Introduction
Wildlife health surveillance is an essential component in the identification and management of potential threats to human and animal health (Ryser-Degiorgis Reference Ryser-Degiorgis2013). One of the main impediments to health surveillance in wild animals is the practical difficulty of obtaining biological samples for testing. For example, for many wild animal species, the only means of collecting a blood sample is under general anaesthesia, but this can be logistically challenging and expensive in the field and may have adverse physiological and behavioural effects on the animal (Soulsbury et al. Reference Soulsbury, Gray, Smith, Braithwaite, Cotter, Elwood, Wilkinson and Collins2020).
Administration of anaesthetic drugs invariably carries a risk, even for healthy animals in carefully controlled conditions (Clarke et al. Reference Clarke, Trim and Hall2014). In the field, anaesthesia of wild animals is often conducted under difficult circumstances or on individuals that are already compromised, and the risk of severe side-effects, injuries and death can never be eliminated (Arnemo et al. Reference Arnemo, Ahlqvist, Andersen, Berntsen, Ericsson, Odden, Brunberg, Segerström and Swenson2006). There are also longer-term impacts of general anaesthesia, including the potential for animals to exhibit behavioural changes post-anaesthesia that may affect their fitness and/or welfare (Machin & Caulkett Reference Machin and Caulkett2000). In addition, induction and maintenance of general anaesthesia require specialist equipment and a high level of training and skill (Soulsbury et al. Reference Soulsbury, Gray, Smith, Braithwaite, Cotter, Elwood, Wilkinson and Collins2020). The risks and challenges associated with the use of general anaesthesia to obtain important biological specimens from wild animals have led to interest in alternative sampling approaches that can be carried out on conscious animals (Luaces et al. Reference Luaces, Rossi, Aldana Marcos and Merani2011; Soulsbury et al. Reference Soulsbury, Gray, Smith, Braithwaite, Cotter, Elwood, Wilkinson and Collins2020; Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021).
The availability of a safe and reliable method for obtaining blood samples from badgers in the field without the need for general anaesthesia would expand options for disease surveillance and control interventions for this species. The European badger (Meles meles) is a medium-sized carnivore that is widely distributed across Europe (Neal Reference Neal1976). In the UK and Republic of Ireland, badgers are considered the most significant wildlife reservoir of the bacterium Mycobacterium bovis (M. bovis), the cause of bovine tuberculosis (bTB) (Krebs et al. Reference Krebs, Anderson, Clutton-Brock, Morrison, Young and Donnelly1997). Consequently, since the 1970s, badgers have been the subject of management interventions such as culling (Downs et al. Reference Downs, Prosser, Ashton, Ashfield, Brunton, Brouwer, Upton, Robertson, Donnelly and Parry2019) and vaccination (Benton et al. Reference Benton, Phoenix, Smith, Robertson, McDonald, Wilson and Delahay2020) in attempts to control infection in domestic cattle. Diagnostic testing of badgers for M. bovis with serological assays can be used to assess seroprevalence, identify infected individuals or populations for control purposes or monitor the success of intervention strategies (Maas et al. Reference Maas, Michel and Rutten2013). However, employing these assays requires blood sampling of free-living animals that has, to date, necessitated general anaesthesia with all the attendant risks and challenges. To address this, the Animal and Plant Health Agency (APHA) developed a bespoke physical restraint cage along with a protocol for obtaining a small blood sample using capillary sampling (skin puncture) from the metatarsal pad of a restrained conscious badger (Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021).
Wildlife management activities, including procedures to collect biological samples, have the potential to negatively impact the welfare of the targeted animals, and such impacts should be minimised as far as possible (Dubois et al. Reference Dubois, Fenwick, Ryan, Baker, Baker, Beausoleil, Carter, Cartwright, Costa, Draper, Griffin, Grogan, Howald, Jones, Littin, Lombard, Mellor, Ramp, Schuppli and Fraser2017; Proulx et al. Reference Proulx, Allen, Cattet, Feldstein, Iossa, Meek, Serfass and Soulsbury2022). A key step, prior to pursuing operational deployment of any new management method, such as the restraint cage approach of blood sampling a conscious badger, is to assess the relative welfare impacts of the method. Assessment of wild animal welfare is challenging, not least because complete data are often not yet available (Rae et al. Reference Rae, Nicol and Simmonds2023), and hence carefully elicited expert opinion may be the best available method to assess the overall welfare impact of any given procedure or scenario (McGreevy et al. Reference McGreevy, Berger, de Brauwere, Doherty, Harrison, Fiedler, Jones, McDonnell, McLean, Nakonechny, Nicol, Preshaw, Thomson, Tzioumis, Webster, Wolfensohn, Yeates and Jones2018). Expert elicitation is a multi-disciplinary systematic process for formalising expert opinions to help fill data gaps and characterise uncertainty where traditional scientific research is not possible, or data are not yet available (European Food Safety Authority [EFSA] 2012). Sharp and Saunders (Reference Sharp and Saunders2011) developed an approach, based on the Five Domains model (Beausoleil & Mellor Reference Beausoleil and Mellor2015; Mellor et al. Reference Mellor, Beausoleil, Littlewood, McLean, McGreevy, Jones and Wilkins2020), to seek expert opinions to assess the relative humaneness of pest animal control methods. The Sharp and Saunders welfare assessment model provides a framework to promote systematic and comprehensive consideration of impacts on the welfare of a subject animal or animals (Beausoleil et al. Reference Beausoleil, Fisher, Littin, Warburton, Mellor, Dalefield and Cowan2016, Reference Beausoleil, Baker and Sharp2022). Application of the model following a clearly articulated process (Hampton et al. Reference Hampton, Hemsworth, Hemsworth, Hyndman and Sandøe2023) by a diverse group of experts can be used to develop a defensible consensus outcome regarding the relative welfare impacts of various methods or procedures, which should increase acceptance of the outcome (Baker et al. Reference Baker, Sharp and Macdonald2016).
The aim of our study was to compare the animal welfare impacts of the novel restraint cage approach of blood sampling conscious badgers with those of the only current alternative for obtaining blood samples from badgers in the field, which involves administration of a general anaesthetic. To achieve this aim, we applied the Sharp and Saunders model, eliciting expert opinion based on available empirical evidence. The overall objective of these assessments was to expand the number of acceptable approaches for obtaining blood samples from badgers in the field.
Materials and methods
Welfare impacts associated with the two methods of blood sampling free-living badgers were evaluated systematically using a modified version of the Sharp and Saunders model. Assessments were made by a panel of experts using information from the scientific literature, field experience and discussion to reach consensus. The assessments were conducted during two online workshops on 15th March 2021 and 25th January 2022.
Panellist selection
Eleven experts were invited to participate as panellists for the workshops. They were selected based on their expertise in animal welfare science (including previous experience of applying the model), their expertise in badger biology, behaviour, and ecology, or their special knowledge of one or both of the methods being evaluated. A number of the panellists had expertise in more than one of these areas. To mitigate the risk of unconscious bias among APHA panellists towards one of the blood-sampling methods, invitations to participate deliberately included independent external experts. The final group comprised five animal welfare scientists (four external and one employed by APHA), plus two wildlife biologists, two badger capture and sampling specialists and two veterinary surgeons (all employed by APHA), providing appropriate breadth and depth of expertise, including practical experience of both blood-sampling methods. The Government department funding the development of the novel restraint cage approach was not represented at either workshop.
Workshops and assessment materials
Ten panellists were present at each workshop, nine of whom attended both. The participants were based in the UK, Australia and New Zealand. Both workshops were chaired by an independent external researcher with relevant multidisciplinary expertise and prior experience of using the model (SEB), who provided guidance on applying the model and facilitated the assessments.
During the first workshop, cage trapping (the method of capturing free-living badgers and the common first stage of both blood-sampling methods) and the two blood-sampling approaches were assessed. At this time, the restraint cage had only been trialled in one area where the badgers were subject to regular trapping as part of a long-term study. At the second workshop, the welfare impacts of the restraint cage method were assessed again, now informed by additional new data from further field trials of the restraint cage method on badgers from other UK populations that were naive to trapping. The second workshop was considered important because the response to restraint of badgers subject to routine trapping may not necessarily be generalisable to a naive population (Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021) and assessments should be refined with the inclusion of new data whenever it becomes available (Hampton et al. Reference Hampton, Jones, Perry, Miller and Hart2016).
Approximately one week before each workshop, panellists were provided with the three relevant APHA Standard Operating Procedures (SOPs): one for each blood-sampling method and one for the common cage trapping component. The SOPs (see Supplementary material) listed the necessary equipment for the procedure, highlighted safety critical activities and described the sequential steps to follow, to ensure the techniques were performed correctly, consistently and in sequence. The panellists were also provided with background reading material and a description of the welfare assessment model and were asked to read the documents prior to attending the workshops. The background reading material (see Supplementary material) comprised peer-reviewed and grey literature on known and potential welfare-relevant impacts of cage trapping and the blood-sampling methods. This information was identified through a literature search undertaken by one of the researchers (AC) using Google Scholar. The search was conducted without date limits, using combinations of the term ‘badger’ with the following: ‘welfare’, ‘trap*’, ‘anaesthesia’, ‘sedation’ and ‘restrain*’, using the Boolean operator ‘AND’. Citation tracking and topic knowledge were used as supplementary search methods. Full texts were reviewed by AC and all potentially relevant articles were shortlisted. The collated information was summarised, and a number of full scientific papers were provided, with relevant parts highlighted for easy reference. Where information was sparse for badgers, relevant evidence from other mammalian species was included.
For the second workshop, the documents provided for the first were supplemented with a new unpublished report on field trials of the restraint cage on badgers naive to trapping. During this second workshop, panellists were also shown video clips of the restraint cage method being applied to previously trap-naive badgers. The video clips were selected by AC to show the different stages of the method, including both successful and failed sampling attempts and the full range of behaviours (including passive and actively resistant behaviour) that badgers exhibited in response to restraint and sampling.
Welfare assessment model
There are two parts to the model (Sharp & Saunders Reference Sharp and Saunders2011): Part A examines the impact of an activity on overall welfare and the duration of this impact, excluding any action that causes death; Part B examines the intensity of suffering and duration of suffering associated with any killing technique applied. Our assessments used only Part A of the model because both blood-sampling methods are non-lethal procedures.
Each blood-sampling method involves four stages (see Table 1) and therefore a multi-stage approach (Humaneness Assessment Panel 2015) was used to evaluate the welfare impacts of each stage of each method separately.
Table 1. Summary of the stages of the two blood-sampling methods (for full details see Supplementary material)
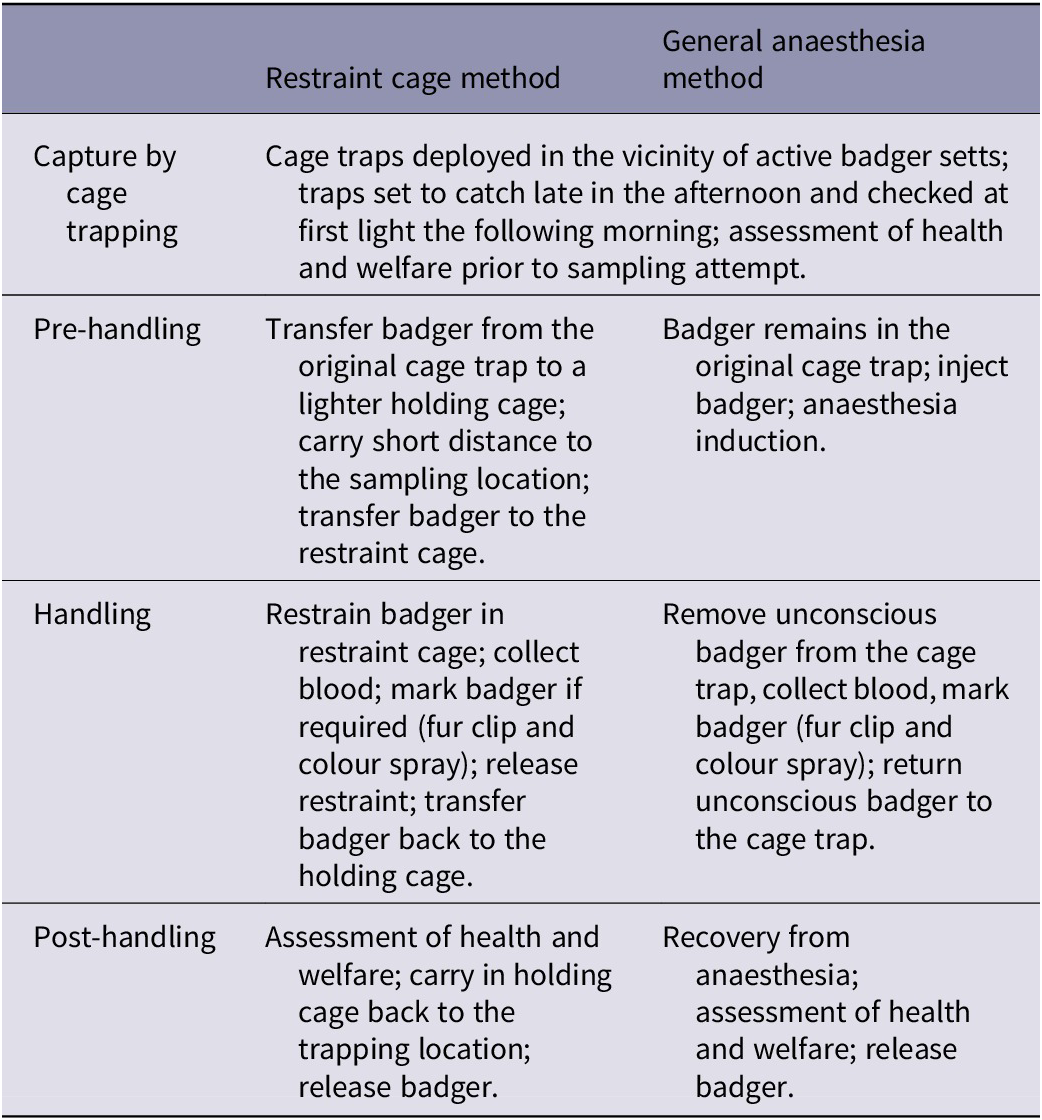
For the purposes of the welfare assessment, it was assumed that the blood sampling and related activities were successfully implemented on badgers in strict accordance with best practice prescribed by the APHA SOPs. The assessments were based on the likely experiences of most animals in the majority of situations, but uncommon scenarios were considered and discussed.
Welfare impacts were considered in each of five inter-related domains based on the Five Domains model (Beausoleil & Mellor Reference Beausoleil and Mellor2015; Mellor et al. Reference Mellor, Beausoleil, Littlewood, McLean, McGreevy, Jones and Wilkins2020). The five domains include four physical/functional domains: Domain 1, nutrition; Domain 2, environment; Domain 3, health; Domain 4, behavioural interaction; and one mental domain: Domain 5, mental state (see Figure 1). For each of Domains 1–4, panellists assigned an impact intensity grade (none, mild, moderate, severe or extreme impact), using the scientific literature provided and with reference to a set of Part A impact scales (see Supplementary material). Impact intensity grades assigned in Domains 1–4 were based on observable/measurable indicators of impacts on the physical/functional state of the animal including pathology, injury and physiological and behavioural responses. The impact in Domain 5 represents mental experiences, such as fear, pain, breathlessness, dizziness and others, arising from impacts in the first four physical/functional domains. Data from Domains 1–4 were used to cautiously infer the animal’s likely mental experiences in Domain 5, which cannot be assessed directly (Mellor & Beausoleil Reference Mellor and Beausoleil2015). The grade assigned in Domain 5 was usually equal to the highest of the Domain 1–4 impact grades (if this was not the case, an explanation was provided).
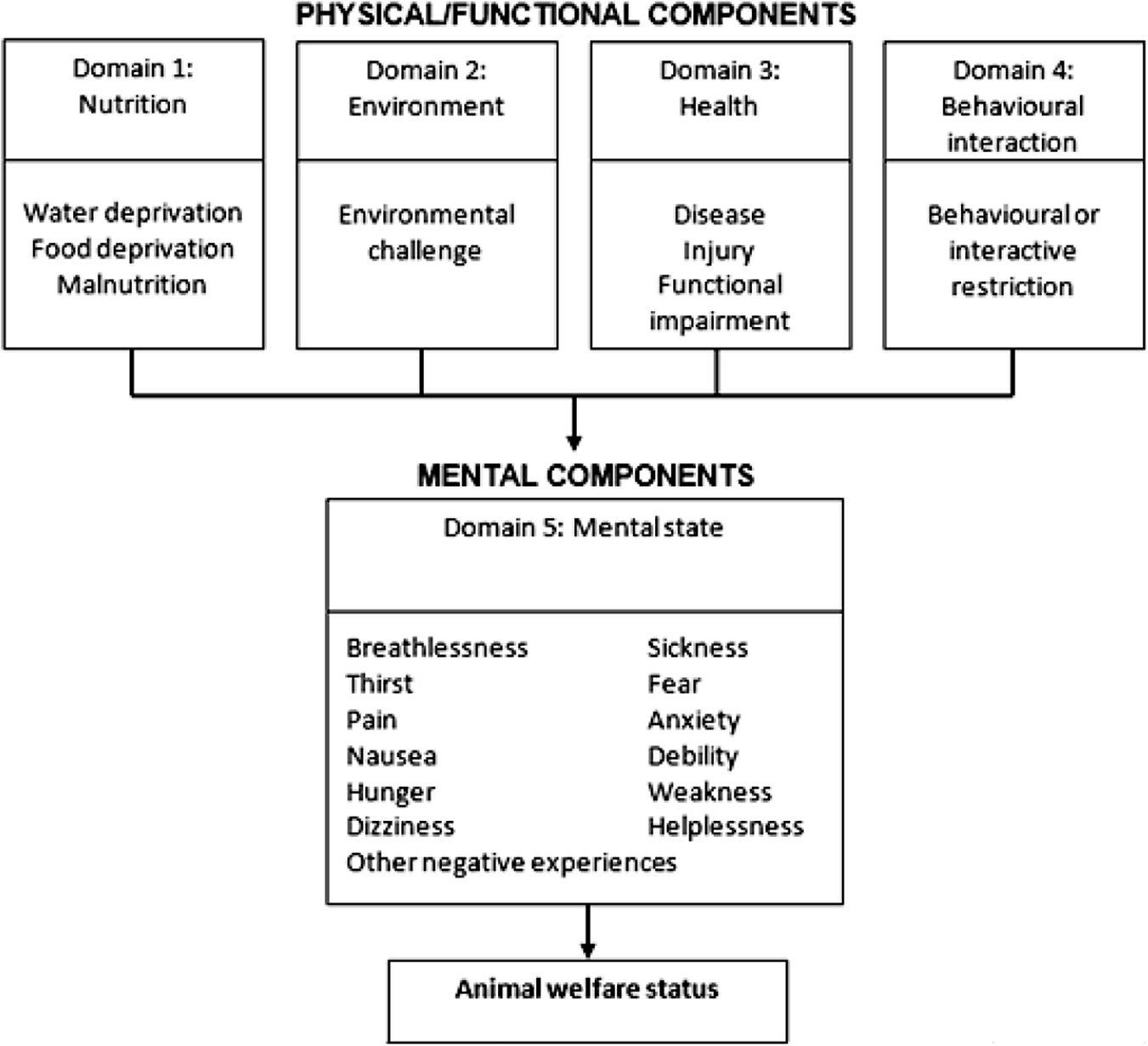
Figure 1. The Five Domains of the Sharp and Saunders Model for Humaneness Assessment with examples of situations or events that could cause negative physical/functional impacts in Domains 1–4, leading to negative mental experiences inferred in Domain 5 (examples listed). Adapted from Beausoleil and Mellor (Reference Beausoleil and Mellor2015).
Ultimately, the panellists assigned an overall impact intensity grade for each stage of each method; this was consistently the grade allocated in Domain 5. The panellists also assigned a category representing the duration for which the impact was likely experienced by the animal (immediate to seconds, minutes, hours, days, weeks). Finally, the overall impact intensity and duration were integrated using a Part A scoring matrix (Figure 2) to assign an overall welfare score for each stage of each method, potentially ranging from 1 (no impact) to 8 (severe/extreme impact for days/weeks). No attempt was made to aggregate the overall welfare scores for all four stages of a method into a single overall welfare score for that method (see Discussion).
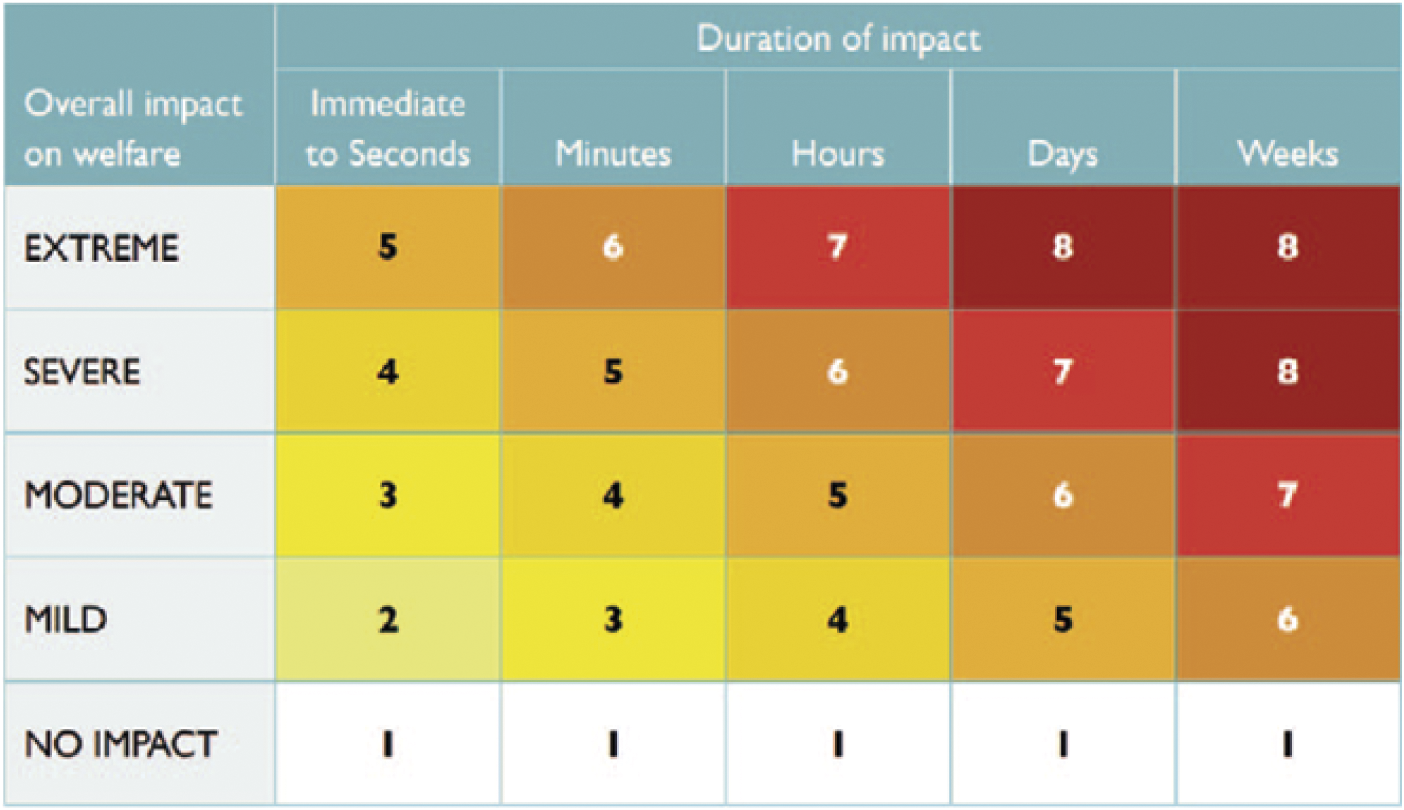
Figure 2. Part A Scoring matrix for integrating the intensity of overall welfare impacts and their duration. Reproduced with permission from Sharp and Saunders (Reference Sharp and Saunders2011).
Process for assigning scores during the workshops
Panellists discussed, assessed and scored each stage of each method in turn. Based on their understanding developed from reading the SOPs and background reading material, and initial discussion during the workshop, each panellist shared impact intensity grades and duration categories, and these were discussed, challenged and defended, and developed collectively to reach consensus. The chairperson (SEB) was responsible for ensuring that no one panellist was able to exercise disproportionate influence, allowing the opinions of all panellists to be heard by inviting contributions in turn.
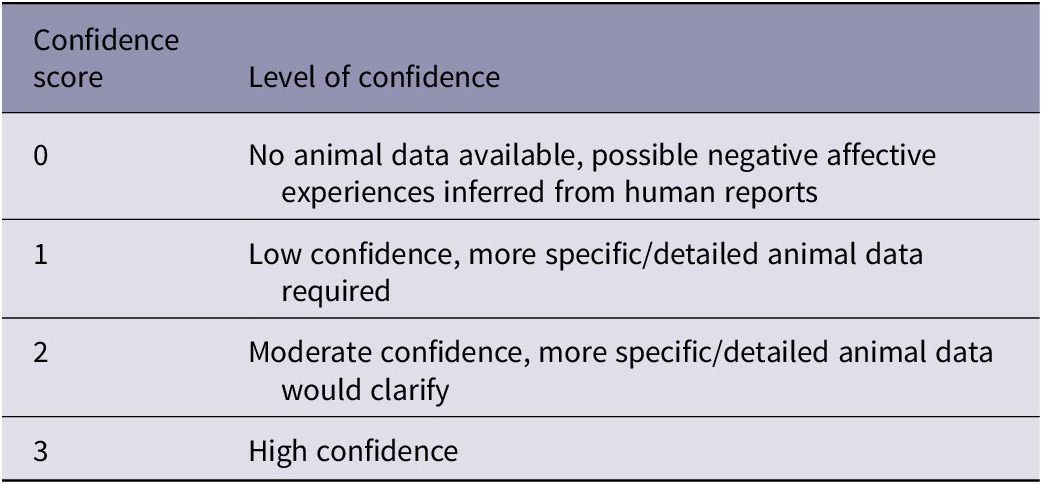
All assessment outcomes were recorded on a worksheet. For the overall impact intensity grades and the duration categories assigned, each panellist also nominated a score between 0 and 3 (see Table 2) reflecting their associated level of confidence in the outcome; the median and range of these scores were recorded.
Table 2. Confidence scores applied to overall impact intensity grades and the duration categories assigned. Adapted from Beausoleil et al. (Reference Beausoleil, Fisher, Littin, Warburton, Mellor, Dalefield and Cowan2016)
Components of the two blood-sampling methods evaluated
Full details of the two methods assessed are provided in the SOPs (see Supplementary material).
(i) Capture/Live cage trapping stage
Irrespective of whether physical or chemical methods are to be used to facilitate collection of a biological sample from a free-living badger, it first needs to be captured. Badgers are largely nocturnal, live in social groups and use underground burrow systems called setts (Neal Reference Neal1976). Capture is typically achieved in the UK by deploying cage traps in the vicinity of active badger setts, with more traps set than the expected number of badgers present (saturation trapping) (Cheeseman & Mallinson Reference Cheeseman, Mallinson, Amlaner and Macdonald1979). Peanuts are used as bait and the traps are pre-baited for a period of 7–10 days, prior to being set to catch for two nights. The traps are set to catch late in the afternoon and checked at first light the following morning; thus, the longest a badger should be held in a trap is approximately 14 h. Trapping is suspended from February 1st to April 30th to reduce the risk of capturing lactating females with their dependent cubs left underground (Woodroffe et al. Reference Woodroffe, Bourne, Cheeseman, Cox, Donnelly, Gettinby, McInerney and Morrison2005a). Prior to the next stage of either blood-sampling method, an observational health and welfare assessment of the badger is performed to confirm it is fit for sampling.
(ii) Pre-handling stage
Restraint cage method
The badger is transferred from the original trap to a lighter holding cage. This is achieved by placing the holding cage on the ground next to the trap; the two adjacent doors are opened. If necessary, the badger is encouraged to move into the holding cage by gently nudging it with a wicket (a pronged metal or plastic insert). The badger is carried in the holding cage a short distance (less than 100 m) to the sampling location where it is transferred (same method as the previous transfer from trap to holding cage) to the bespoke restraint cage.
General anaesthesia method
The badger is anaesthetised, in the trap where it was captured, by intramuscular injection in the thigh region of a triple combination of ketamine hydrochloride (100 mg ml–1, Ketavet, Zoetis UK Ltd), medetomidine hydrochloride (1 mg ml–1, Domitor, Vetoquinol UK Ltd) and butorphanol tartrate (10 mg ml–1, Dolerex, MSD Animal Health UK Ltd) at a ratio of 2:1:2 by volume, respectively, and a dose rate of approximately 0.2 ml kg–1 (equivalent to 8 mg kg–1 ketamine hydrochloride, 0.04 mg kg–1 medetomidine hydrochloride and 0.8 mg kg–1 butorphanol tartrate) (de Leeuw et al. Reference de Leeuw ANS, Spyvee, Brash and Delahay2004) (each badger’s bodyweight is estimated by visual assessment).
(iii) Handling stage
Restraint cage method
This approach is described in detail by Smith et al. (Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021). The restraint cage measures 610 × 285 × 280 mm (length × width × height) and is constructed from PPA 571-coated steel mesh. It has a cushioned movable internal wall, a solid floor and two sliding panels through which the hindfoot of a restrained badger can be accessed (Figures 3 and 4). Wickets (pronged metal inserts) can be inserted horizontally if required to restrict internal cage height which, together with the movable internal wall, provide physical restraint. The movable wall is operated by a ratchet system, making it possible to increase the degree of restraint gradually, and the cushions attached to the wall provide a soft contact surface with one side of the badger. Once the badger is securely restrained, a hind leg is extracted by hand through one of the access panels (left or right) in the front of the cage. The metatarsal pad is cleaned and dried before a thin smear of petroleum jelly is applied to its surface. A 4-mm lancet is used to make a small puncture wound through the epidermis of the foot pad to produce blood flow and blood is collected from the surface of the pad into a capillary collection device. On completion of blood collection, gentle pressure is applied to the pad to stem any residual blood flow. If necessary, the badger is marked by clipping an area of fur on the rump or back with curved scissors to remove the outer dark guard hairs; the clipped area is then sprayed with a brightly coloured livestock marker (this temporary mark avoids badgers captured on consecutive trapping nights being sampled twice). An animal can be marked whilst being restrained or after removal of restraint. The badger is then transferred from the restraint cage back to the holding cage (same method as previous cage transfers).
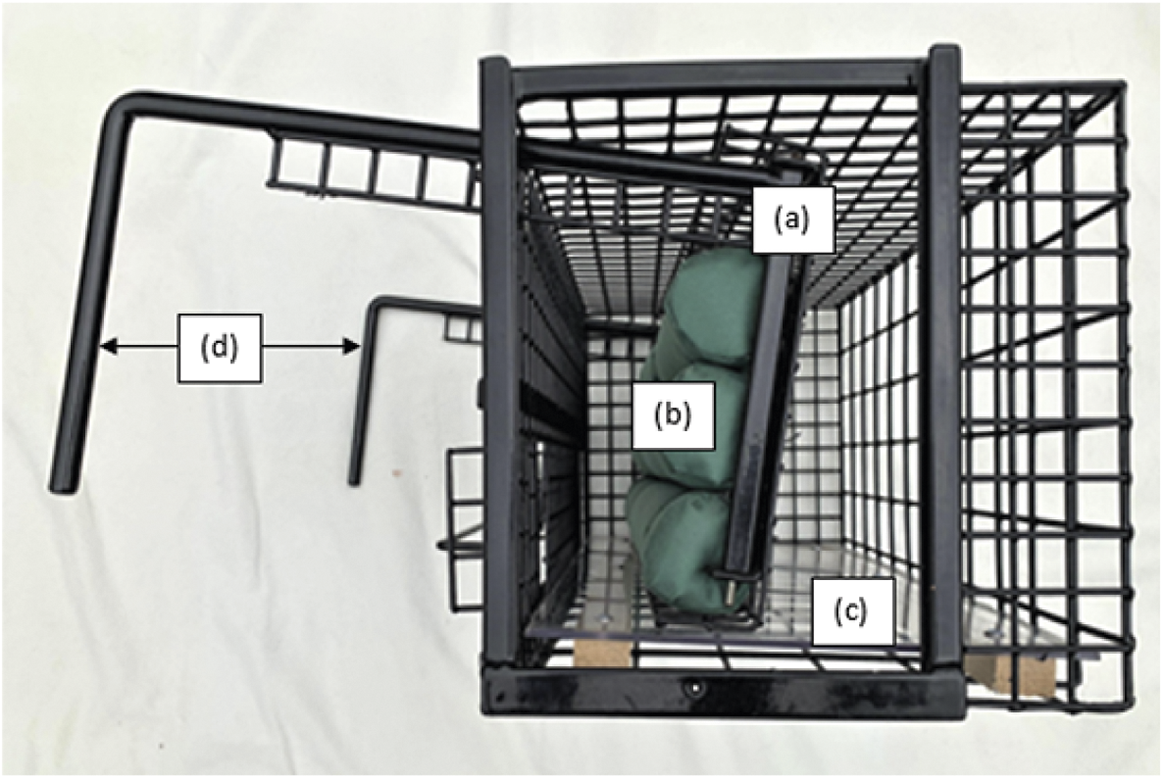
Figure 3. View of end of restraint cage (with door removed) showing (a) movable internal wall, (b) cushions attached to movable wall, (c) solid floor and (d) ratchet arms.
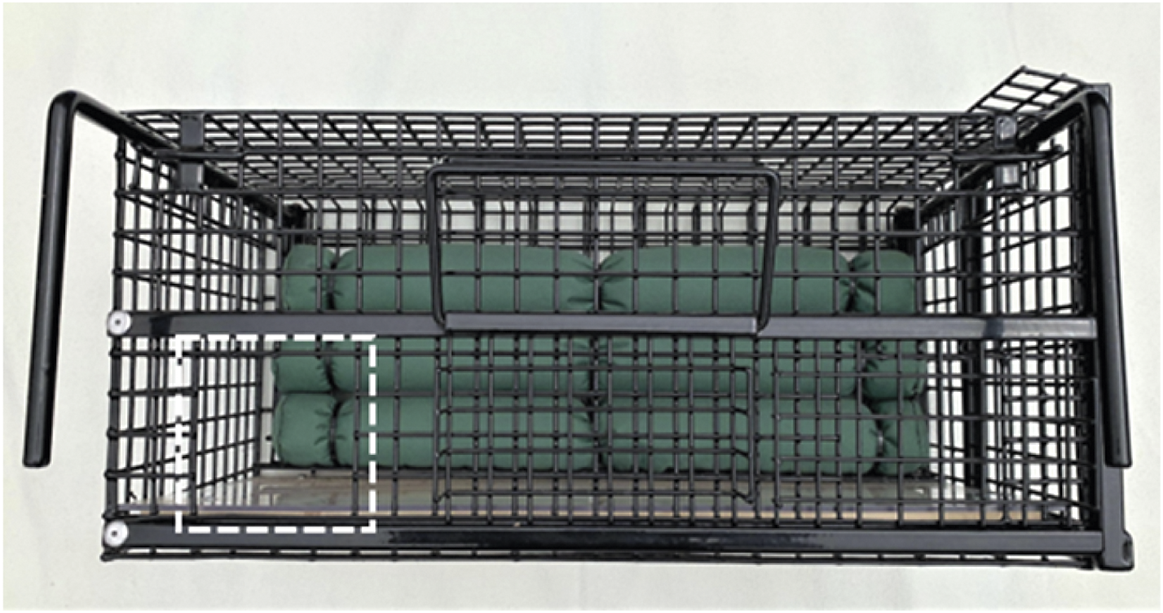
Figure 4. View of front of restraint cage showing left sliding panel (to access hindfoot) open (dashed white line). An identical sliding panel is present on the right side of the cage, to accommodate a badger facing in either direction.
General anaesthesia method
The anaesthetised (unconscious) badger is removed from the trap and 2 ml of blood is collected via jugular venepuncture into a blood collection tube. The animal is then marked using the same technique described for the restraint cage method. The unconscious badger is then returned to the trap to recover from the anaesthetic.
(iv) Post-handling stage
Restraint cage method
After a satisfactory observational health assessment to confirm it is fit for release, the badger is carried in the holding cage back to the trap location where it is released.
General anaesthesia method
Once recovered from the anaesthetic (awake and showing normal responsiveness and movement), and after a satisfactory observational health assessment to confirm it is fit for release, the badger is released.
Ethical considerations
The welfare assessments conducted for this study did not use any animals; ethical approval was not required to carry out this study.
Results
Capture/Live cage trapping
Cage trapping was assigned an overall welfare score of 4–5, based on mild to moderate impact lasting hours (Table 3 and Figure 5). The badger may experience mild to moderate nutritional impacts in Domain 1; although bait (peanuts) is present in the trap, water is not available, and so the animal may be deprived of water for a number of hours. The badger may experience mild impacts in Domains 2 and 3. Environmental exposure could last for a number of hours, but trapping is suspended when adverse weather conditions are expected, and traps are positioned to take advantage of natural shelter. The badger may sustain injuries when trying to escape from the trap, but these are usually only minor skin abrasions and severe injuries are rare. Woodroffe et al. (Reference Woodroffe, Bourne, Cox, Donnelly, Gettinby, McInerney and Morrison2005b) assessed trap-related injuries in badgers captured in cage traps, finding that 88% had no detectable injuries, whilst 72% of those injured had only minor skin abrasions and 1.8% had damage to the teeth or jaws that may have caused serious pain. A moderate impact was assigned to Domain 4 because, although the captured badger can move freely within the confines of the trap (current trap dimensions: 102 × 36 × 36 cm), its behaviour and movement are restricted, including an inability to perform normal behaviour such as foraging and social interactions. In terms of experiences under Domain 5 (mental experience), it is likely that a badger will experience some thirst (based on Domain 1), mild pain if injured (based on Domain 3) and anxiety/fear and frustration for hours related to being behaviourally restricted (Domain 4), resulting in mild to moderate mental impacts in Domain 5. Although it is likely that being captured is a stressful experience for a badger (Schütz et al. Reference Schütz, Ågren, Amundin, Röken, Palme and Mörner2006), with a moderate impact in Domain 4, only mild to moderate mental impacts were assigned to Domain 5 because many individual badgers can be repeatedly recaptured (Tuyttens et al. Reference Tuyttens, Macdonald, Delahay, Rogers, Mallinson, Donnelly and Newman1999). The high recapture rate observed suggests that the overall experience is not sufficiently aversive to cause long-term avoidance of the cage trap (Paul et al. Reference Paul, Edgar, Caplen and Nicol2018), although other factors such as the attractiveness of the bait and the interval between captures must be considered.
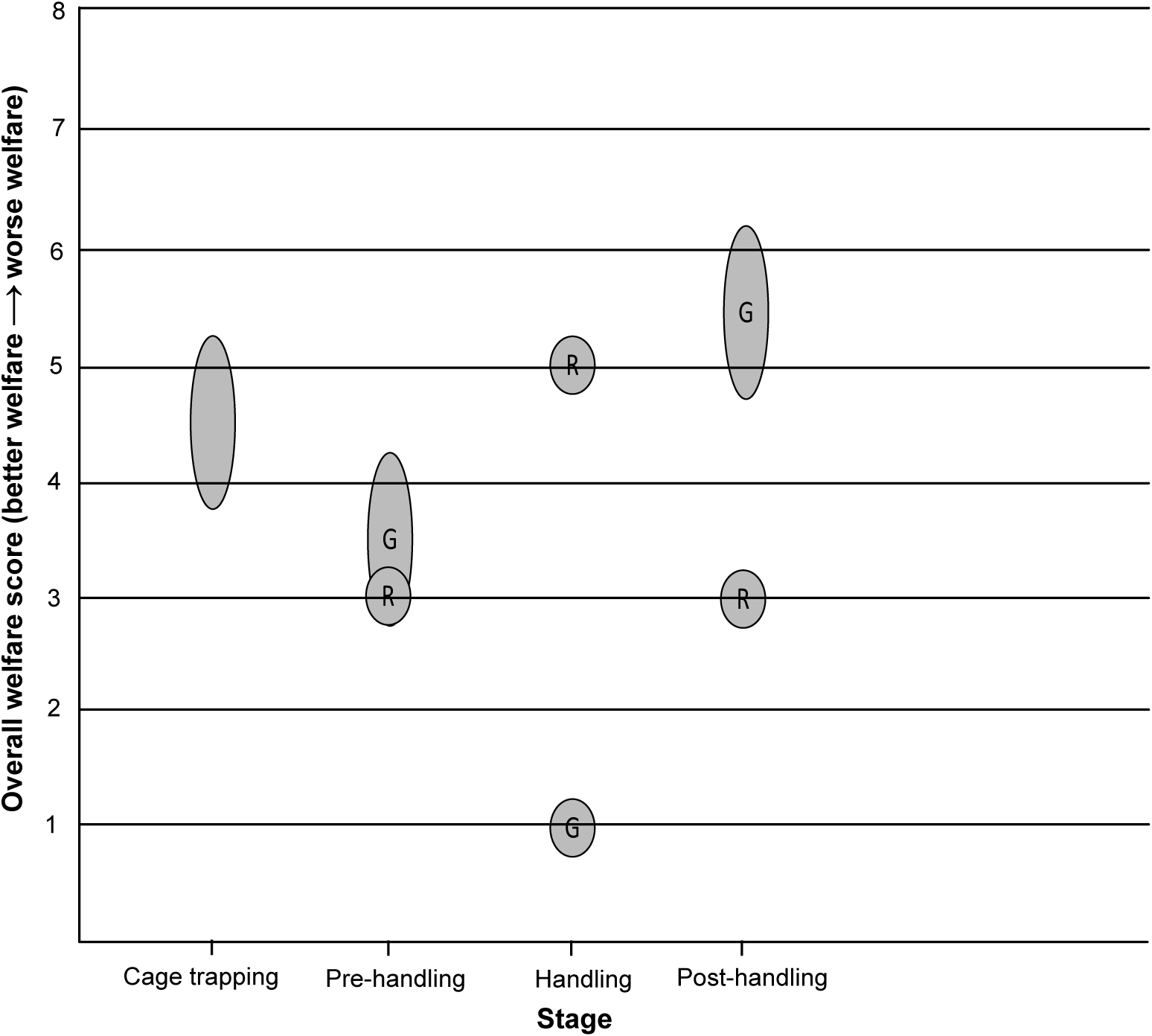
Figure 5. Overall welfare scores for the two blood-sampling methods for free-living European badgers (Meles meles). A circular bubble represents a discrete overall welfare score. An oval bubble represents a range of overall welfare scores. R = Restraint cage method. G = General anaesthesia method.
Table 3. Welfare assessment results for each stage of two blood-sampling methods for free-living European badgers (Meles meles). Relative impact intensity grades in each of four physical/functional domains and one mental domain, overall impact intensity grade (none, mild, moderate, severe, extreme) and duration category (immediate to seconds, minutes, hours, days, weeks). Overall welfare scores (shown in bold) were derived from Overall impact and Duration using the Part A scoring matrix (Figure 2)
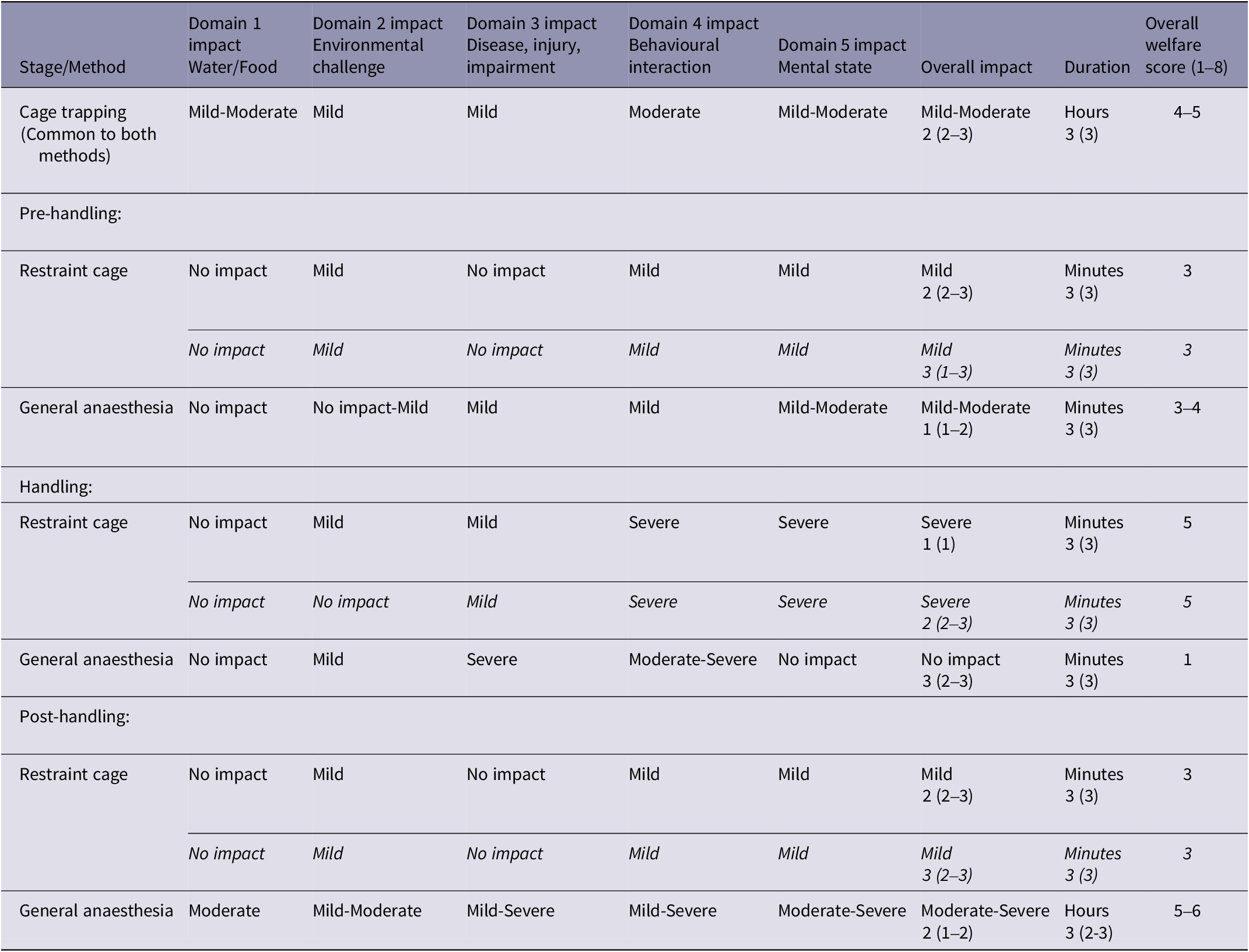
The numbers in the Overall impact and Duration columns are the median confidence scores (and the range of confidence scores), explained in Table 2.
The restraint cage method was assessed at both workshops. The results from the first workshop are presented in italics below the updated results from the second workshop.
Restraint cage pre-handling stage
The restraint cage pre-handling stage was assigned an overall welfare score of 3, based on mild impact lasting minutes (Table 3 and Figure 5). This score resulted from mild impacts in Domains 2 and 4. Environmental exposure is brief and consists mainly of low-level noise from human voices and movement. A badger may experience mild behavioural impacts because some individuals must be encouraged to move during the transfer between cages, but this is achieved by a light touch using a wicket. It is likely that the badger will experience mild anxiety/fear (Domain 5) for minutes related to human proximity and handling during this stage.
Restraint cage handling stage
The restraint cage handling stage was assigned an overall welfare score of 5, based on severe impact lasting minutes (Table 3 and Figure 5). During the several minutes that physical restraint is applied, the badger is almost completely immobilised and is incapable of most normal defensive or escape behaviours, thus eliciting a severe impact intensity grade for Domain 4. The badger may experience mild impacts in Domains 2 and 3. Environmental exposure is brief, and a quiet location is chosen for restraint and sampling, although there is a degree of low-level human noise. Some badgers will be exposed to the odour and noise of a livestock marker spray. Although the density of pain receptors in a badger’s metatarsal pad is unknown, the lancet puncture wound required to achieve blood flow is a minor injury and likely to cause only momentary pain. When sampled badgers have subsequently been examined under anaesthesia on the same day, the lancet incision was barely visible and in all cases was free from bruising, swelling or continued bleeding (Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021). The animal is unlikely to sustain any injuries as a direct result of the physical restraint (Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021). The high level of physical handling when restraint is applied would likely result in severe anxiety/fear and/or frustration and possibly breathlessness (Domain 5) for minutes, but there is a lack of data on the overall impact of such restraint on badgers.
Restraint cage post-handling stage
The restraint cage post-handling stage was assigned an overall welfare score of 3, based on mild impact lasting minutes (Table 3 and Figure 5). This score resulted from mild impacts in Domains 2 and 4. The human proximity and low-level noise during this stage would be expected to result in mild anxiety/fear in Domain 5.
General anaesthesia pre-handling stage
The general anaesthesia pre-handling stage was assigned an overall welfare score of 3–4, based on mild to moderate impact lasting minutes (Table 3 and Figure 5). During this stage, the badger may experience mild impacts in Domains 2, 3 and 4. General anaesthesia can disrupt thermoregulation in mammals (Pottie et al. Reference Pottie, Dart, Perkins and Hodgson2007), but field data show that a badger’s rectal temperature is usually within the normal range shortly after induction of anaesthesia (M Siwonia, personal communication 2021). The intramuscular injection of the anaesthetic cocktail causes a minor injury. There is brief human proximity and interaction when the anaesthetic is injected, but the badger is subsequently left undisturbed, unless a ‘top-up’ injection is required. Some badgers are difficult to inject, and a wicket will be used to restrict their movement in the trap, but this is not required for the majority (Sun et al. Reference Sun, Stevens, Newman, Buesching and Macdonald2015). Although induction of anaesthesia appears to be smooth (the transition from conscious to unconscious occurs in the absence of problems such as excitement) in most cases, it is impossible to ascertain the degree of various negative mental impacts a badger may experience, including anxiety/fear, dizziness, or confusion, as a consequence of the anaesthetic drugs and the progressive functional impairment that occurs while the animal is still conscious. This uncertainty, combined with the principle of giving the animal the benefit of doubt, accounts for the mild to moderate mental impacts assigned to Domain 5, higher than the maximum of the scores for Domains 1–4.
General anaesthesia handling stage
The general anaesthesia handling stage was assigned an overall welfare score of 1, based on no impact lasting minutes (Table 3 and Figure 5). During this stage, there are impacts in Domains 2, 3 and 4. Due to the potential impact of anaesthesia on thermoregulation, management measures to help maintain normothermia (e.g. wrapping cold badgers in bubble-wrap) are implemented as required. There is temporary severe functional impairment, including a total inability to move. The badger is also temporarily unable to perform any natural behaviour. However, it is assumed that the badger is unconscious throughout this stage and thus incapable of any mental experiences in Domain 5.
General anaesthesia post-handling stage
The general anaesthesia post-handling stage was assigned an overall welfare score of 5–6, based on moderate to severe impact lasting hours (Table 3 and Figure 5). The badger may experience moderate nutritional impacts in Domain 1. It could be moderately dehydrated by this stage; water is not provided in the trap because of the risk of an incapacitated badger dropping its head into the drinker and experiencing breathing problems. Anorexia is reported as an uncommon undesirable effect of ketamine anaesthesia in humans (Electronic Medicines Compendium 2022). The badger may experience mild to moderate impacts in Domain 2. It is likely that the badger’s physiological capacity to thermoregulate remains compromised for the duration of this stage and could remain compromised to some degree for a period after release. The badger is severely functionally impaired (Domain 3) at the start of this stage, but functional capacity is progressively regained as recovery from general anaesthesia progresses and this is accompanied by a return to consciousness. The possibility of some long-term effects of general anaesthesia on functional capacity, beyond release, cannot be excluded. It is also possible that a badger could sustain undetected injuries during the recovery period, particularly if it is confused or agitated at a point where full consciousness has not been regained. It is unable to perform any natural behaviour (Domain 4) at the start of this stage, but this ability also progressively returns as consciousness is regained, although there is some degree of behavioural restriction up to the point of release. Although there is gradual amelioration of the functional and behavioural impacts in Domains 3 and 4 as this stage progresses, the impact in each of these domains at the start of the stage is severe and the badger is likely to be conscious for much of this time. Thus, it is likely that the badger will experience moderate thirst based on Domain 1 and moderate to severe anxiety/fear, frustration or confusion related to initially being very functionally (Domain 3) and behaviourally (Domain 4) restricted, resulting in moderate to severe mental impacts in Domain 5. Overall, it is likely that there will be moderate to severe welfare impacts for over an hour.
Differences in grading/scoring the restraint cage method between the first and second workshops
Only Domain 2 of the handling stage of the restraint cage method was graded differently by the panel at the second workshop. This reflected the panel at the second workshop acknowledging the mild environmental impacts of very low-level human noise during this stage and exposure of some badgers to the odour and noise of a livestock marker spray. The overall impact grade and duration category (and hence the overall welfare score) assigned to this stage were the same at both workshops (Table 3).
The median confidence scores nominated by the panel for the overall impact grades for the pre-handling, handling and post-handling stages of the restraint cage method were consistently lower at the second workshop (Table 3).
Discussion
There are both moral (Littin et al. Reference Littin, Mellor, Warburton and Eason2004) and regulatory (Home Office 2014; Natural England 2019) obligations to reduce, wherever possible, any negative impacts of wildlife research and management activities on the welfare of the animals involved. Approaches should seek to minimise negative impacts and be subject to continual improvement (Baker et al. Reference Baker, Ayers, Beausoleil, Belmain, Berdoy, Buckle, Cagienard, Cowan, Fearn-Daglish, Goddard, Golledge, Mullineaux, Sharp, Simmons and Schmolz2022). Here, we used an established (Cowan et al. Reference Cowan, Forrester and Warburton2013; Beausoleil et al. Reference Beausoleil, Fisher, Littin, Warburton, Mellor, Dalefield and Cowan2016; Baker et al. Reference Baker, Ayers, Beausoleil, Belmain, Berdoy, Buckle, Cagienard, Cowan, Fearn-Daglish, Goddard, Golledge, Mullineaux, Sharp, Simmons and Schmolz2022) welfare assessment model (Sharp & Saunders Reference Sharp and Saunders2011) to systematically assess the relative impacts of two methods of blood sampling a live badger in the field. The outcomes clearly demonstrated that both methods have negative impacts on badger welfare. There was no evidence of the restraint cage approach being worse for welfare and this method possibly has a lower overall negative welfare impact than the use of general anaesthesia. The panellists’ confidence scores indicated that there are gaps in our understanding of the welfare impacts of both blood-sampling methods. Panellists were generally less confident about grading the intensity of impacts than about categorising their duration, probably because it is easier to estimate how long inferred affective states might be present than to estimate the degree/intensity of the inferred state.
The cage trapping stage is common to both blood-sampling methods and therefore, by definition, does not differentiate between them. The overall welfare score (4–5) reflects hours of mild to moderate impact when a badger is captured in a trap. Panellist confidence was medium for this impact, indicating that more data would be useful, particularly information on the effects of behavioural restriction on the welfare of cage-trapped badgers. The impact of cage trapping could potentially be reduced by checking traps at night, which would reduce the time badgers spent in them. However, this would mean working in the dark and, as badgers are more active at night, handling may be more difficult (Woodroffe et al. Reference Woodroffe, Bourne, Cox, Donnelly, Gettinby, McInerney and Morrison2005b). Checking traps at night could also reduce capture success because of the additional human disturbance in the trapping location.
Relative welfare impacts of the restraint cage method
The pre- and post-handling stages of the restraint cage method were both assigned an overall welfare score of 3, reflecting minutes of mild impact. Panellist confidence was medium for the overall impact grades, indicating that more specific data would clarify the type and intensity of any negative mental experiences. The impact of the restraint cage method could potentially be reduced if it were possible to reduce the number of cage transfers. For operator safety and comfort, the current SOP prescribes that the badgers are moved from the cage trap location, which is often inaccessible to vehicles, to the tailgate of a pick-up truck, where the restraint cage can be operated at a safe and comfortable working height. As the cage trap and restraint cage are both unsuitable for carrying badgers any distance, this current practice necessitates transferring them to a holding cage to move them to the truck, where they are transferred to the restraint cage for blood sampling. Systematic evaluation of the restraint cage method conducted for this study indicated that the current SOP could be revised, with the aim of reducing the number of cage transfers, possibly by moving to a procedure where the badger is transferred directly from the cage trap to the restraint cage at the trap location, and the restraint cage is then operated whilst placed on top of the trap. A potential further refinement of the method would be to modify the cage trap so it could also be used for restraint and blood sampling, eliminating the requirement for any cage transfers, although no practical means of achieving such modification has been identified to date.
The overall welfare score for the restraint cage handling stage (5) reflects minutes of severe impact. Panellist confidence was low because of a lack of badger-specific data on the effects of such physical restraint, but it is very likely to be stressful as suggested by studies in other mammals. For example, manual restraint in ferrets (Mustela putorius furo) (Schoemaker et al. Reference Schoemaker, Mol, Lumeij, Thijssen and Rijnberk2003), which are closely related to badgers, and squeeze cage restraint and venepuncture in female rhesus monkeys (Macaca mulatta) (Fuller et al. Reference Fuller, Hobson, Reyes, Winter and Faiman1984), resulted in a significant increase in plasma cortisol (a stress hormone) concentration. However, use of the restraint cage method in regularly captured UK badgers was not associated with a decrease in subsequent recapture probability (Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021), suggesting that the experience of physical restraint did not increase aversion to subsequent cage trapping.
Although more data on the impact of physical restraint on wild badgers are required, the panellists adopted a cautious approach during our assessment. The welfare assessment model has only one impact grade higher than ‘severe’ (‘extreme’), and the Part A impact scales used to support the assessment process are clear that this highest level of impact would usually be associated with death of the animal (Sharp & Saunders Reference Sharp and Saunders2011). The restraint cage method does not appear to cause a badger any permanent disability or physical harm (Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021) and a physical restraint device has also been used without any discernible deleterious physical effects on sea otters (Enhydra lutris) (closely related to badgers) to accomplish various procedures including blood sampling (Williams et al. Reference Williams, Baylis, Downey and Clark1990).
Although the overall impact grades and duration categories (and hence the overall welfare scores) assigned to all stages of the restraint cage approach were the same at both workshops, the median confidence scores assigned to the overall impact grades were consistently lower at the second workshop, despite the panel composition being almost identical at both workshops (just one panellist present at the first workshop was different for the second). This decrease in confidence was reported even though additional data including video clips of the restraint cage procedure were available at the second workshop. The psychology of human confidence is outside the scope of this paper, but it is interesting to speculate that a type of Dunning-Kruger effect (individuals with limited knowledge fail to accurately appraise their own knowledge) (Dunning Reference Dunning2011) resulted in overconfidence at the first workshop, this being adjusted downward by improved knowledge of the restraint cage method at the second workshop.
Relative welfare impacts of the general anaesthesia method
The overall welfare score for the pre-handling stage of the general anaesthesia method (3–4) reflects minutes of mild to moderate impact. In contrast, the score for the post-handling stage (5–6) reflects hours of moderate to severe impact. For these stages, our assessments were complicated by the fact that the badger is experiencing a transition between consciousness and unconsciousness. Assessment of consciousness in animals is a key challenge for robust assessment of animal welfare state in a number of settings (Steiner et al. Reference Steiner, Flammer, Beausoleil, Berg, Bettschart-Wolfensberger, Garcia Pinillos, Golledge, Marahrens, Meyer, Schnitzer, Toscano, Turner, Weary and Gent2019). We attempted to address this by assigning impact grades considering only the parts of the stages when the badger is likely to be conscious and capable of mental experiences, but the lack of certainty about when unconsciousness begins, or consciousness returns, and the level of consciousness required for welfare-relevant experiences is reflected by the confidence of panellists in the overall impact grades for these stages. Panellist confidence was low for the mild to moderate grade assigned to the pre-handling stage and medium for the moderate to severe grade for the post-handling stage. Nevertheless, the panel again adopted a cautious approach for both stages, giving the animal the benefit of the doubt with respect to its capacity to experience negative mental impacts.
The overall welfare score for the general anaesthesia handling stage (1) relates to minutes of no impact. For transparency, assessment of this stage was undertaken by assigning an impact grade to each of the four physical/functional domains, but then assuming that the badger is unconscious and incapable of any mental experiences during this stage, resulting in ‘no impact’ being assigned to Domain 5. Panellist confidence was high for the ‘no impact’ grading. A badger retaining some awareness of its surroundings was not considered likely by the panellists. Although ketamine-based anaesthesia has been associated with intraoperative awareness in human adults (Villegas et al. Reference Villegas, Suarez, Owuor, Wuyke, Nelson, Imbamba, Rogo, Rogo and Burke2019), the triple combination of ketamine hydrochloride, medetomidine hydrochloride and butorphanol tartrate was reported to generally produce a state of balanced anaesthesia in badgers with the animals presumed to be completely unconscious and exhibiting relaxed muscle tone (de Leeuw et al. Reference de Leeuw ANS, Spyvee, Brash and Delahay2004).
While technically possible, it is unlikely that chemical reversal of general anaesthesia would reduce the overall welfare score assigned to the post-handling stage. The effects of the alpha-2 adrenoreceptor agonist, medetomidine hydrochloride, can be eliminated using the specific antagonist, atipamezole (Veterinary Medicines Directorate 2019). This can be useful in situations where reversal of the effects of medetomidine is clinically indicated, such as respiratory or cardiac depression or prolonged recovery. However, routine reversal of all anaesthetised badgers using atipamezole is not recommended in the APHA SOP because of the risk of poor-quality recovery, possibly due to undesirable effects of residual ketamine once unopposed by the sedative effects of medetomidine or due to a direct excitatory effect of atipamezole (Thornton et al. Reference Thornton, Newman, Johnson, Buesching, Baker, Slater, Johnson and Macdonald2005). While reversal with atipamezole would reduce the recovery time from anaesthesia (e.g. average 17 min [range 2–47] from injection to sternal recumbency [de Leeuw et al. Reference de Leeuw ANS, Spyvee, Brash and Delahay2004]), it is unlikely to change the duration of impacts during recovery to full mobility from ‘hours’ and may increase the type/intensity of negative impacts experienced by recovering badgers in the post-handling stage.
Qualitative assessment of multiple stages of blood-sampling methods
In this assessment, we chose to separately evaluate each of four stages comprising the two approaches to blood sampling free-living badgers, whilst acknowledging that the cumulative effects of the multiple stages may compound the overall impact on animal welfare (Humaneness Assessment Panel 2015). We did not attempt to aggregate numeric scores from across the different stages but rather discussed the overall qualitative impacts of each method. This is because numerical aggregation can imply precision in scoring welfare impacts, which is not possible when the approach to assessment is predicated on understanding welfare state through the animals’ various affective experiences, which cannot be measured or quantified (Beausoleil & Mellor Reference Beausoleil and Mellor2015). In addition, the methods chosen for aggregation have ethical implications and any approach to aggregation should be carefully considered and transparently represented (Sandøe et al. Reference Sandøe, Corr, Lund and Forkman2019).
Both blood-sampling methods have negative welfare impacts, but their overall welfare scores were not higher than intermediate, never exceeding a qualitative score of 5–6 out of a possible 8. Our assessments suggest that the restraint cage approach may have a lower cumulative negative impact as only one stage (the handling stage) has a higher score than general anaesthesia and the highest scoring stage for either method was the post-handling stage for general anaesthesia. Although physical restraint is very likely to be stressful for a wild badger, the average duration is less than four minutes (Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021). Sampling under restraint not only avoids the much lengthier general anaesthesia procedure, resulting in the animal spending substantially less time away from its sett, but it also avoids the adverse physiological and behavioural effects of general anaesthesia and any unpleasant mental experiences associated with induction and/or subsequent recovery. Sampling under restraint also circumvents the potential negative impacts of a badger being inadvertently returned to the wild before it has completely recovered from general anaesthesia, when it may be more susceptible to conspecific aggression and other threats (Soulsbury et al. Reference Soulsbury, Gray, Smith, Braithwaite, Cotter, Elwood, Wilkinson and Collins2020).
In addition to the potential animal welfare gains from using the restraint cage approach over the general anaesthesia approach, blood sampling under restraint is likely to be achieved at lower financial cost, including reduced requirement for specialist veterinary support. Restraint cages can be used repeatedly and avoid the costs associated with anaesthetic drugs and the time required for field anaesthesia (Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021). The availability of the restraint cage method for operational deployment could expand current options for bTB surveillance and disease control interventions in badgers by permitting more efficient trap-side sampling and testing (Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021). However, a limitation of the restraint cage method is that, because it employs capillary blood sampling from a foot-pad, only a very small volume of blood can be collected, which may present challenges for diagnostic tests. Fortunately, at least one of the currently available serological assays (Dual-Path Platform [DPP®] VetTB assay, Chembio Diagnostic Systems Inc, USA) for detection of M. bovis infection in badgers can be performed using just 10 μl of whole blood (Ashford et al. Reference Ashford, Anderson, Waring, Davé, Smith, Delahay, Gormley, Chambers, Sawyer and Lesellier2020), although this is not sufficient volume for other diagnostic assays. Another limitation of the restraint cage method is that restraint is more difficult to achieve for badger cubs, particularly smaller ones (Smith et al. Reference Smith, Rogers, Tomlinson, Arnold, Benton, Spyvee, Boxall, Whiteside and Delahay2021).
Utility of the Five Domains model as an animal welfare assessment tool
The modified version of the Sharp and Saunders model used for our assessments is based on the Five Domains model. Application of the Five Domains model as an operational tool for welfare assessment has recently been explored and several useful recommendations made for improving the clarity of outcomes (Hampton et al. Reference Hampton, Hemsworth, Hemsworth, Hyndman and Sandøe2023). Of particular relevance to our study is the recommendation to provide explicit information regarding the selection criteria for panellists and about the processes used for sourcing the data (scientific literature) and developing the welfare impact grades/scores, including accounting for uncertainty.
There will always be inherent limitations in elicitation of expert opinion; expert judgements have the potential to be biased, inaccurate or self-serving (Martin et al. Reference Martin, Burgman, Fidler, Kuhnert, Low-Choy, McBride and Mengersen2012). However, we have described transparently how our expert panel was convened, the diverse expertise of the panellists and the measures taken to facilitate equitable interaction amongst them and mitigate the risk of biased outcomes. We are confident our panel collectively had appropriate detailed biological knowledge of wild badgers, understanding of animal behaviour, physiology and animal welfare science, and experience of the model as an assessment tool, to effectively interpret complex data and produce defensible and useful outcomes.
For our assessments, we chose to use a consensus-development workshop method, resulting in a single overall welfare score for each stage of each blood-sampling approach. Hence, no statistical analysis of welfare scores was required. An alternative approach, to mitigate the risk of a dominant panellist influencing others and to formally account for variability among panellists, could have been to collect independently derived individual scores (based on the literature and videos provided) and subject them to non-parametric statistical analysis to detect any differences between the methods. However, we chose not to take this approach owing to the low statistical power offered by a small number of data-points, and to avoid suggesting an inappropriate level of precision in our assessment of relative welfare impacts. The consensus-development method also offered the benefits of group interaction, including sharing of knowledge and better appreciation of disciplinary viewpoints (Knol et al. Reference Knol, Slottje, van der Sluijs and Lebret2010).
Although we assigned numerical overall welfare scores for the different stages of the two blood-sampling approaches, it should be emphasised that these scores are ordinal and so, for example, designating a score of six to the general anaesthesia post-handling stage does not indicate that its impacts are twice as negative as the restraint cage post-handling stage, which scored three. The information provided by our assessments allows us to rank the two blood-sampling approaches according to their relative welfare impacts, but it is important to note that the overall welfare scores are fundamentally qualitative, rather than quantitative in nature (Beausoleil & Mellor Reference Beausoleil and Mellor2015). We also acknowledge the general challenges associated with integrating information on the intensity and duration of welfare impacts. For example, is a moderately intense impact for a prolonged period better or worse than a severe impact experienced for a short time? The answer to this question probably depends on the inherent unpleasantness of the experience, in addition to its intensity and duration, but as we do not have a common metric for this, the question remains unresolved (Beausoleil & Mellor Reference Beausoleil and Mellor2015).
We addressed the issue of uncertainty arising from incomplete information by providing confidence scores for the overall impact intensity grades and duration categories assigned using a clear approach relating to data availability (Beausoleil et al. Reference Beausoleil, Fisher, Littin, Warburton, Mellor, Dalefield and Cowan2016). These confidence scores represent a caveat to the tentative overall welfare scores reported, as well as revealing areas requiring additional empirical research. Thus far, only a few wildlife management-related welfare assessments have explicitly represented uncertainty associated with the outcomes (Hampton et al. Reference Hampton, Hemsworth, Hemsworth, Hyndman and Sandøe2023); these include Fisher et al. (Reference Fisher, Beausoleil, Warburton, Mellor, Campion and Booth2010), Beausoleil et al. (Reference Beausoleil, Fisher, Littin, Warburton, Mellor, Dalefield and Cowan2016) and Baker et al. (Reference Baker, Ayers, Beausoleil, Belmain, Berdoy, Buckle, Cagienard, Cowan, Fearn-Daglish, Goddard, Golledge, Mullineaux, Sharp, Simmons and Schmolz2022). This addition to the Sharp and Saunders model reflects the iterative refinement and improvement expected of such frameworks with experience of their application (Sharp & Saunders Reference Sharp and Saunders2011), improvement which will continue as long as the philosophy and operational approaches to welfare assessment are discussed and debated (Hampton et al. Reference Hampton, Hemsworth, Hemsworth, Hyndman and Sandøe2023).
Repeatability is a fundamental requirement of any reliable scientific measurement method. Our study repeated the assessment of the restraint cage blood-sampling approach, using new additional data once it were available, and the overall welfare scores assigned to all stages were the same at both workshops, although the confidence scores for the overall impact grades were lower at the second workshop. These outcomes indicate that the panel’s original opinion remained consistent with the new data, but with less certainty, suggesting an absence of fixed opinions relating to bias or vested interest. The Sharp and Saunders model stipulates that the assessment panel should include a range of experts relating to the species and methods being assessed (Sharp & Saunders Reference Sharp and Saunders2011), so it may not always be possible to repeat assessments with a completely new panel that has the same breadth and depth of expertise. Notwithstanding this challenge, we acknowledge that it could be informative to further examine the reliability of our assessment outcomes, through different panels performing Five Domains model assessments of the two blood-sampling methods, to determine whether these panels repeat our findings.
It is important that the limitations of any animal welfare assessment approach relying on expert opinion are understood (Sharp & Saunders Reference Sharp and Saunders2011), but the utility of available qualitative information and expert knowledge should not be ignored or diminished (EFSA 2012). If researchers refrain from systematic expert evaluation of animal welfare impacts until all relevant empirical data are available, we will fail to move forward in our understanding of these important impacts, particularly where the empirical data are not practically obtainable (EFSA 2012).
We acknowledge the limitations of the Sharp and Saunders model discussed above (Sharp & Saunders Reference Sharp and Saunders2011; Beausoleil & Mellor Reference Beausoleil and Mellor2015; Baker et al. Reference Baker, Sharp and Macdonald2016; Hampton et al. Reference Hampton, Jones, Perry, Miller and Hart2016, Reference Hampton, Hemsworth, Hemsworth, Hyndman and Sandøe2023). However, we contend that when applied by a diverse panel with relevant expertise, informed by an appropriate broad range of empirical evidence from the scientific literature, the model provides a versatile and practical tool to helpfully advance evaluation of the animal welfare impacts of wildlife management activities. The utility of the model should not be disregarded, particularly in the absence of an equivalent alternative.
Animal welfare implications
Assessing animal welfare is challenging, but robust, transparent, science-based systems for assessing the welfare impacts of wildlife management interventions should be pursued to improve processes and ensure genuine consideration of the welfare of wild animals (Beausoleil et al. Reference Beausoleil, Baker and Sharp2022). Although requiring cautious interpretation and cognisance of the limitations of the assessment model (Beausoleil & Mellor Reference Beausoleil and Mellor2015; Hampton et al. Reference Hampton, Hemsworth, Hemsworth, Hyndman and Sandøe2023), our results suggest that selection of the restraint cage method of blood sampling for operational deployment, over general anaesthesia, would ameliorate to some degree the negative welfare impacts of blood sampling badgers in the field. However, the low level of confidence in some of the impact grades assigned to each method suggests that our assessment would be more reliable if better underpinning data were available. Although gaps in our scientific understanding remain, it seems reasonable to cautiously move forward with operational deployment of the restraint cage method, expanding the number of acceptable approaches for obtaining blood samples from badgers in the field, provided the number of animals subjected to the method is minimised as field experience is gathered and all operators receive sufficient guidance to implement the method in accordance with best practice. As further field experience of the method is acquired and more empirical data are collected, ongoing monitoring and assessment of the welfare impacts will be crucial to ensure refinements are made as necessary in the interests of continual improvement of animal welfare (Hampton et al. Reference Hampton, Jones, Perry, Miller and Hart2016).
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/awf.2024.16.
Acknowledgements
SEB advised on study design and use of the welfare assessment methodology, as well as facilitating the workshops. AC organised the workshops, prepared assessment materials and wrote the first draft of the manuscript. Nine of the eleven participants in the workshops are authors on this paper. All authors have reviewed and commented on the drafts of the manuscript. All authors have read and approved the final manuscript. This work was funded by Defra (project APHATBOM0430).
Competing interest
The authors declare that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. AC, JL, RC, MS and RD are employed by the APHA. SEB, NJB, TS and HG were remunerated as expert consultants to the APHA for their contributions to the two workshops. HG is employed as Chief Executive/Scientific Director of the Universities Federation for Animal Welfare (UFAW) and is Joint Editor-in-Chief of Animal Welfare which is published by UFAW in conjunction with Cambridge University Press; he was not involved in the editorial processing of this paper. The funder of this work played no role in the study design, welfare assessment process and writing of the paper.









