associated with mortality. The level of statistical significance was set at P<0.05. Receiver-operating characteristics (ROC) curve analysis selected the optimal threshold values for event prediction (MedCalc® for Windows, v.14.12.0, Ostend, Belgium). All remaining statistical analyses were performed using SPSS® statistical package (v.22.0.0.1, Chicago, USA).
Results
During the year of baseline assessment for inclusion (2014), 550 patients were evaluated. Of these, 301 had both spirometry and echocardiography: 160 (53%) with COPD on isolation; 100 (33%) with CHF on isolation; and 41 (14%) with overlap COPD plus CHF. The mean follow-up of the present cohort was 20.9±8.5 months, with similar mortality among the groups: COPD 17/160 (11%); CHF 12/100 (12%); and CHF–COPD 7/41 (17%) (P=0.73).
The baseline characteristics of the overlap group was compared according to survival status as presented in Table 1. On average, they presented moderate dyspnea and functional capacity reduction, high prevalence of other co-morbidities and moderate-to-severe reduction in FEV1 (Vogelmeier et al., Reference Vogelmeier, Criner, Martinez, Anzueto, Barnes, Bourbeau, Celli, Chen, Decramer, Fabbri, Frith, Halpin, López Varela, Nishimura, Roche, Rodriguez-Roisin, Sin, Singh, Stockley, Vestbo, Wedzicha and Agustí2017).
Table 1 Clinical and functional baseline parameters of the overlap chronic obstructive pulmonary disease + chronic heart failure group according vital status during the follow-up period
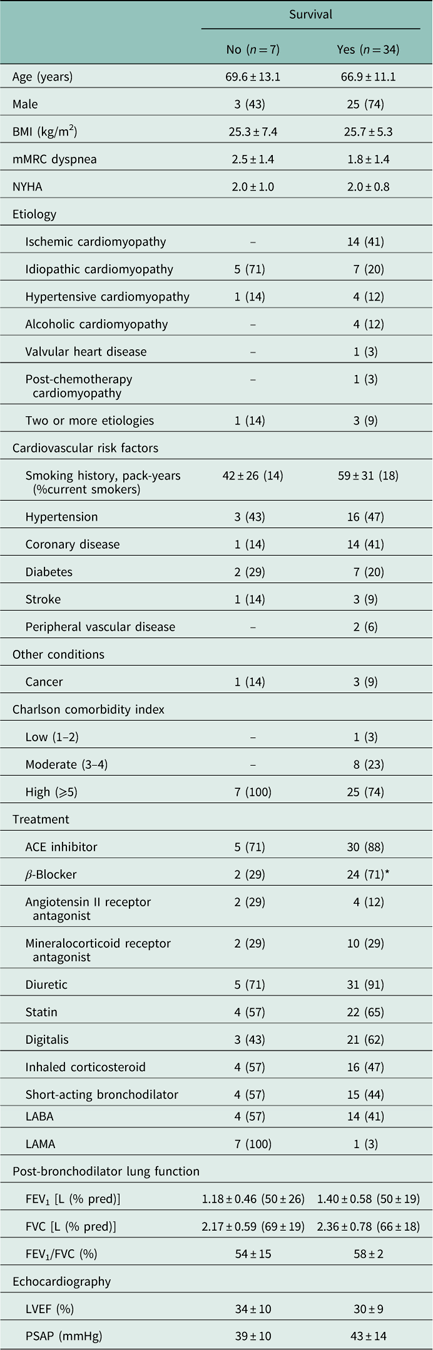
BMI=body mass index; mMRC=modified Medical Research Council dyspnea scale; NYHA=New York Heart Association Functional Classification; ACE=angiotensin-converting enzyme; LABA=long-acting β 2-agonist; LAMA=long-acting muscarinic antagonist; FEV1=forced expired volume in 1 s; FVC=forced vital capacity; % pred=% of predicted; LVEF=left ventricular ejection fraction; PSAP=pulmonary systolic arterial pressure.
Data are presented as mean±SD or n (%).
*P<0.05.
The proportion of patients with inability to perform a clinical exercise test [4/7 (57%) versus 6/34 (18%); P=0.03] was higher among non-survivors. The main reasons to consider a patient unable to perform an exercise test were: moderate-to-severe dyspnea at rest (n=4); intolerance to walk less than few meters (n=2); excessive lower limb pain (n=2), intolerable exercise angina with clinical optimized treatment and without indication/condition to invasive treatment (n=2). The hospitalization rate also tended to be higher among non-survivors (2.29±1.98 versus 0.74±0.99; P=0.08).
Accordingly, inability to exercise (nominal variable: yes/no) and hospitalization rate (ordinal variable: number of hospitalizations in the year preceding study inclusion) were associated with higher mortality (Table 2). Although the proportion of patients on β-blocker therapy was significantly higher among survivors (Table 1), no association was found between the use of β-blockers and survival (P>0.05).
Table 2 Mortality prediction based on univariate logistic regression analyses

CI=confidence interval; BMI=body mass index; mMRC=modified Medical Research Council dyspnea score; NYHA=New York Heart Association; FEV1=forced expired volume in 1 s; % pred=% of predicted; FVC=forced vital capacity; LVEF=left ventricular ejection fraction.
*P<0.05.
ROC curve analysis [area under the curve (95% CI)=0.794 (0.639–0.904); P<0.001] showed absence of hospitalizations as having a 100% sensitivity and >2 hospitalizations/year with 91% of specificity to predict mortality.
Discussion
The present study suggests that the history of previous COPD and/or CHF-related hospitalizations and clinical judgment of exercise incapacity to perform an exercise test may be potential predictors of future risk of death in patients with COPD plus CHF coexistence. Classical parameters of lung and heart function, as well as dyspnea and clinical functional capacity, were not associated with mortality in this group. Of particular clinical relevance, >2 hospitalizations in the preceding year before study inclusion were highly specific for a bad outcome (just 9% of false positive).
There are several therapeutic options to improve survival in each disease on isolation. The main challenge is to improve their utilization (Bender, Reference Bender2014; Thorvaldsen et al., Reference Thorvaldsen, Benson, Dahlström, Edner and Lund2016). Some of these options, however, are expensive and of limited availability. Therefore, optimal identification of patients with increased risk of mortality or more suitable for a given intervention is of clear relevance (DeCamp et al., Reference DeCamp, Blackstone, Naunheim, Krasna, Wood, Meli, McKenna and Group2006; Lund et al., Reference Lund, Matthews and Aaronson2010). Notwithstanding, despite the growing aged population with the coexistence of both conditions (Fabbri et al., Reference Fabbri, Luppi, Beghé and Rabe2008; Rutten, Reference Rutten, Cramer, Grobbee, Sachs, Kirkels, Lammers and Hoes2013) and the reciprocal modulation of the diseases (Apostolo et al., Reference Apostolo, Laveneziana, Palange, Agalbato, Molle, Popovic, Bussotti, Internullo, Sciomer, Bonini, Alencar, Godinas, Arbex, Garcia, Neder and Agostoni2015; Arbex et al., Reference Arbex, Alencar, Souza, Mazzuco, Sperandio, Rocha, Hirai, Mancuso, Berton, Borghi-Silva, Almeida, O’Donnell and Neder2016), prognostic studies in the context of coexistent diseases are still scarce in the literature.
Interestingly, clinical and physiological parameters currently incorporated in several prognostic models recommended by specific COPD (Vogelmeier et al., Reference Vogelmeier, Criner, Martinez, Anzueto, Barnes, Bourbeau, Celli, Chen, Decramer, Fabbri, Frith, Halpin, López Varela, Nishimura, Roche, Rodriguez-Roisin, Sin, Singh, Stockley, Vestbo, Wedzicha and Agustí2017) and CHF (Yancy et al., Reference Yancy, Jessup, Bozkurt, Butler, Casey, Drazner, Fonarow, Geraci, Horwich, Januzzi, Johnson, Kasper, Levy, Masoudi, McBride, McMurray, Mitchell, Peterson, Riegel, Sam, Stevenson, Tang, Tsai and Wilkoff2013) guidelines were not associated with mortality. Although our best efforts to include the highest number of patients, our sample is underpowered to detect some associations between baseline parameters and mortality depending on the magnitude of the association (Hsieh, Reference Ho, Pinsky, Kannel and Levy1989). Confirming the diagnosis of both diseases needs complementary exams and the usual more severe clinical condition of these patients challenges the recruitment and follow-up for research. Accordingly, the average sample size of the majority of previous studies in the scarce literature available is similar to ours (Rutten et al., Reference Rutten2005; Beghé et al., Reference Beghé, Verduri, Bottazzi, Stendardo, Fucili, Balduzzi, Leuzzi, Papi, Mantovani, Fabbri, Ceconi and Boschetto2013; Boschetto et al., Reference Boschetto, Fucili, Stendardo, Malagù, Parrinello, Casimirri, Potena, Ballerin, Fabbri, Ferrari and Ceconi2013; Alencar et al., Reference Alencar, Arbex, Souza, Mazzuco, Sperandio, Rocha, Hirai, Mancuso, Berton, Borghi-Silva, Almeida, OʼDonnel and Neder2016). Even though, some statistically significant associations were found. The small number of deaths in the present study, however, precludes the performance of multivariate logistic regression analyses evaluating the independent significance of these associations. We can verify, on the other hand, that these currently observed significant predictors have higher odds ratio to predict mortality than those without statistical significance (Hsieh, Reference Ho, Pinsky, Kannel and Levy1989) and represent potential candidates to refine survival models of COPD+CHF in next studies. If our results are confirmed in the future, from a clinical perspective, overlap patients presenting hospitalization(s) in the previous year and/or a clinical judgment of incapacity to perform an exercise test may be at high risk and considered potential candidates for add-on therapeutic options or palliation. For those able to exercise and, when available, a CPET could be performed in order to improve risk stratification (Mancini et al., Reference Mancini, Eisen, Kussmaul, Mull, Edmunds and Wilson1991; Oga et al., Reference Oga, Nishimura, Tsukino, Sato and Hajiro2003; Poggio et al., Reference Poggio, Arazi, Giorgi and Miriuka2010; Alencar et al., Reference Alencar, Arbex, Souza, Mazzuco, Sperandio, Rocha, Hirai, Mancuso, Berton, Borghi-Silva, Almeida, OʼDonnel and Neder2016; Neder et al., Reference Neder, Alharbi, Berton, Alencar, Arbex, Hirai, Webb and O’Donnell2016).
Finally, it was shown that even in stable outpatient subjects, the coexistence of COPD and CHF should not be neglected (14% in the present sample). Patients initially diagnosed with one disorder even without clear evidence of the other may pass unnoticed, once they share clinical, etiological and epidemiological factors (Fabbri et al., Reference Fabbri, Luppi, Beghé and Rabe2008; Rutten, Reference Rutten, Cramer, Grobbee, Sachs, Kirkels, Lammers and Hoes2013). Notwithstanding, the mortality rate was not significantly different among the groups (COPD=11%; CHF=12%; overlap=17%), possibly related to an underpowered sample (Macchia et al., Reference Macchia, Rodriguez Moncalvo, Kleinert, Comignani, Gimeno, Arakaki, Laffaye, Fuselli, Massolin, Gambarte, Romero and Tognoni2012).
To conclude, hospitalization rate and clinical judgment of incapacity to perform an exercise test were the unique investigated parameters associated with mortality in our sample. Nevertheless, the lack of association between other variables and mortality may be resultant of an underpowered sample.
Acknowledgments
The authors are thankful to all healthcare professionals who work in the care of COPD and heart failure patients in the outpatient clinic of Hospital de Clinicas de Porto Alegre (HCPA) and to the research unit (Grupo de Pesquisa e Pós-Graduação; GPPG) of HCPA due to statistical analysis support.
Financial Support
This study was funded by Fundo de Incentivo à Pesquisa do Hospital de Clinicas de Porto Alegre (FIPE-HCPA) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CAPES, Brazil).
Conflicts of Interest
The authors report no conflicts of interest.




